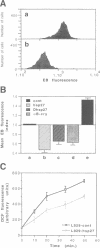
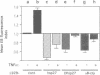
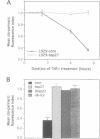
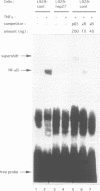
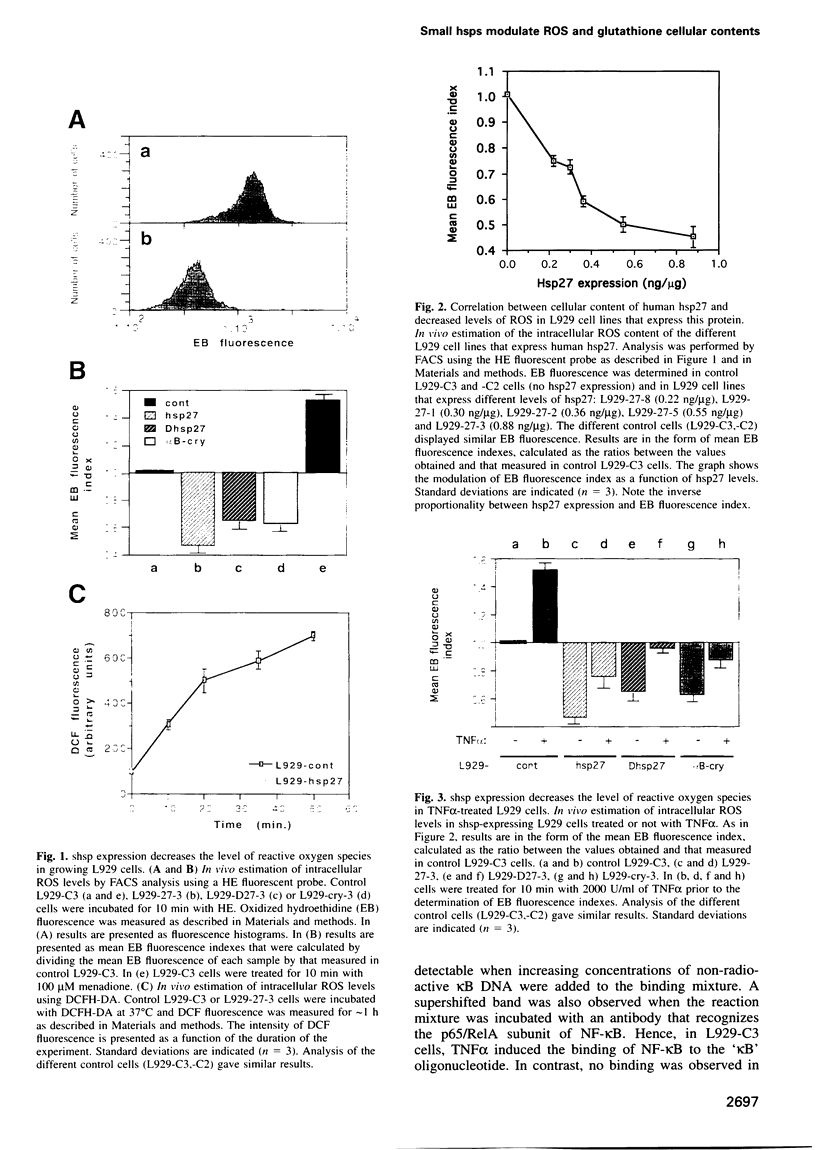
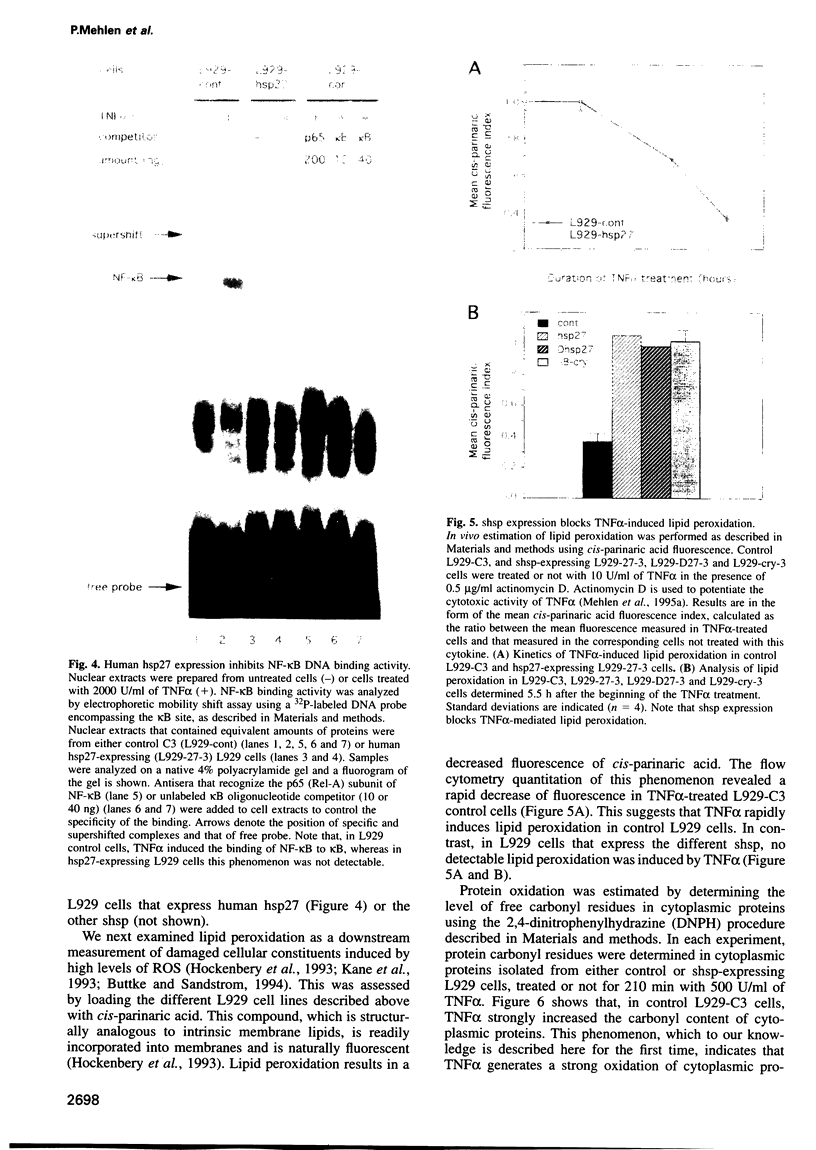
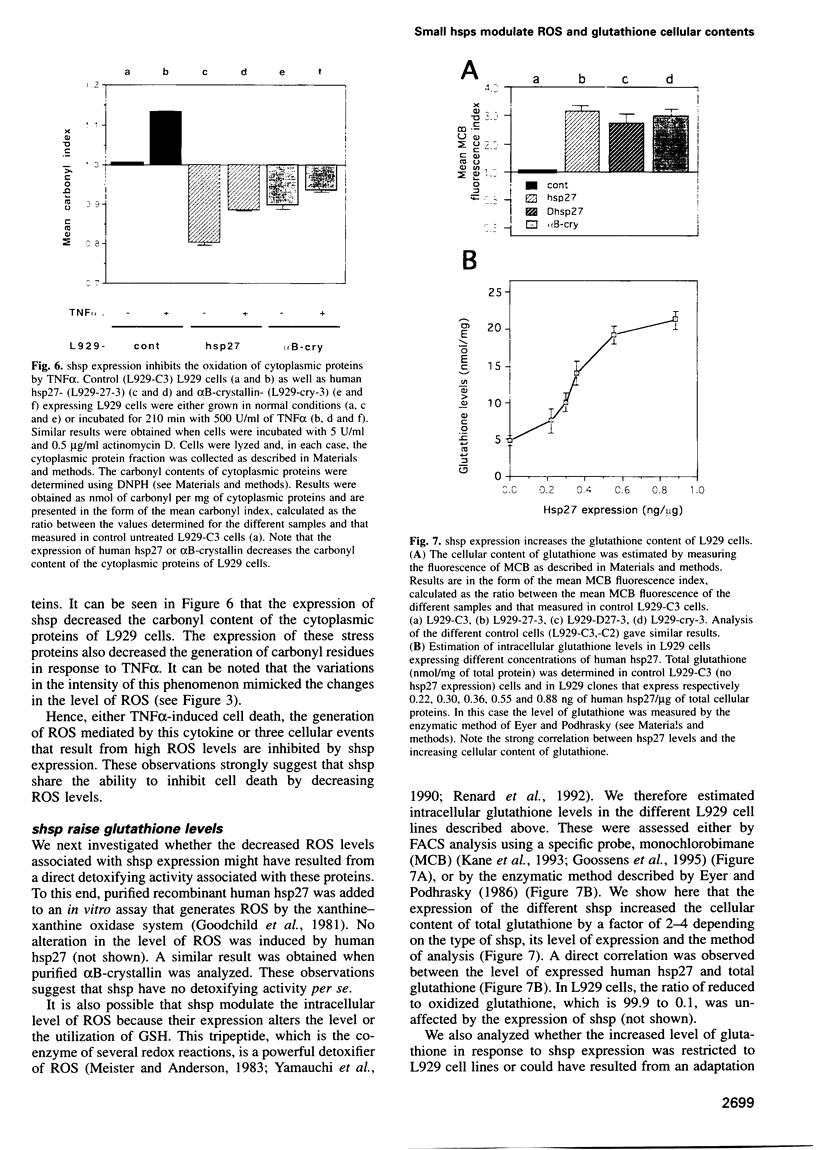
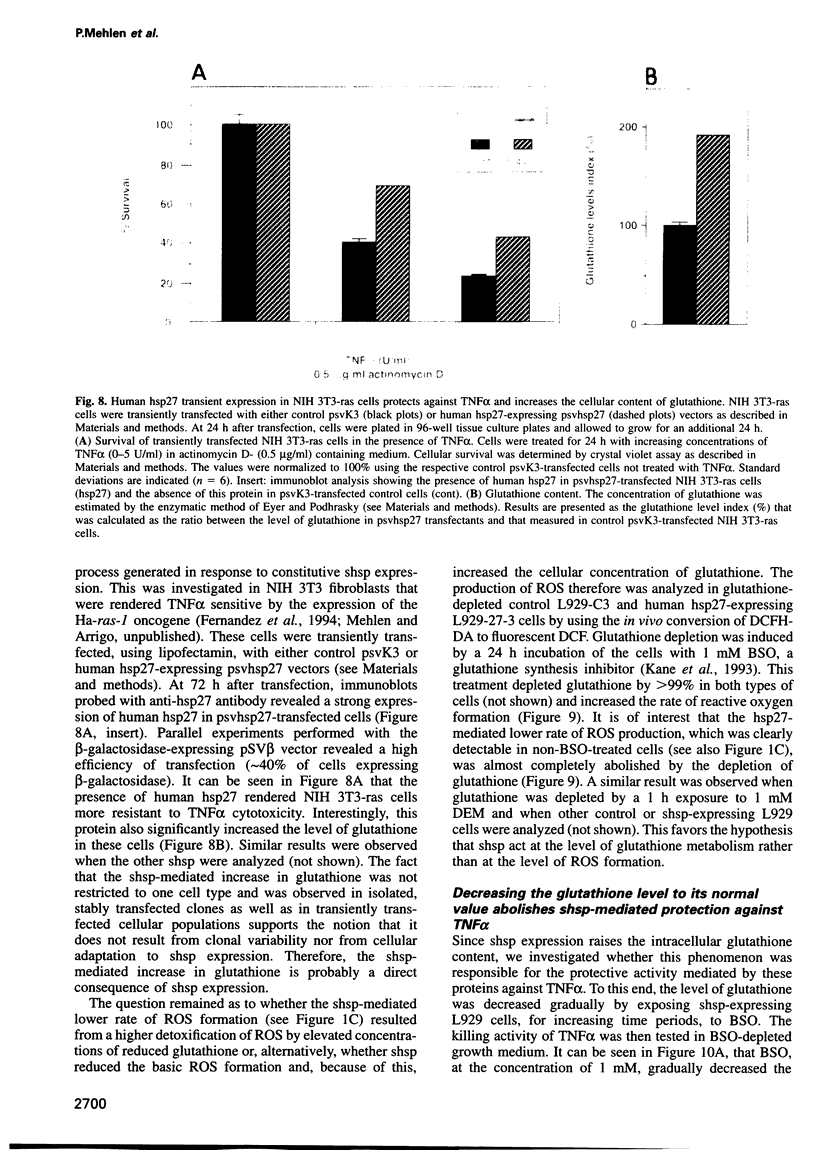
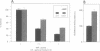Abstract
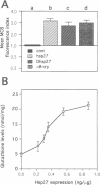
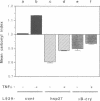
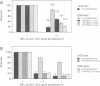
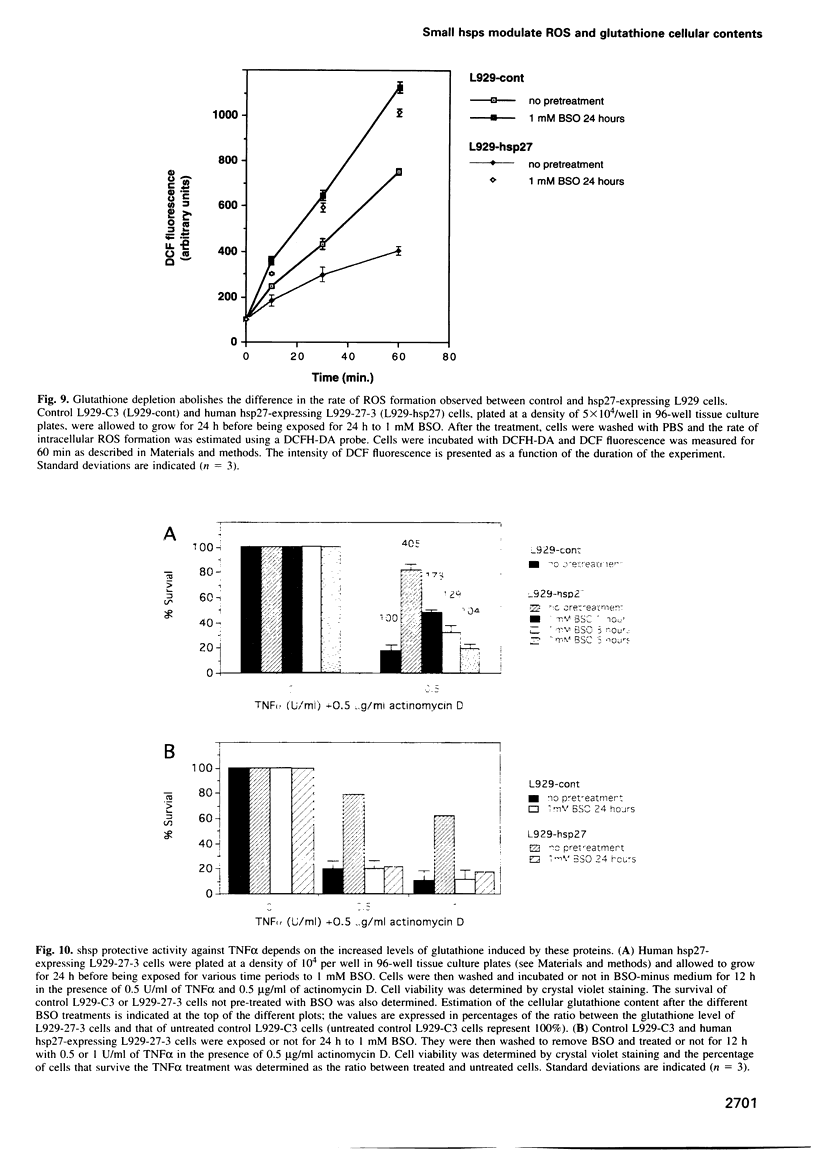
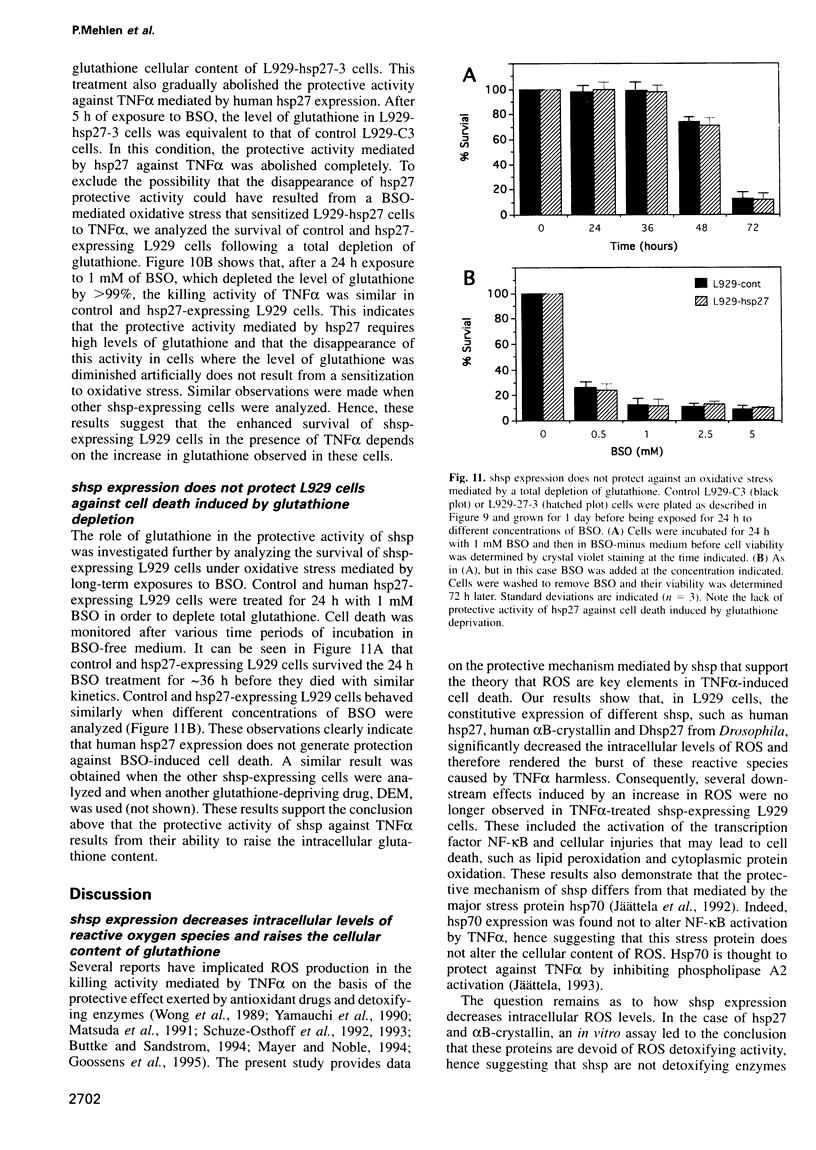
Expression of small stress proteins (shsp) enhances the survival of mammalian cells exposed to heat or oxidative injuries. Recently, we have shown that the expression of shsp from different species, such as human hsp27, Drosophila hsp27 or human alphaB-crystallin protected murine L929 cells against cell death induced by tumor necrosis factor (TNFalpha), hydrogen peroxide or menadione. Here, we report that, in growing L929 cell lines, the presence of these shsp decreased the intracellular level of reactive oxygen species (ROS). shsp expression also abolished the burst of intracellular ROS induced by TNFalpha. Several downstream effects resulting from the TNFalpha-mediated ROS increment, such as NF-kappaB activation, lipid peroxidation and protein oxidation, were inhibited by shsp expression. We also report that the expression of these different shsp raised the total glutathione level in both L929 cell lines and transiently transfected NIH 3T3-ras cells. This phenomenon was essential for the shsp-mediated decrease in ROS and resistance against TNFalpha. Our results therefore suggest that the protective activity shared by human hsp27, Drosophila hsp27 and human alphaB-crystallin against TNFalpha-mediated cell death and probably other types of oxidative stress results from their conserved ability to raise the intracellular concentration of glutathione.