Abstract
Autoantibodies to IA-2 in Type 1 diabetes are associated with HLA-DR4, suggesting influences of HLA-DR4 restricted T-cells on IA-2-specific B-cell responses. The aim of this study was to investigate possible T-B-cell collaboration by determining whether autoantibodies to IA-2 epitopes are associated with T-cell responses to IA-2 peptides presented by DR4. T-cells secreting the cytokines interferon-γ and interleukin-10 in response to seven peptides known to elicit T-cell responses in Type 1 diabetes were quantified by cytokine ELISPOT in HLA-typed patients characterised for antibodies to IA-2 epitopes. T-cell responses were detected to all peptides tested, but only interleukin-10 responses to 841-860 and 853-872 peptides were associated with DR4. Phenotyping by RT-PCR of FACS-sorted CD45ROhi T-cells secreting interleukin-10 in response to these two peptides indicated that these expressed GATA-3 or T-bet, but not FoxP3, consistent with these being Th2 or Th1 memory T-cells rather than of regulatory phenotype. T-cell responses to the same two peptides were also associated with specific antibodies; those to 841-860 peptide with antibodies to juxtamembrane epitopes, which appear early in pre-diabetes, and those to peptide 853-872 with antibodies to an epitope located in the 831-862 central region of the IA-2 tyrosine phosphatase domain. Antibodies to juxtamembrane and central region constructs were both DR4-associated. This study identifies a region of focus for B- and T-cell responses to IA-2 in HLA-DR4 diabetic patients that may explain HLA- associations of IA-2 autoantibodies and this region may provide a target for future immune intervention to prevent disease.
Introduction
Type 1 diabetes is the result of an autoimmune destruction of beta cells and is associated with autoimmunity to multiple islet cell autoantigens, including (pro)insulin, glutamic acid decarboxylase (GAD65), zinc transporter-8 (ZnT8), and the secretory granule protein IA-2 (1). A role for T-cells in disease pathogenesis was demonstrated by experiments in NOD mice where transfer of CD4+ and CD8+ T-cells from diabetic mice into irradiated recipients was sufficient to initiate disease (2) and in the human disease is implicated by a dominance of T-cells in the islet infiltration and genetic susceptibility conferred at the MHC class II locus (3-5). There is now substantial evidence that B-cells also play a critical role in the development of disease. The presence of autoantibodies to multiple islet autoantigens is highly predictive of disease progression (6), and direct evidence for a role of B-cells in pathogenesis was demonstrated by partial preservation of beta cell function in patients with new-onset diabetes by anti-CD20 (Rituximab)-mediated depletion of B-cells (7). B-cell depletion also prevents disease development in animal models of Type 1 diabetes (8-10). The contribution of B-cells to the disease process is largely attributed to their role as professional antigen presenting cells (11), with the high affinity surface B-cell receptor facilitating uptake, processing and presentation of islet autoantigen to T-cells. If such a mechanism operates in Type 1 diabetes, then one would expect to see associations between autoantibody and T-cell responses to islet antigens in the disease and with the HLA gene products involved in antigen presentation. To date, studies describing links between B-cell and T-cell responses in human Type 1 diabetes are rare, and there are no convincing reports of associations between T-cell responses to individual peptides derived from autoantigens and disease-associated HLA alleles.
Autoantibodies to IA-2 are detected in 60-70% of Type 1 diabetic patients at disease onset, appear within the first 5 years of life in family members of a diabetic proband, after which they are strongly predictive of subsequent diabetes development (12-16). Several epitopes on IA-2 have been defined and the antibody responses to these are progressive, with early responses directed to epitopes in the juxtamembrane domain of the molecule, subsequently spreading to those in the tyrosine phosphatase domain (17). Antibodies to IA-2 are positively associated with expression of HLA-DR4 (18-19), suggesting that B-cell autoimmunity to the protein may be linked to T-cell responses restricted by this major Type 1 diabetes susceptibility allele. Furthermore, several naturally processed peptides derived from IA-2 have been identified that both bind HLA-DR4 and stimulate T-cell responses in Type 1 diabetic patients (20). These properties make the IA-2 autoimmune response an ideal system to investigate links of T- and B-cell responses with HLA-DR4 in human patients. The aim of the current study was to investigate associations between T- and B-cell responses at an epitope level and to study the influence of HLA-DR4 on these responses.
