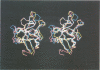Abstract
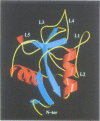
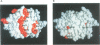
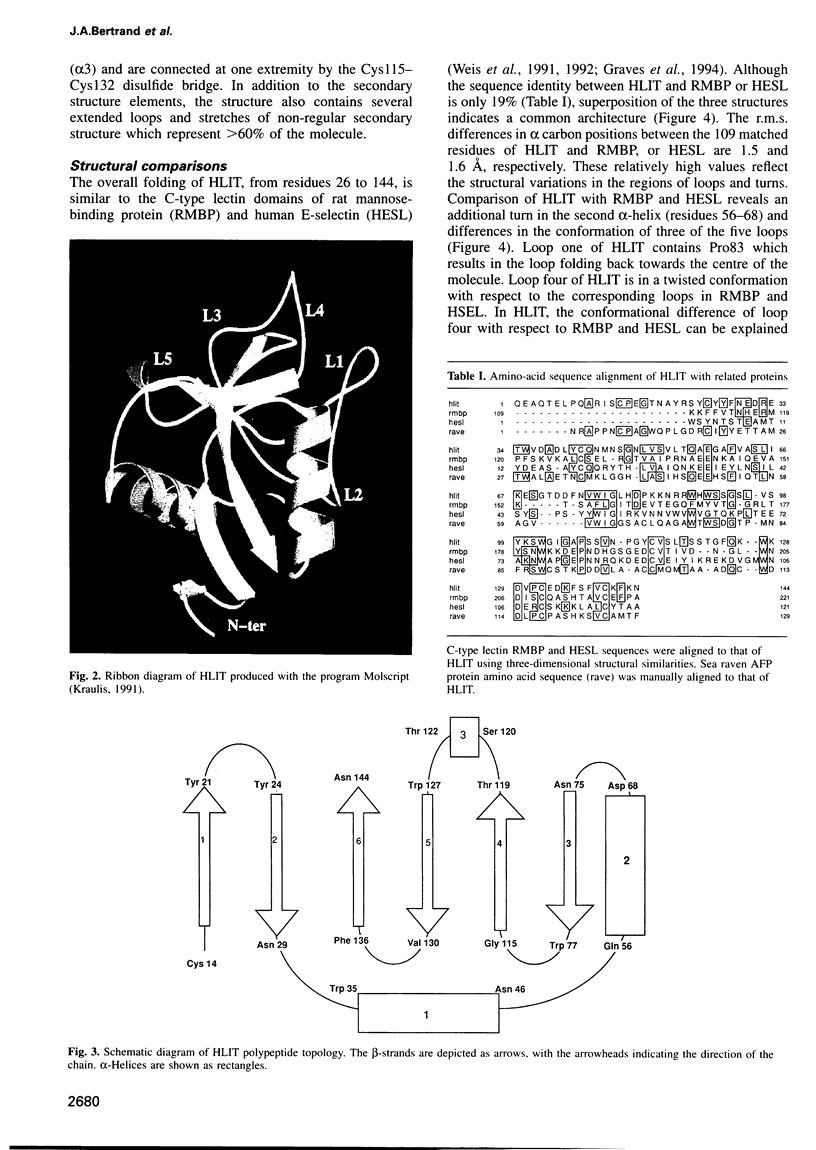
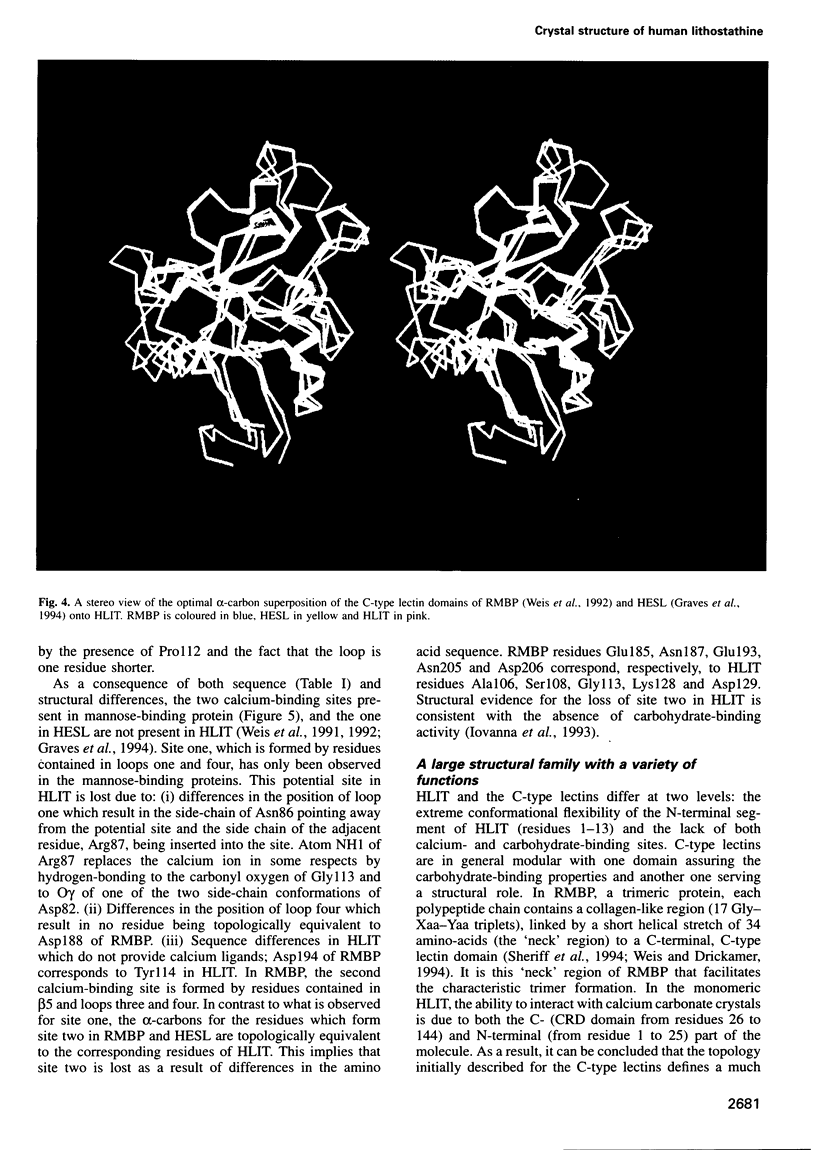
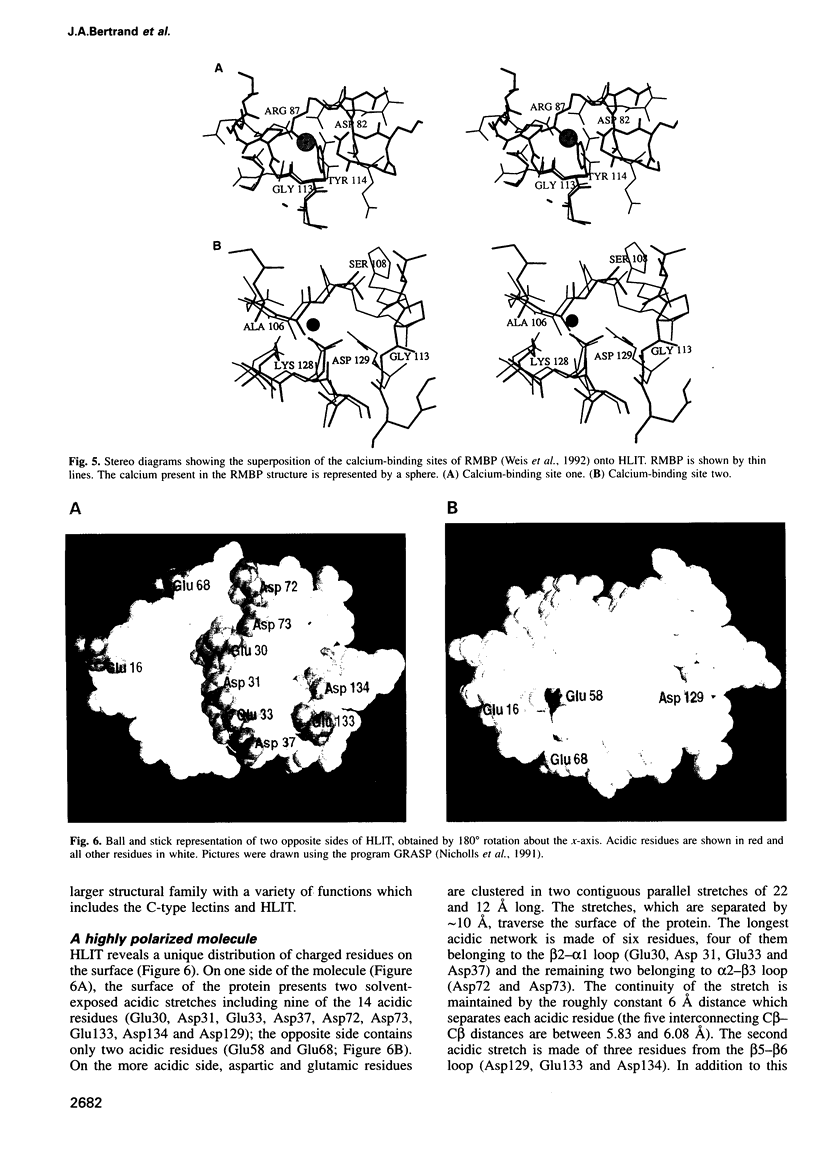
Human lithostathine (HLIT) is a pancreatic glycoprotein which inhibits the growth and nucleation of calcium carbonate crystals. The crystal structure of the monomeric 17 kDa HLIT, determined to a resolution of 1.55 angstroms, was refined to a crystallographic R-factor of 18.6%. Structural comparison with the carbohydrate-recognition domains of rat mannose-binding protein and E-selectin indicates that the C-terminal domain of HLIT shares a common architecture with the C-type lectins. Nevertheless, HLIT does not bind carbohydrate nor does it contain the characteristic calcium-binding sites of the C-type lectins. In consequence, HLIT represents the first structurally characterized member of this superfamily which is not a lectin. Analysis of the charge distribution and calculation of its dipole moment reveal that HLIT is a strongly polarized molecule. Eight acidic residues which are separated by regular 6 angstrom spacings form a unique and continuous patch on the molecular surface. This arrangement coincides with the distribution of calcium ions on certain planes of the calcium carbonate crystal; the dipole moment of HLIT may play a role in orienting the protein on the crystal surface prior to the more specific interactions of the acidic residues.
Full text
PDF
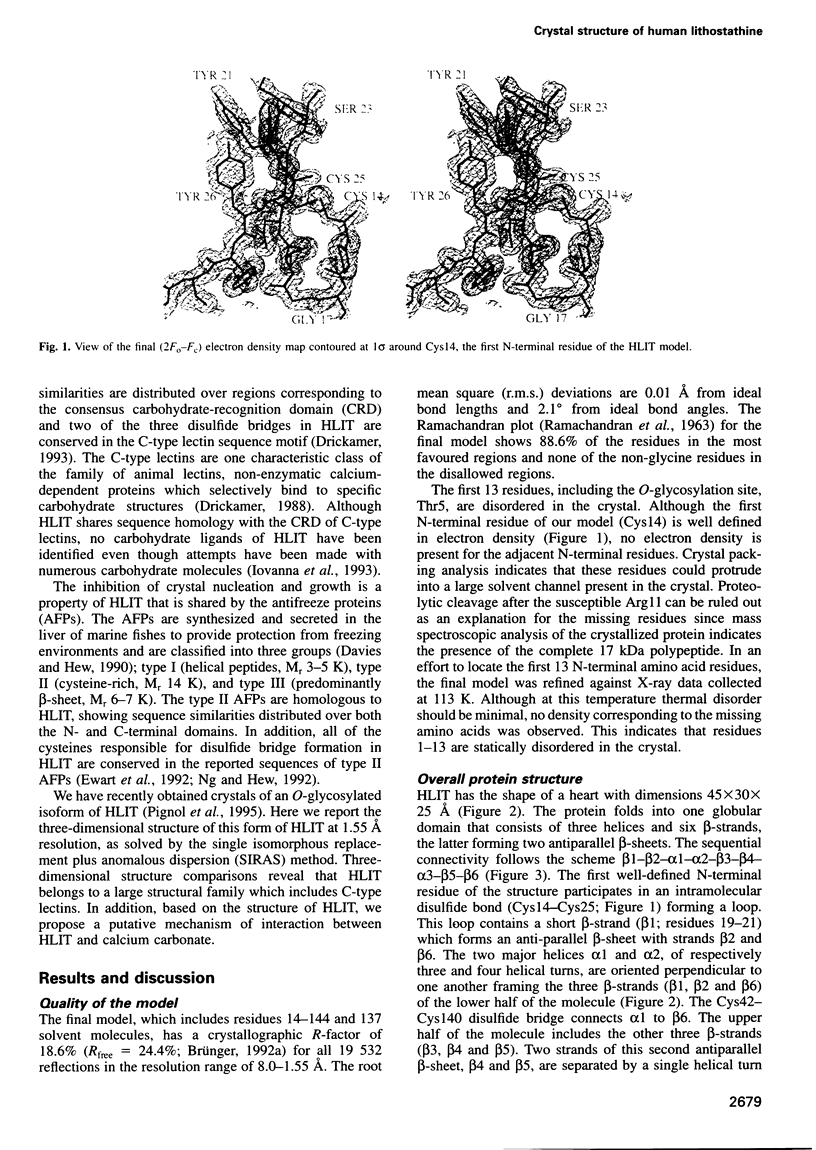


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard J. P., Adrich Z., Montalto G., De Caro A., De Reggi M., Sarles H., Dagorn J. C. Inhibition of nucleation and crystal growth of calcium carbonate by human lithostathine. Gastroenterology. 1992 Oct;103(4):1277–1284. doi: 10.1016/0016-5085(92)91516-7. [DOI] [PubMed] [Google Scholar]
