Abstract
Large cranial defects of complex geometric shapes are challenging to reconstruct. The cranial implants has to be fabricated prior to the cranioplastic surgery. The ideal material for cranial implant has to be inert, light weight, easy to fit and adaptable to the defect, offering the best aesthetic and functional results. Here is a clinical case report of a patient who was operated for osteomyelitis in the parieto-temporal region. The defect was reconstructed with heat cure polymethylmethacrylate (PMMA). Operative closure of the defect was facilitated with ligature titanium wires with minimal prosthesis contouring. The heat cure PMMA cranial implant is a safe, easy and economic alternative with great adaptability to cranial vault defects. The cosmetic results in this patient was excellent. No post-operative complications occurred.
Keywords: Craniofacial prosthesis, Reconstruction, Heat cure PMMA
Introduction
Cranioplasty is defined as a neurosurgical procedure to cover an injured bone in the skull [1]. This procedure is carried out in order to protect and restore intracranial structures and to restore the appearance and psychological stability of the patient [2]. In the absence of the bone fragments, the brain is covered only by the dura mater, subcutaneous tissue and the scalp, thus have both aesthetic and functional deficits. Seizure episodes occasionally occurs as a secondary complication. The brain is left exposed to trauma and there is an increased possibility of meningitis [3]. Reconstruction of the bones of the skull is a complex procedure and represents a challenge for both prosthetic and surgical team.
The goals of cranioplasty are to restore aesthetics, protect the underlying brain, and maintains intracranial pressure relationships [4]. Throughout history, different materials have been used in the manufacture of cranial implant [5] among them the most common are (i) acrylics such as heat cure polymethylmethacrylate (PMMA) [6–9], (ii) implants designed from bone grafts [10–14], (iii) ceramic materials such as hydroxyapatite [15]. Furthermore, in the group of biocompatible metals, titanium alloys are highlighted [16, 17]. (iv) Polyetheretherketone (PEEK) computer-designed implant based on the defect evaluated by computed tomography [3]. The ideal material is inert, lightweight, easy to fit and adaptable to the defect, offering the best aesthetic and functional results.
Advances in medical imaging, such as MRI and CT, have allowed the 3D reconstruction of anatomical structures for several medical applications, including the design of custom-made cranial implants [1]. The CAD–CAM technology and Rapid prototyping makes the fabrication of cranial implants much easier and precise based on the CBCT imaging but these advancement in this field hikes the cost of the whole procedure.
Heat cure PMMA had been used extensively as the material for cranioplasty [18] in near past. This clinical report highlights the methodology for the fabrication of the custom made acrylic implant in a simpler methodology and the surgical approach for the successful management of parieto-temporal cranial defect.
Clinical Report
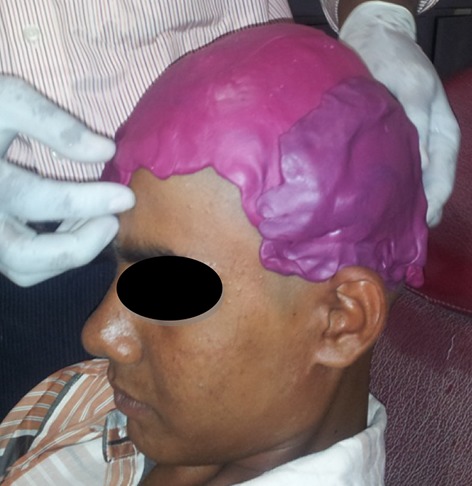
A young boy aged 14 years came to our Department of Prosthodontics, Vinayaka Mission’s Sankarachariyar Dental College, Salem, India in Oct 2012 with the chief complaint of bulging in the left side of the head in the place of previous surgery giving an unpleasant appearance. He had history of fall at the age of 9 years and had sub-dural hematoma. On evacuation of hematoma, patient had repeated pus discharge till the age of 12 years and diagnosed as chronic osteomyelitis. Patient had seizures and upper limb weakness. Craniotomy and excision of calcified dural membrane was done followed by duroplasty. The loss of bone resulted in the outward growth of the soft tissue (muscle) and cartilages. The herniation was in the left parieo-temporal region measuring about 10 × 8 cm in diameter and about 12 mm projected (Fig. 1). The patient had no neurological problems after craniotomy. On examination the bony margins were felt and the enlargement was painless, soft, non edematous and not pedunculated.
Fig. 1.