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P. Tumor necrosis factor induces the rapid phosphorylation of the mammalian heat shock protein hsp28. Mol Cell Biol. 1990 Mar;10(3):1276–1280. doi: 10.1128/mcb.10.3.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle P. A., Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Bellomo G., Mirabelli F., Vairetti M., Iosi F., Malorni W. Cytoskeleton as a target in menadione-induced oxidative stress in cultured mammalian cells. I. Biochemical and immunocytochemical features. J Cell Physiol. 1990 Apr;143(1):118–128. doi: 10.1002/jcp.1041430116. [DOI] [PubMed] [Google Scholar]
- Benchekroun M. N., Pourquier P., Schott B., Robert J. Doxorubicin-induced lipid peroxidation and glutathione peroxidase activity in tumor cell lines selected for resistance to doxorubicin. Eur J Biochem. 1993 Jan 15;211(1-2):141–146. doi: 10.1111/j.1432-1033.1993.tb19880.x. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Borner C., Martinou I., Mattmann C., Irmler M., Schaerer E., Martinou J. C., Tschopp J. The protein bcl-2 alpha does not require membrane attachment, but two conserved domains to suppress apoptosis. J Cell Biol. 1994 Aug;126(4):1059–1068. doi: 10.1083/jcb.126.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttke T. M., Sandstrom P. A. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994 Jan;15(1):7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Carter W. O., Narayanan P. K., Robinson J. P. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J Leukoc Biol. 1994 Feb;55(2):253–258. doi: 10.1002/jlb.55.2.253. [DOI] [PubMed] [Google Scholar]
- Eyer P., Podhradský D. Evaluation of the micromethod for determination of glutathione using enzymatic cycling and Ellman's reagent. Anal Biochem. 1986 Feb 15;153(1):57–66. doi: 10.1016/0003-2697(86)90061-8. [DOI] [PubMed] [Google Scholar]