Material and Methods
Study subjects
Patients (n=127) of up to 30 years of age were recruited within 6 months of diagnosis of Type 1 diabetes from clinics in West Yorkshire, Durham and King’s College Hospital, London U.K., and provided blood samples for analysis of autoantibody responses and for HLA genotyping with informed consent and approval from appropriate Ethics Committees (Reference 08/H1313/70). A subgroup of 58 of these patients aged between 12 and 30 years provided sufficient volumes of heparinised blood (>20 ml) delivered within 24h of sample collection to laboratories at King’s College London for analyses of T-cell responses to IA-2 peptides by cytokine ELISPOT. The characteristics of the subjects studied for autoantibody associations with HLA gene expression and T-cell responses are summarised in Table I. There were no significant differences in characteristics between these two study groups. Five additional patients (three male, mean age 22 years) were recruited for phenotypic analysis of peptide responsive T-cells.
Table I.
Characteristics of groups of Type 1 diabetic patients analysed for HLA associations with HLA gene expression and for T-cell responses
| HLA-Ab associations | T-cell analysis | |
|---|---|---|
|
| ||
| Number | 127 | 58 |
| Age at diagnosis (mean years ± SD) | 17 ± 5.6 | 18 ± 5.1 |
| Sex (% male) | 68.5 | 74.1 |
| Autoantibodies | ||
| IA-2A (% positive) | 68.5 | 55.2 |
| GADA (% positive) | 79.5 | 82.7 |
| ZnT8A (% positive) | 59.0 | 55.2 |
| HLA | ||
| DR3/4 (%) | 27.6 | 30.9 |
| DR4/x (%) | 33.9 | 34.5 |
| DR3/x (%) | 26.0 | 23.6 |
| non-DR3/4 (%) | 12.6 | 10.9 |
DNA extraction and HLA genotyping
Study subjects were genotyped for class II HLA-DRB1 and DQB1 loci. Genomic DNA was extracted from cell pellets obtained after density gradient centrifugation of heparinised whole blood by proteinase K digestion followed by salt-ethanol precipitation. DNA was further purified using the Genomic DNA Clean and Concentrator kit (Zymo Research Corporation, Irvine, CA). Genotyping was performed by PCR amplification of genomic DNA, using sequence specific primers (21-22).
Antibody analysis
Autoantibodies to radiolabelled GAD65, IA-2 and ZnT8 constructs were analysed by radioligand binding assays, as previously described (23-24). Antibodies to GAD65 were analysed using constructs representing the full length coding region of the human protein, whereas ZnT8 antibodies were determined using constructs representing the C-terminus of the molecule (amino acids 268-369) with both 325Trp and 325Arg variants. IA-2 constructs used were the cytoplasmic domain of IA-2 (IA-2ic, residues 605-979) and truncated constructs (25) representing the juxtamembrane domain (JM, residues 605-693) tyrosine phosphatase domain (PTP, residues 643-979) and the central region of the cytoplasmic domain (residues 643-937).