Pre operative
Initially, the patient’s zero magnification X-rays in both posterio anterior and lateral views were obtained to allow for accurate sizing and identification of bony irregularities. CT scan was also used for the measurement of the dimension of the defect. Comparison of the bony margins were done with the palpation, X-ray and CT findings and marked with eosin pencil. After ensuring the bony margins the prosthetic phase of rehabilitation was started.
A custom tray was fabricated using the low fusing impression compound (Fig. 2), vents were made for the escape of the excess impression material and these acts as retentive plug for the impression material thus helps to retain the impression material in custom tray and for easy withdrawal of the impression from the skull without tear of the impression material. Cranial moulage was made with irreversible hydrocolloid impression material (Hydrogum soft, Zhermack, Italy)] (Fig. 3) and the master cast was fabricated using Type II gypsum material (AAC Super Grade, Pollachi, India) (Fig. 4). Here the master cast was used as the diagnostic cast. Sectional impression of the herniated portion alone was made using irreversible hydrocolloid impression material (Hydrogum soft, Zhermack, Italy) and reinforced with guaze cloth and Type III gypsum material (Goldstone, Kalabhai, India). Then working cast was made. The boundary of the defective area was transferred to the working cast. The orientation grooves were made in the marked area (Fig. 5). The working cast was contoured to the desired shape (Fig. 6). The hard wax (Accu byte-Ivoclar) was used to reconstruct the defective area to the desired dimensions on the working cast. Multiple holes of 2.5 mm diameter were made over the entire wax pattern at a distance of 2.5–3 mm from each other (Fig. 7). The perforation in the custom made implant is a definite advantage since they allow accumulated fluid seep out into the sub-galeal space, permit adhesions between the prosthesis and the soft tissue which helps to secure the former and allows adequate blood supply to the overlying flap i.e. scalp. Finished wax pattern was flasked in a customised flask (Fig. 8) and heat cure Polymethylmethacrylate (PMMA)-clear was processed by compression molding technique (Acralyn-H, Asian acrylates, India). Long curing cycle of 74 °C for 8 h was done [19]. This reduces the residual monomer in the prosthesis. The prosthesis was trimmed and polished well. The finished prosthesis was then autoclaved (Fig. 9).
Fig. 2.
Impression tray
Fig. 3.

Impression made
Fig. 4.
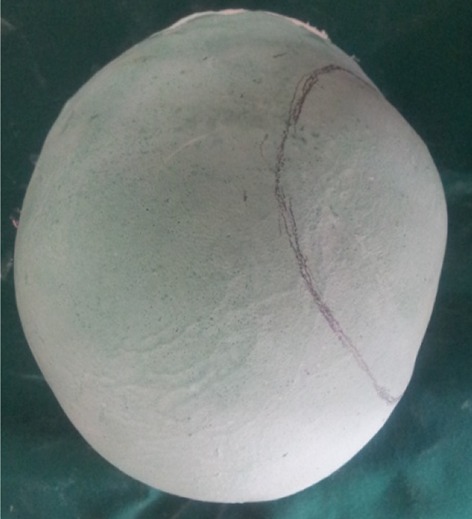
Master cast
Fig. 5.

Orientation groove
Fig. 6.

Prepared cast
Fig. 7.

Wax pattern
Fig. 8.

Customised flask
Fig. 9.

Acrylic cranial implant
Surgical procedure was carried out with a team of Neuro surgeons, Oral surgeons and the Prosthodontist at Hi-tech Medical College, Salem. Scalp tissues were reflected by blunt dissection method (Fig. 10) to ensure good vascular supply and adequate exposure of the defect. Fabricated prosthesis was then checked for proper adaptation to the bony margin and its over-all conformity with the face. Minor corrections of the prosthesis were done with the help of lab micromotor. Ligature wire holes were made to receive the wires in four points around its perimeter and then immersed in betadine solution and washed in saline. Once the acrylic implant was ready it was positioned on the defect area and ligated with 27 gauge titanium wires (Fig. 11). Hemostasis was achieved, suction drain (Acu-Vac) was placed and closure was done by using silk suture material (00 Ethylon). Post operative radiograph PA view and LAT view and CT was taken to ensures the proper placement of the acrylic implant placement (Fig. 12). Suture material was removed after 10 days. Post operative recovery was uneventful. Patient’s was happy when he came for follow up after one month (Fig. 13). He had no neurological defects. Patient was reviewed after 6 months and 1 year, he was in his regular work and esthetic was restored to his expectation.
Fig. 10.