- Fernandez A., Marin M. C., McDonnell T., Ananthaswamy H. N. Differential sensitivity of normal and Ha-ras-transformed C3H mouse embryo fibroblasts to tumor necrosis factor: induction of bcl-2, c-myc, and manganese superoxide dismutase in resistant cells. Oncogene. 1994 Jul;9(7):2009–2017. [PubMed] [Google Scholar]
- Goossens V., Grooten J., De Vos K., Fiers W. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8115–8119. doi: 10.1073/pnas.92.18.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grooten J., Goossens V., Vanhaesebroeck B., Fiers W. Cell membrane permeabilization and cellular collapse, followed by loss of dehydrogenase activity: early events in tumour necrosis factor-induced cytotoxicity. Cytokine. 1993 Nov;5(6):546–555. doi: 10.1016/s1043-4666(05)80003-1. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Harris C., Juchau M. R., Mirkes P. E. Role of glutathione and hsp 70 in the acquisition of thermotolerance in postimplantation rat embryos. Teratology. 1991 Mar;43(3):229–239. doi: 10.1002/tera.1420430307. [DOI] [PubMed] [Google Scholar]
- Hennet T., Bertoni G., Richter C., Peterhans E. Expression of BCL-2 protein enhances the survival of mouse fibrosarcoid cells in tumor necrosis factor-mediated cytotoxicity. Cancer Res. 1993 Mar 15;53(6):1456–1460. [PubMed] [Google Scholar]
- Hirose K., Longo D. L., Oppenheim J. J., Matsushima K. Overexpression of mitochondrial manganese superoxide dismutase promotes the survival of tumor cells exposed to interleukin-1, tumor necrosis factor, selected anticancer drugs, and ionizing radiation. FASEB J. 1993 Feb 1;7(2):361–368. doi: 10.1096/fasebj.7.2.8440412. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot J., Roy G., Lambert H., Chrétien P., Landry J. Increased survival after treatments with anticancer agents of Chinese hamster cells expressing the human Mr 27,000 heat shock protein. Cancer Res. 1991 Oct 1;51(19):5245–5252. [PubMed] [Google Scholar]