- Bernard J. P., Barthet M., Gharib B., Michel R., Lilova A., Sahel J., Dagorn J. C., De Reggi M. Quantification of human lithostathine by high performance liquid chromatography. Gut. 1995 Apr;36(4):630–636. doi: 10.1136/gut.36.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao H., Sönnichsen F. D., DeLuca C. I., Sykes B. D., Davies P. L. Structure-function relationship in the globular type III antifreeze protein: identification of a cluster of surface residues required for binding to ice. Protein Sci. 1994 Oct;3(10):1760–1769. doi: 10.1002/pro.5560031016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. L., Hew C. L. Biochemistry of fish antifreeze proteins. FASEB J. 1990 May;4(8):2460–2468. doi: 10.1096/fasebj.4.8.2185972. [DOI] [PubMed] [Google Scholar]
- De Caro A. M., Bonicel J. J., Rouimi P., De Caro J. D., Sarles H., Rovery M. Complete amino acid sequence of an immunoreactive form of human pancreatic stone protein isolated from pancreatic juice. Eur J Biochem. 1987 Oct 1;168(1):201–207. doi: 10.1111/j.1432-1033.1987.tb13405.x. [DOI] [PubMed] [Google Scholar]
- De Caro A., Multigner L., Dagorn J. C., Sarles H. The human pancreatic stone protein. Biochimie. 1988 Sep;70(9):1209–1214. doi: 10.1016/0300-9084(88)90186-1. [DOI] [PubMed] [Google Scholar]
- Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem. 1988 Jul 15;263(20):9557–9560. [PubMed] [Google Scholar]
- Ewart K. V., Rubinsky B., Fletcher G. L. Structural and functional similarity between fish antifreeze proteins and calcium-dependent lectins. Biochem Biophys Res Commun. 1992 May 29;185(1):335–340. doi: 10.1016/s0006-291x(05)90005-3. [DOI] [PubMed] [Google Scholar]