IA-2, GAD and ZnT8 cDNAs were transcribed and translated in vitro in the presence of 35S-methionine using the TNT®-Quick-Coupled Transcription/Translation System (Promega, Southampton, UK). Incorporated radioactivity was determined by precipitation of the translated protein with 10% trichloroacetic acid, followed by scintillation counting. Radiolabelled protein (20,000 cpm in 20 μl) was incubated with 5 μl of test sera for 16 hours at 4C in wash buffer (10 mM HEPES, pH7.4, 150mM NaCl, 20 mM methionine, 0.5 mg/mml BSA and 0.5 % Triton X-100). Immune complexes were immunoprecipitated with Protein A-Sepharose and washed five times under vacuum filtration with wash buffer, followed by two washes in water. The quantity of immunoprecipitated radiolabelled antigen was determined by liquid scintillation counting (Plate Chameleon V, LabLogic, Sheffield, UK). Antibody levels (as WHO units/ml) were calculated by comparison with a standard curve of an in-house standard positive serum, pre-calibrated against the WHO reference standard, run in each assay. Cut offs for positive values were defined as the mean + 3SD of normal control sera and were as follows: IA-2ic >5 U/ml; GAD65 >13 U/ml and ZnT8 >10 U/ml. The performance (sensitivity and specificity) of each assay in the 2012 IASP workshop were: IA-2ic: 62% and 100%; GAD65: 70% and 96.7%; ZnT8: 62% and 98.9%.
Antibodies to the IA-2 JM domain were analysed using radiolabelled IA-2605-693 construct in the absence or presence of 2 μg of synthetic 20-mer peptides representing amino acids 601-620, 611-630 and 621-630. Subjects were only considered positive for JM antibodies if reactivity was significantly inhibited in the presence of one or more of these peptides. The IA-2643-937 construct detects antibody reactivity to epitopes represented by amino acids within the 831-860 region of IA-2 (23), whereas the IA-2643-979 construct detects antibody reactivity to additional epitopes in the PTP domain. Levels of antibodies to truncated IA-2 constructs were standardised to those of a rabbit polyclonal anti-IA-2 serum (26) included in each assay.
T-cell ELISPOT assays
Seven peptides within the tyrosine phosphatase domain of IA-2, previously reported to contain T-cell epitopes (27-28), were synthesised by fmoc chemistry and purified by reverse phase HPLC (GenScript, Piscataway, NJ). Peptides tested represented aa 709-736, 752-775, 793-817, 831-850, 841-860, 853-872 and 955-976 of IA-2. T-cell responses were also determined to phytohaemagglutinin (PHA) as a mitogenic control. T-cells responding to the synthetic IA-2 peptides or PHA were enumerated using human cytokine ELISPOT systems (U-CyTech, Utrecht, The Netherlands). All reagents and consumables used in the assay were of low endotoxin content, to minimise background responses. Immunosorb 96-well plates were coated overnight at 4°C with interferon-γ, (IFN-γ), interleukin-10 (IL-10) or interleukin-17 (IL-17) capture antibodies and then blocked. Peripheral blood mononuclear cells (PBMC) were isolated from heparinised blood by density gradient centrifugation. Where there were insufficient cells for analysis of all peptides with the three cytokines, test stimuli were systematically allocated to samples, giving preference to IFN-γ and IL-10 assays because of the low sensitivity of detection of IL-17 responding cells. Medium only and PHA tests were included in all assays. Because of the fast kinetics of IFN-γ secretion in response to antigen stimulation, for IFN-γ assays, cells were seeded directly into coated plates at a density of 3×105/well and incubated in triplicate in the presence of 10 μg/ml IA-2 peptide or 1 μg/ml PHA for 48 hours at 37°C in a 5% CO2 atmosphere. For IL-10 and IL-17 assays, cells were pre-incubated in 48 well plates at a density of 2×106/well in the presence of 10 μg/ml of IA-2 peptide or 1 μg/ml PHA. Non-adherent cells were recovered, washed in culture medium and seeded in triplicate at 3×105/well into coated plates and incubated for a further 24 hours at 37°C. Plates were developed according to the manufacturer’s instructions. Spots were counted using a BioReader 3000 ELISPOT counter (Bio-Sys GmbH, Karben, Germany). Numbers of cells per 106 PBMC responding to peptides or PHA were calculated by subtracting the number of spots in the medium only wells from the spots in the test sample wells. Median numbers of spots with medium alone were 3.3, 5.5 and 0 per 106 PBMC for IL-10, IFN-γ and IL-17, respectively.