- Ishii Y., Partridge C. A., Del Vecchio P. J., Malik A. B. Tumor necrosis factor-alpha-mediated decrease in glutathione increases the sensitivity of pulmonary vascular endothelial cells to H2O2. J Clin Invest. 1992 Mar;89(3):794–802. doi: 10.1172/JCI115658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. D., Raff M. C. Programmed cell death and Bcl-2 protection in very low oxygen. Nature. 1995 Apr 27;374(6525):814–816. doi: 10.1038/374814a0. [DOI] [PubMed] [Google Scholar]
- Jakob U., Gaestel M., Engel K., Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993 Jan 25;268(3):1517–1520. [PubMed] [Google Scholar]
- Jättelä M. Overexpression of major heat shock protein hsp70 inhibits tumor necrosis factor-induced activation of phospholipase A2. J Immunol. 1993 Oct 15;151(8):4286–4294. [PubMed] [Google Scholar]
- Jättelä M., Wissing D., Bauer P. A., Li G. C. Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J. 1992 Oct;11(10):3507–3512. doi: 10.1002/j.1460-2075.1992.tb05433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane D. J., Sarafian T. A., Anton R., Hahn H., Gralla E. B., Valentine J. S., Ord T., Bredesen D. E. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993 Nov 19;262(5137):1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- Kretz-Remy C., Arrigo A. P. The kinetics of HIV-1 long terminal repeat transcriptional activation resemble those of hsp70 promoter in heat-shock treated HeLa cells. FEBS Lett. 1994 Oct 24;353(3):339–344. doi: 10.1016/0014-5793(94)00828-0. [DOI] [PubMed] [Google Scholar]