- Graves B. J., Crowther R. L., Chandran C., Rumberger J. M., Li S., Huang K. S., Presky D. H., Familletti P. C., Wolitzky B. A., Burns D. K. Insight into E-selectin/ligand interaction from the crystal structure and mutagenesis of the lec/EGF domains. Nature. 1994 Feb 10;367(6463):532–538. doi: 10.1038/367532a0. [DOI] [PubMed] [Google Scholar]
- Gross J., Carlson R. I., Brauer A. W., Margolies M. N., Warshaw A. L., Wands J. R. Isolation, characterization, and distribution of an unusual pancreatic human secretory protein. J Clin Invest. 1985 Dec;76(6):2115–2126. doi: 10.1172/JCI112216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovanna J., Frigerio J. M., Dusetti N., Ramare F., Raibaud P., Dagorn J. C. Lithostathine, an inhibitor of CaCO3 crystal growth in pancreatic juice, induces bacterial aggregation. Pancreas. 1993 Sep;8(5):597–601. doi: 10.1097/00006676-199309000-00011. [DOI] [PubMed] [Google Scholar]
- Itoh T., Tsuzuki H., Katoh T., Teraoka H., Matsumoto K., Yoshida N., Terazono K., Watanabe T., Yonekura H., Yamamoto H. Isolation and characterization of human reg protein produced in Saccharomyces cerevisiae. FEBS Lett. 1990 Oct 15;272(1-2):85–88. doi: 10.1016/0014-5793(90)80454-q. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Ng N. F., Hew C. L. Structure of an antifreeze polypeptide from the sea raven. Disulfide bonds and similarity to lectin-binding proteins. J Biol Chem. 1992 Aug 15;267(23):16069–16075. [PubMed] [Google Scholar]
- Nicholls A., Sharp K. A., Honig B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins. 1991;11(4):281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- Ozturk M., de la Monte S. M., Gross J., Wands J. R. Elevated levels of an exocrine pancreatic secretory protein in Alzheimer disease brain. Proc Natl Acad Sci U S A. 1989 Jan;86(2):419–423. doi: 10.1073/pnas.86.