Isolation of interleukin-10 secreting CD4+ T-cells by fluorescence-activated cell sorting
To obtain preparations of T-cells enriched in those responding to IA-2 peptides suitable for further phenotyping, PBMC were seeded at a density of 2×106/tube into 5ml round bottomed polystyrene tubes in medium alone or with 10 μg/ml IA-2 peptide (841-860 or 853-872) and incubated for 18 hours at 37°C in a 5% CO2 atmosphere. Where there were sufficient cells, PBMC were also incubated with 20 μl/ml CytoStim (Miltenyi Biotec, Bisley, UK) as a non-antigen specific stimulus. Cells were recovered and IL-10 secreting cells labelled using the IL-10 secretion assay – detection kit (Miltenyi Biotec) according to the manufacturer’s instructions. Cells were co-stained with CD4 and CD45RO antibodies (Miltenyi Biotec) and a live/dead fixable stain (BioLegend, San Diego, USA). Sorting of live CD4+ CD45RO+ IL-10+ cells was performed on a FACS Aria (BD, Oxford, UK) and data analysed using FCS Express (De Novo Software, Los Angeles, USA).
Phenotyping of interleukin-10 secreting T-cells by PCR
Total RNA was isolated from CD4+ CD45RO+ IL-10+ T-cells immediately post sort using a PureLink RNA Micro Scale Kit (Life Technologies, Carlsbad, USA), and cDNA generated using Sensiscript Reverse Transcriptase (Qiagen, Hilden, Germany). PCR was performed with GoTaq G2 (Promega) for 40 cycles with annealing temperature of 60°C using the following primer pairs: T-bet forward 5-GAT GTT TGT GGA CGT GGT CTT G-3, T-bet reverse 5-CTT TCC ACA CTG CAC CCA CTT-3 (29); GATA-3 forward 5′-ACC GGC TTC GGA TGC AA-3′, GATA-3 reverse 5′-TGC TCT CCT GGC TGC AGA C-3′ (30); RORγt forward 5-TTT TCC GAG GAT GAG ATT GC-3, RORγt reverse 5-CTT TCC ACA TGC TGG CTA CA-3 (31); FoxP3 forward CAG CAC ATT CCC AGA GTT CCT C, FoxP3 reverse GCG TGT GAA CCA GTG GTA GAT C (32); β-actin forward 5′-GGC CAA CCG CGA GAA GAT-3′, β-actin reverse, 5′-CGT CAC CGG AGT CCA TCA C-3′ (30). Control RT-PCR reactions were performed with RNA from total PBMC to confirm appropriate functioning of all primer pairs. PCR products were run on a 3% agarose gel stained with ethidium bromide and visualised using a Gene Genius Bio Imaging System (Syngene, Cambridge, U.K.).
Statistical analysis
The analysis of T-cell responses to autoantigens by cytokine ELISPOT presents a challenge because of the very low frequency of responding T-cells in the samples, which introduces a high degree of uncertainty in assigning positive and negative responses in T-cell assays. The frequent absence of cytokine-secreting cells with medium alone make the conventional approach to analysis of T-cell responses by calculation of stimulation index impossible without further data manipulation, and cut-off values for positive responses established for assays detecting cell proliferation after several days of antigen stimulation may be inappropriate for acute responses detected by ELISPOT. Our T-cell response data contained a high proportion of zero values (see results) and a skewed distribution of numbers of cells responding to IA-2 peptides. Because confidence in assigning positive values to T-cell ELISPOT data increases with number of spot forming cells detected, the analysis needs to take into account differences in both the frequency of detectable (non-zero) responses and the magnitude of the responses detected. Lachenbruch’s 2-part statistic (33), which has been applied previously to immune response data with similar characteristics (34), was used to analyse T-cell responses between different patient groups. In this model, the test statistic is the sum of the squared test statistics from a Chi-squared test and a Mann-Whitney U test (df=2). Associations between antibody positivity and HLA type were analysed by the two-sided Fisher’s exact test. All data were analysed using Prism 5 software (GraphPad Software, San Diego, CA).