- Landry J., Chrétien P., Lambert H., Hickey E., Weber L. A. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989 Jul;109(1):7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie J. N., Gingras-Breton G., Tanguay R. M., Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem. 1993 Feb 15;268(5):3420–3429. [PubMed] [Google Scholar]
- Lim K., Chae C. B. A simple assay for DNA transfection by incubation of the cells in culture dishes with substrates for beta-galactosidase. Biotechniques. 1989 Jun;7(6):576–579. [PubMed] [Google Scholar]
- Matsuda M., Masutani H., Nakamura H., Miyajima S., Yamauchi A., Yonehara S., Uchida A., Irimajiri K., Horiuchi A., Yodoi J. Protective activity of adult T cell leukemia-derived factor (ADF) against tumor necrosis factor-dependent cytotoxicity on U937 cells. J Immunol. 1991 Dec 1;147(11):3837–3841. [PubMed] [Google Scholar]
- Mayer M., Noble M. N-acetyl-L-cysteine is a pluripotent protector against cell death and enhancer of trophic factor-mediated cell survival in vitro. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7496–7500. doi: 10.1073/pnas.91.16.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonagh J., Fossel E. T., Kradin R. L., Dubinett S. M., Laposata M., Hallaq Y. A. Effects of tumor necrosis factor-alpha on peroxidation of plasma lipoprotein lipids in experimental animals and patients. Blood. 1992 Dec 15;80(12):3217–3226. [PubMed] [Google Scholar]
- Mehlen P., Briolay J., Smith L., Diaz-latoud C., Fabre N., Pauli D., Arrigo A. P. Analysis of the resistance to heat and hydrogen peroxide stresses in COS cells transiently expressing wild type or deletion mutants of the Drosophila 27-kDa heat-shock protein. Eur J Biochem. 1993 Jul 15;215(2):277–284. doi: 10.1111/j.1432-1033.1993.tb18032.x. [DOI] [PubMed] [Google Scholar]