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthy L. Homology of human pancreatic stone protein with animal lectins. Biochem J. 1988 Jul 1;253(1):309–311. doi: 10.1042/bj2530309b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen T. E. The amino-terminal domain of thrombomodulin and pancreatic stone protein are homologous with lectins. FEBS Lett. 1988 Apr 11;231(1):51–53. doi: 10.1016/0014-5793(88)80700-2. [DOI] [PubMed] [Google Scholar]
- Pignol D., Bertrand J. A., Bernard J. P., Verdier J. M., Dagorn J. C., Fontecilla-Camps J. C. Crystallization and preliminary crystallographic study of human lithostathine. Proteins. 1995 Dec;23(4):604–606. doi: 10.1002/prot.340230418. [DOI] [PubMed] [Google Scholar]
- RAMACHANDRAN G. N., RAMAKRISHNAN C., SASISEKHARAN V. Stereochemistry of polypeptide chain configurations. J Mol Biol. 1963 Jul;7:95–99. doi: 10.1016/s0022-2836(63)80023-6. [DOI] [PubMed] [Google Scholar]
- Sheriff S., Chang C. Y., Ezekowitz R. A. Human mannose-binding protein carbohydrate recognition domain trimerizes through a triple alpha-helical coiled-coil. Nat Struct Biol. 1994 Nov;1(11):789–794. doi: 10.1038/nsb1194-789. [DOI] [PubMed] [Google Scholar]
- Sicheri F., Yang D. S. Ice-binding structure and mechanism of an antifreeze protein from winter flounder. Nature. 1995 Jun 1;375(6530):427–431. doi: 10.1038/375427a0. [DOI] [PubMed] [Google Scholar]
- Stewart T. A. The human reg gene encodes pancreatic stone protein. Biochem J. 1989 Jun 1;260(2):622–623. doi: 10.1042/bj2600622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönnichsen F. D., Sykes B. D., Chao H., Davies P. L. The nonhelical structure of antifreeze protein type III. Science. 1993 Feb 19;259(5098):1154–1157. doi: 10.1126/science.8438165. [DOI] [PubMed] [Google Scholar]