Results
HLA association with IA-2 antibody epitopes
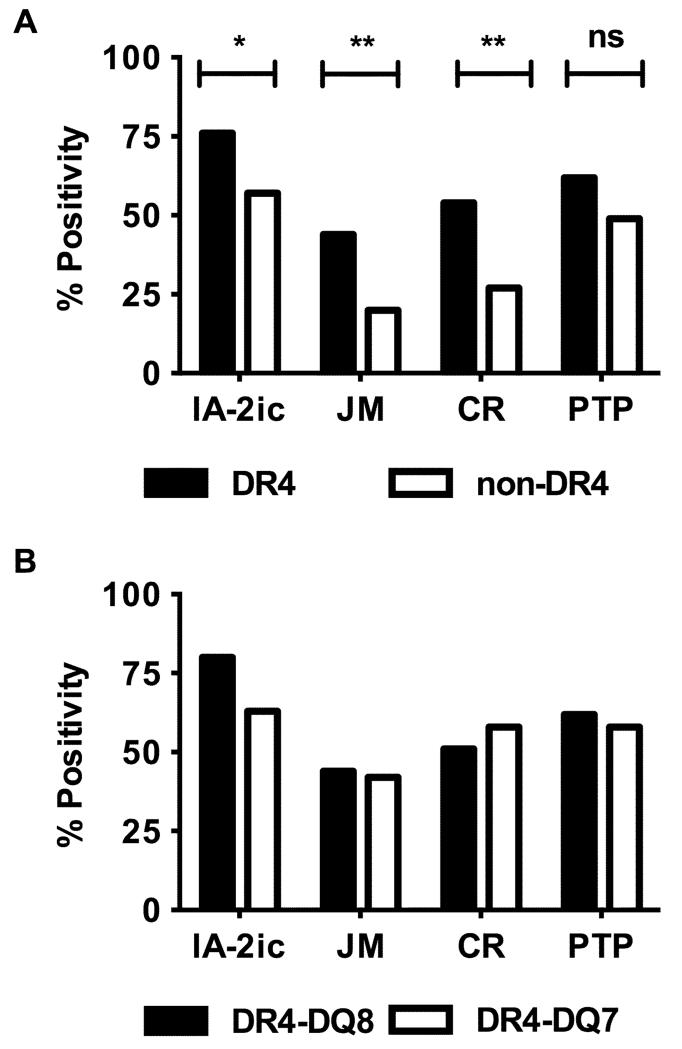
IA-2 antibodies have been shown previously to be associated with the expression of HLA-DR4 (18). To further explore the role of this allele in regulation of IA-2 autoantibodies, we investigated HLA associations with antibodies to constructs representing distinct epitopes in the JM and PTP domains of IA-2. In common with previous studies (18), a weak association of HLA-DR4 with antibodies to the cytoplasmic domain of IA-2 was observed in 127 recent onset Type 1 diabetic patients (p=0.03, Fisher’s exact test, Figure 1A). Truncated constructs were used to assess the contribution of individual epitopes to the HLA-DR4 association observed. Antibodies to the IA-2605-693 construct, which contains 3 overlapping linear epitopes in the 611-640 region of IA-2, were more strongly associated with HLA-DR4 (p=0.008; Fig 1A). Likewise, antibodies to the IA-2643-937 construct, representing a dominant conformational epitope within the PTP domain (831-860) of IA-2 (23), also showed a strong DR4 association (p=0.003; Fig 1A). In contrast, antibodies to other epitopes in the PTP domain, as detected by reactivity to the IA-2643-979 construct, showed no significant HLA-DR4 association. To determine whether the HLA-DR4 association was secondary to a primary association at the linked diabetes-susceptibility HLA-DQ8 allele, patients were also typed for HLA-DQB1 alleles. The prevalence of IA-2 antibody specificities in HLA-DR4-DQ8 and HLA-DR4-DQ7 patients were similar, suggesting a primary association with HLA-DR4 (Fig 1B). No associations of autoantibodies with other HLA-DRB1 or HLA-DQB1 alleles were detected.