- Mehlen P., Kretz-Remy C., Briolay J., Fostan P., Mirault M. E., Arrigo A. P. Intracellular reactive oxygen species as apparent modulators of heat-shock protein 27 (hsp27) structural organization and phosphorylation in basal and tumour necrosis factor alpha-treated T47D human carcinoma cells. Biochem J. 1995 Dec 1;312(Pt 2):367–375. doi: 10.1042/bj3120367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P., Mehlen A., Guillet D., Preville X., Arrigo A. P. Tumor necrosis factor-alpha induces changes in the phosphorylation, cellular localization, and oligomerization of human hsp27, a stress protein that confers cellular resistance to this cytokine. J Cell Biochem. 1995 Jun;58(2):248–259. doi: 10.1002/jcb.240580213. [DOI] [PubMed] [Google Scholar]
- Mehlen P., Preville X., Chareyron P., Briolay J., Klemenz R., Arrigo A. P. Constitutive expression of human hsp27, Drosophila hsp27, or human alpha B-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol. 1995 Jan 1;154(1):363–374. [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Miron T., Vancompernolle K., Vandekerckhove J., Wilchek M., Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991 Jul;114(2):255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. B., Russo A., Kinsella T. J., Glatstein E. Glutathione elevation during thermotolerance induction and thermosensitization by glutathione depletion. Cancer Res. 1983 Mar;43(3):987–991. [PubMed] [Google Scholar]
- Mårtensson J., Steinherz R., Jain A., Meister A. Glutathione ester prevents buthionine sulfoximine-induced cataracts and lens epithelial cell damage. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8727–8731. doi: 10.1073/pnas.86.22.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholl I. D., Quinlan R. A. Chaperone activity of alpha-crystallins modulates intermediate filament assembly. EMBO J. 1994 Feb 15;13(4):945–953. doi: 10.1002/j.1460-2075.1994.tb06339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli D., Tonka C. H., Tissieres A., Arrigo A. P. Tissue-specific expression of the heat shock protein HSP27 during Drosophila melanogaster development. J Cell Biol. 1990 Sep;111(3):817–828. doi: 10.1083/jcb.111.