Blunt dissection of scalp done
Fig. 11.

Cranial implant ligated
Fig. 12.
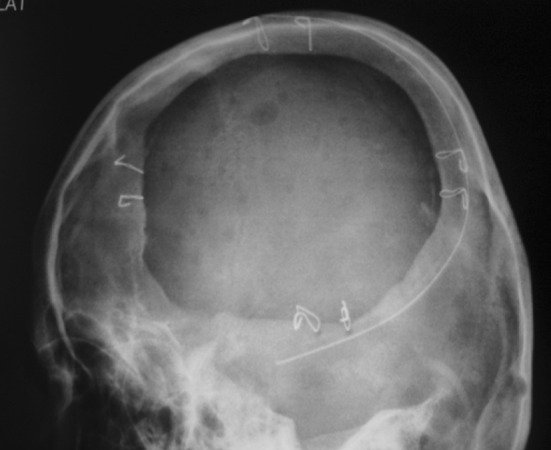
Post operative radiograph
Fig. 13.
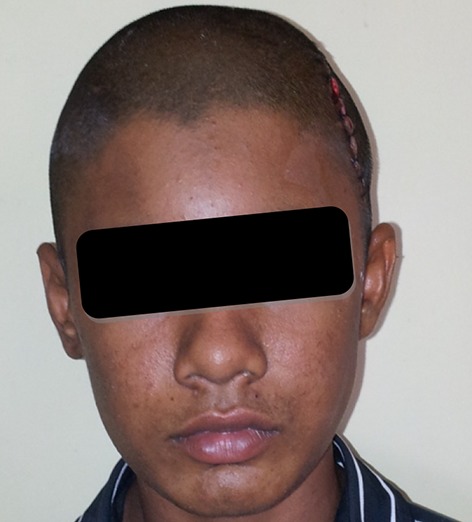
Post operative
Discussion
The area of the skull must be rebuilt and alloplastic implants are the best option because they provide rigid and adequate coverage with good aesthetic and functional results. There is no routine method to define the “ideal or standard.” This should be adapted for each individual patient. The advent of CAD–CAM, rapid prototyping, titanium custom-made, polyethylene implants have titanium mesh embedded (MEDPOR TITAN™), laser-sintered PEEK (poly-ether-ether ketone) and PEKK (poly-ether-ketone–ketone) for cranial implant prosthesis promotes adaptability, precision and facilitates the reconstruction process. Main disadvantages of these material is, laser economically challenging and minor correction during the time of surgery is not possible. Advantages of heat cure acrylic cranial implants are economically accepted, minor corrections in the acrylic implant margins can be done at the time of surgery and the material is inert and does not produce artifacts on tomography or magnetic resonance, which makes the oncological follow-up possible. We had a promising result and thus shows that acrylic implants is a very useful for reconstruction of the cranial bones.
Conclusion
The use of a combined staged cranioplasty for patients with large, geometrically complex defects allows for definitive, safe reconstruction that fulfills the goals of cranioplasty. This technique is especially useful for patients with anatomically complex defects in cosmetically challenging areas. Volumetric reconstruction of the cranial defect and overlying soft tissue enhances the symmetry of the reconstruction, ensuring a cosmetically pleasing result.
Contributor Information
Paul Simon, Email: drpaulsimonmds@yahoo.co.in.
Jayashree Mohan, Email: mohanjay@rediffmail.com.
Sunantha Selvaraj, Email: drsunujai@yahoo.co.in, Email: drsunujai@gmail.com.
References
- 1.Juan F, Santiago, Adolfo, et al. Design and manufacturing of a custom skull implant. Am J Eng Appl Sci. 2011;4(1):169–174. doi: 10.3844/ajeassp.2011.169.174. [DOI] [Google Scholar]
- 2.Joffe JM, Nicoll SR, Richards R, Linney AD, Harris M. Validation of computer-assisted manufacture of titanium plates for cranioplasty. Int J Oral Maxillofac Surg. 1999;28(309–313):1999. [PubMed] [Google Scholar]
- 3.Chacon E, Jose FH, Sandra PC, Fabricio CZ, et al. Cranial vault reconstruction using computer-designed Polyetheretherketone (PEEK) implant: case report. Cir Ciruj. 2009;77(437–440):2009. [PubMed] [Google Scholar]
- 4.Origitano TC, Ricardo I, Louis BS. Reconstructing complex cranial defects with a preformed cranial prosthesis. Skull Base Surg. 1995;5(5):109–116. doi: 10.1055/s-2008-1058941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artico M, Ferrante L, Pastore FS, Ramundo EO, Cantarelli D, et al. Bone autografting of the calvaria and craniofacial skeleton: Historical background, surgical results in a series of 15 patients, and review of the literature. Surg Neurol. 2003;60:71–79. doi: 10.1016/S0090-3019(03)00031-4. [DOI] [PubMed] [Google Scholar]