FIGURE 1.
Association of IA-2 antibodies with HLA. Subjects grouped according to the expression of DR4 (black bars) or non-DR4 (open bars) HLA alleles (A) or DR4-DQ8 (black bars) or DR4-DQ7 (open bars) haplotypes (B) were analysed for antibody reactivity to the IA-2 cytoplasmic domain (IA-2ic) or, juxtamembrane (JM), central region (CR) or tyrosine phosphatase (PTP) domain constructs. Data are presented as percentage positivity for each construct. N=127. The significance of differences between frequencies of antibody positive patients in DR4 and non-DR4 groups are indicated: * p<0.05, ** p<0.01, ns: not significant.
T-cell responses to IA-2 peptides
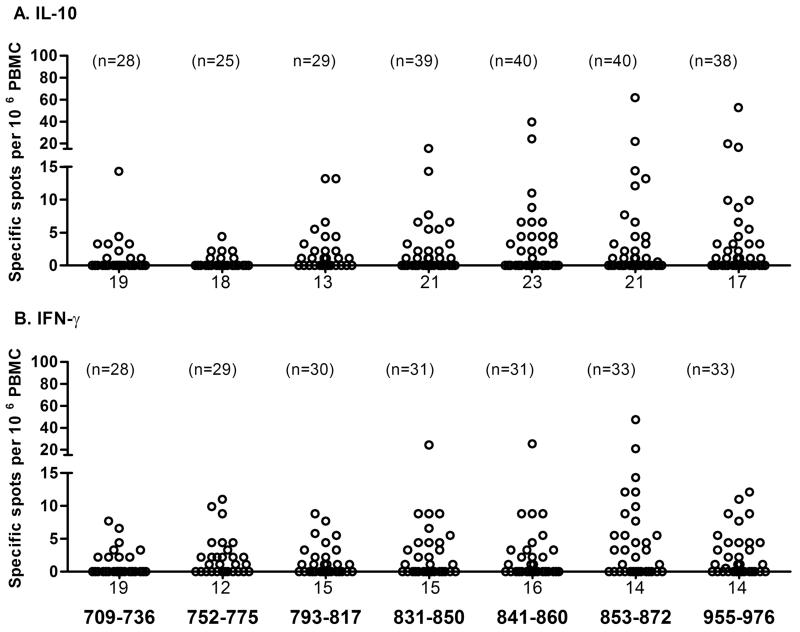
Responses to seven known IA-2 T-cell determinants were analysed in a subset of the patients by cytokine ELISPOT (Fig 2). The magnitude of the responses varied considerably between subjects (range 1-62 spots per 106 PBMC), with the highest frequency of responding T-cells observed for peptides in the region of 831-976 of the IA-2 molecule. There was no effect of gender on T-cell responses and the mean age at onset of responders and non-responders to each peptide was similar in both the IL-10 or IFN-γ ELISPOT assays. IL-17 responses were too low for meaningful analysis (all responses <5 cells per 106 PBMC).
FIGURE 2.
T-cell responses to IA-2 peptides. The number of responding cells per million PBMC detected in ELISPOT assays and representing cells responding to each IA-2 peptide tested are presented as scatter plots for IL-10 (A) and IFN-γ (B). The numbers of patients tested for each peptide are indicated above the relevant scatter plot and the number of zero values for each peptide below the plot.