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard D. C., Seitz M. B., Thomas A. P. Oxidized glutathione causes sensitization of calcium release to inositol 1,4,5-trisphosphate in permeabilized hepatocytes. Biochem J. 1992 Jun 1;284(Pt 2):507–512. doi: 10.1042/bj2840507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick A. Z., Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. 1994;233:357–363. doi: 10.1016/s0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- Richter C. Pro-oxidants and mitochondrial Ca2+: their relationship to apoptosis and oncogenesis. FEBS Lett. 1993 Jun 28;325(1-2):104–107. doi: 10.1016/0014-5793(93)81423-w. [DOI] [PubMed] [Google Scholar]
- Rothe G., Valet G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2',7'-dichlorofluorescin. J Leukoc Biol. 1990 May;47(5):440–448. [PubMed] [Google Scholar]
- Scanlon M., Laster S. M., Wood J. G., Gooding L. R. Cytolysis by tumor necrosis factor is preceded by a rapid and specific dissolution of microfilaments. Proc Natl Acad Sci U S A. 1989 Jan;86(1):182–186. doi: 10.1073/pnas.86.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K. N., Amstad P., Cerutti P., Baeuerle P. A. The roles of hydrogen peroxide and superoxide as messengers in the activation of transcription factor NF-kappa B. Chem Biol. 1995 Jan;2(1):13–22. doi: 10.1016/1074-5521(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Osthoff K., Bakker A. C., Vanhaesebroeck B., Beyaert R., Jacob W. A., Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992 Mar 15;267(8):5317–5323. [PubMed] [Google Scholar]