- Sönnichsen F. D., Sykes B. D., Davies P. L. Comparative modeling of the three-dimensional structure of type II antifreeze protein. Protein Sci. 1995 Mar;4(3):460–471. doi: 10.1002/pro.5560040313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terazono K., Yamamoto H., Takasawa S., Shiga K., Yonemura Y., Tochino Y., Okamoto H. A novel gene activated in regenerating islets. J Biol Chem. 1988 Feb 15;263(5):2111–2114. [PubMed] [Google Scholar]
- Watanabe T., Yonekura H., Terazono K., Yamamoto H., Okamoto H. Complete nucleotide sequence of human reg gene and its expression in normal and tumoral tissues. The reg protein, pancreatic stone protein, and pancreatic thread protein are one and the same product of the gene. J Biol Chem. 1990 May 5;265(13):7432–7439. [PubMed] [Google Scholar]
- Weis W. I., Drickamer K., Hendrickson W. A. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature. 1992 Nov 12;360(6400):127–134. doi: 10.1038/360127a0. [DOI] [PubMed] [Google Scholar]
- Weis W. I., Drickamer K. Trimeric structure of a C-type mannose-binding protein. Structure. 1994 Dec 15;2(12):1227–1240. doi: 10.1016/S0969-2126(94)00124-3. [DOI] [PubMed] [Google Scholar]
- Weis W. I., Kahn R., Fourme R., Drickamer K., Hendrickson W. A. Structure of the calcium-dependent lectin domain from a rat mannose-binding protein determined by MAD phasing. Science. 1991 Dec 13;254(5038):1608–1615. doi: 10.1126/science.1721241. [DOI] [PubMed] [Google Scholar]
- Yang D. S., Sax M., Chakrabartty A., Hew C. L. Crystal structure of an antifreeze polypeptide and its mechanistic implications. Nature. 1988 May 19;333(6170):232–237. doi: 10.1038/333232a0. [DOI] [PubMed] [Google Scholar]
- de la Monte S. M., Ozturk M., Wands J. R. Enhanced expression of an exocrine pancreatic protein in Alzheimer's disease and the developing human brain. J Clin Invest. 1990 Sep;86(3):1004–1013. doi: 10.1172/JCI114762. [DOI] [PMC free article] [PubMed] [Google Scholar]