HLA association of T-cell responses
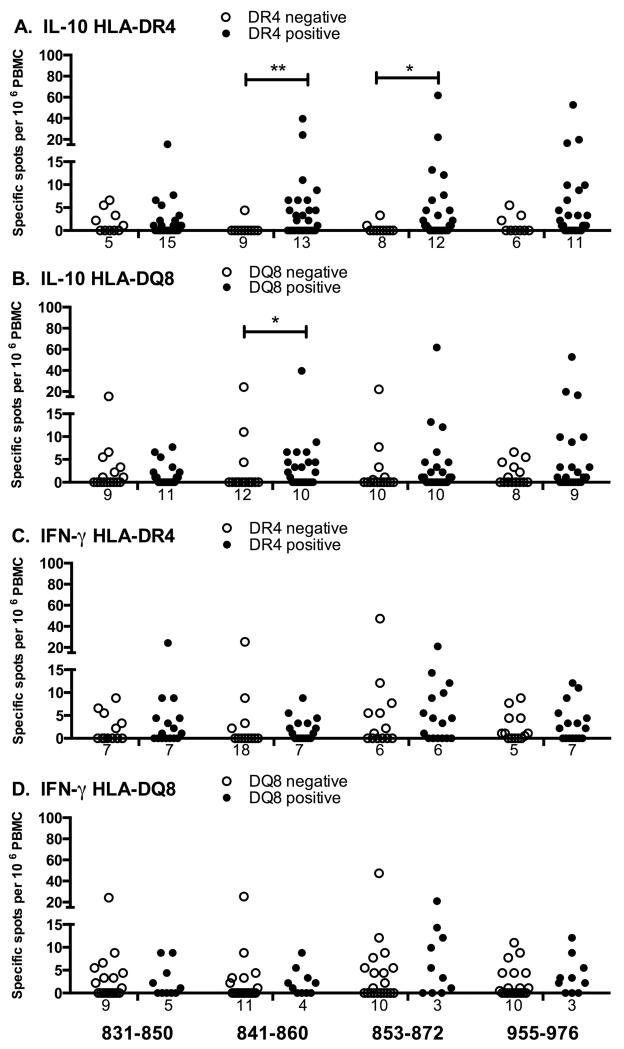
The association of the major diabetes susceptibility HLA alleles with individual peptide responses was studied for IL-10 (Figure 3 A, B) and IFN-γ (Figure 3 C, D). The IL-10 response to peptide 841-860 was significantly associated with the expression of DR4 (Fig 3A; p=0.005, Lachenbruch’s 2-part model) and also with the linked DQ8 allele (Fig 3B; p=0.015). However, no significant difference was observed in the frequency and magnitude of responses to this peptide between DR4-DQ8 and DR4-DQ7 subjects (p=0.15), indicating that, like autoantibody responses, the primary HLA association is likely to be with DR4. IL-10 responses to peptide 853-872 were also significantly associated with DR4 (Fig 3A; p=0.046), but not to DQ8. No significant associations were detected for IFN-γ responses to IA-2 peptides with DR4, DQ8 (Fig 3 C, D) or DR3 (not shown). Neither IL-10 nor IFN-γ responses to PHA were associated with HLA alleles.
FIGURE 3.
Association of T-cell responses with HLA. Type 1 diabetic patients analysed for T-cell responses to IA-2 peptides by IL-10 (A, B) or IFN-γ (C, D) ELISPOT were grouped according to the expression of HLA-DR4 (A, C) or HLA-DQ8 (B, D). T-cell responses of patients expressing (black bars), or not expressing (open bars) the relevant HLA allele are presented as scatter plots, with numbers of zero values indicated below each plot. Data were analysed for differences in T-cell responses to peptides using Lachenbruch’s 2-part model, that assesses differences in both proportions of responders and magnitude of responses; * p<0.05, **p<0.01.
Subtyping of DR4 positive subjects indicated that whilst the majority (22/34) expressed DRB1*0401, the DR4 subtypes DRB1*0402 (5/34), 0404 (4/34), 0405 (1/34), 0408 (1/34), and 0409 (2/34) were also represented; one patient was heterozygous for DRB1*0405 and 0409. These subtypes differed from DRB1*0401 at 1-3 amino acids within the peptide binding site. The frequency and magnitude of IL-10 T-cell responses to peptides 841-860 and 853-872 were not significantly different between DRB1*0401 subjects and in those with other DR4 subtypes (peptide 841-860: frequency 53% vs 50%, magnitude 3.3 vs 6.6 responders per 106 PBMC; peptide 853-872: frequency 65% vs 40%, magnitude 4.4 vs 1.7 responders per 106 PBMC for 0401 and non-0401 patients, respectively). No additional associations of T-cell responses with HLA were detected following DR4 subtyping.