- 6.Malis L. Titanium mesh and acrylic cranioplasty. Neurosurg. 1989;25:351–355. doi: 10.1227/00006123-198909000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Yanai MDA. Resin sealant: a new method of methyl methacrylate cranioplasty. Technical note. J Neurosurg. 1991;75:328–330. doi: 10.3171/jns.1991.75.2.0328. [DOI] [PubMed] [Google Scholar]
- 8.Gronet PM, Waskewicz GA, Richardson C. Preformed acrylic cranial implants using fused deposition modeling: a clinical report. J Prosthet Dent. 2003;90:429–433. doi: 10.1016/j.prosdent.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Joffe JM, Mcdermott PJC, Linney AD, Mosse CA, Harris M. Computer-generated titanium cranioplasty: report of a new technique for repairing skull defects. Br J Neurosurg. 1992;6:343–350. doi: 10.3109/02688699209023793. [DOI] [PubMed] [Google Scholar]
- 10.Santoni-Rugiu P. Repair of skull defects by outer table osteoperiosteal free grafts. Plast Reconstr Surg. 1969;43:157–161. doi: 10.1097/00006534-196902000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Kulali A, Kayaale S. Single-table autogenous calvarial grafting for cranioplasty. J Craniomaxillofac Surg. 1991;19:208–211. doi: 10.1016/S1010-5182(05)80549-9. [DOI] [PubMed] [Google Scholar]
- 12.Psillakis JM, Nocchi VL, Zanini SA. Repair of large defect of frontal bone with free graft of outer table of parietal bones. Plast Reconstr Surg. 1979;64:827–830. doi: 10.1097/00006534-197912000-00023. [DOI] [PubMed] [Google Scholar]
- 13.Prolo DJ, Oklund S. Composite autogeneic human cranioplasty: frozen skull supplemented with fresh iliac orticocancellous bone. Neurosurgery. 1984;15:846–851. doi: 10.1227/00006123-198412000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Osawa M, Hara H, Ichinose Y, Koyama T, Kobayashi S, et al. Cranioplasty with a frozen and autoclaved bone flap. Acta Neurochir. 1990;102:38–41. doi: 10.1007/BF01402184. [DOI] [PubMed] [Google Scholar]
- 15.Hollier LH, Stal S. The use of hydroxyapatite cements in craniofacial surgery. Clin Plast Surg. 2004;31:423–428. doi: 10.1016/j.cps.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Koppel DA, Moos KF, Walker FS. Skull reconstruction with a two-part interlocking custom made titanium plate. Br J Oral Maxillofac Surg. 1999;37:70–72. doi: 10.1054/bjom.1998.0349. [DOI] [PubMed] [Google Scholar]
- 17.Kuttenberger JJ, Hardt N. Long-term results following reconstruction of craniofacial defects with titanium micro-mesh systems. J Craniomaxillofac Surg. 2001;29:75–81. doi: 10.1054/jcms.2001.0197. [DOI] [PubMed] [Google Scholar]
- 18.Solaro P, Pierangeli E, Pizzoni C, Boffi P, Scalese G, Di Lorenzo N, Pirillo V. From computerized tomography data processing to rapid manufacturing of custom-made prostheses for cranioplasty: case report. J Neurosurg Sci. 2008;52(4):113–116. [PubMed] [Google Scholar]
- 19.Anusavice JK. Phillip’s science of dental materials (ch-22) 11. St. Louis: Elsevier; 2003. p. 733. [Google Scholar]