- Schulze-Osthoff K., Beyaert R., Vandevoorde V., Haegeman G., Fiers W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 1993 Aug;12(8):3095–3104. doi: 10.1002/j.1460-2075.1993.tb05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Osthoff K., Krammer P. H., Dröge W. Divergent signalling via APO-1/Fas and the TNF receptor, two homologous molecules involved in physiological cell death. EMBO J. 1994 Oct 3;13(19):4587–4596. doi: 10.1002/j.1460-2075.1994.tb06780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Osthoff K. The Fas/APO-1 receptor and its deadly ligand. Trends Cell Biol. 1994 Dec;4(12):421–426. doi: 10.1016/0962-8924(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Eguchi Y., Kosaka H., Kamiike W., Matsuda H., Tsujimoto Y. Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-xL. Nature. 1995 Apr 27;374(6525):811–813. doi: 10.1038/374811a0. [DOI] [PubMed] [Google Scholar]
- Shrieve D. C., Bump E. A., Rice G. C. Heterogeneity of cellular glutathione among cells derived from a murine fibrosarcoma or a human renal cell carcinoma detected by flow cytometric analysis. J Biol Chem. 1988 Oct 5;263(28):14107–14114. [PubMed] [Google Scholar]
- Stadtman E. R. Protein oxidation and aging. Science. 1992 Aug 28;257(5074):1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Elwell J. H., Oberley L. W., Goeddel D. V. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989 Sep 8;58(5):923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- Yamauchi N., Watanabe N., Kuriyama H., Neda H., Maeda M., Himeno T., Tsuji Y., Niitsu Y. Suppressive effects of intracellular glutathione on hydroxyl radical production induced by tumor necrosis factor. Int J Cancer. 1990 Nov 15;46(5):884–888. doi: 10.1002/ijc.2910460522. [DOI] [PubMed] [Google Scholar]
- Zhong L. T., Sarafian T., Kane D. J., Charles A. C., Mah S. P., Edwards R. H., Bredesen D. E. bcl-2 inhibits death of central neural cells induced by multiple agents. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4533–4537. doi: 10.1073/pnas.90.10.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]