Association of T-cell responses with antibodies
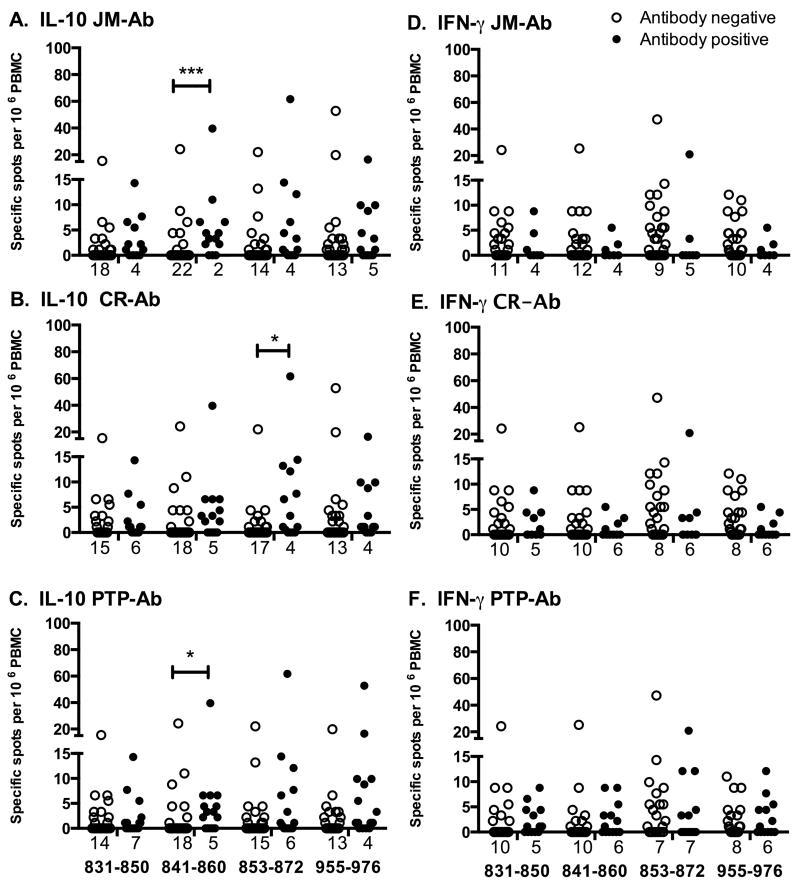
T-cell responses to individual peptides were further analysed to investigate associations with antibodies to sub-domains of IA-2 (Fig 4). IL-10 responses to peptide 841-860 were significantly associated with antibodies to the JM domain (Fig 4A; p<0.001, Lachenbruch’s 2-part model) and, to a lesser extent, with antibodies to the PTP domain (Fig 4C; p=0.037). IL-10 responses to peptide 853-872 were significantly associated with antibodies to the central region (Fig 4B; p=0.032). No further antibody associations were observed with T-cell responses to individual peptides for either IL-10 or IFN-γ.
FIGURE 4.
Association of T-cell responses with IA-2-related antibodies. Type 1 diabetic patients analysed for T-cell responses to IA-2 peptides by IL-10 (A-C) or IFN-γ (D-F) ELISPOT were grouped according to the presence or absence of antibodies to the JM domain (A, D), the central region of the IA-2 PTP domain (B, E) or the entire PTP domain (C, F). T-cell responses to peptides 831-850, 841-860, 853-872 and 955-976 in antibody negative (open bars), or positive (black bars) patients are presented as scatter plots, with numbers of zero values indicated under each plot. T-cell responses to other peptides were not associated with autoantibodies. Data were analysed for differences in T-cell responses to peptides using Lachenbruch’s 2-part model; * p<0.05, ***p<0.001.
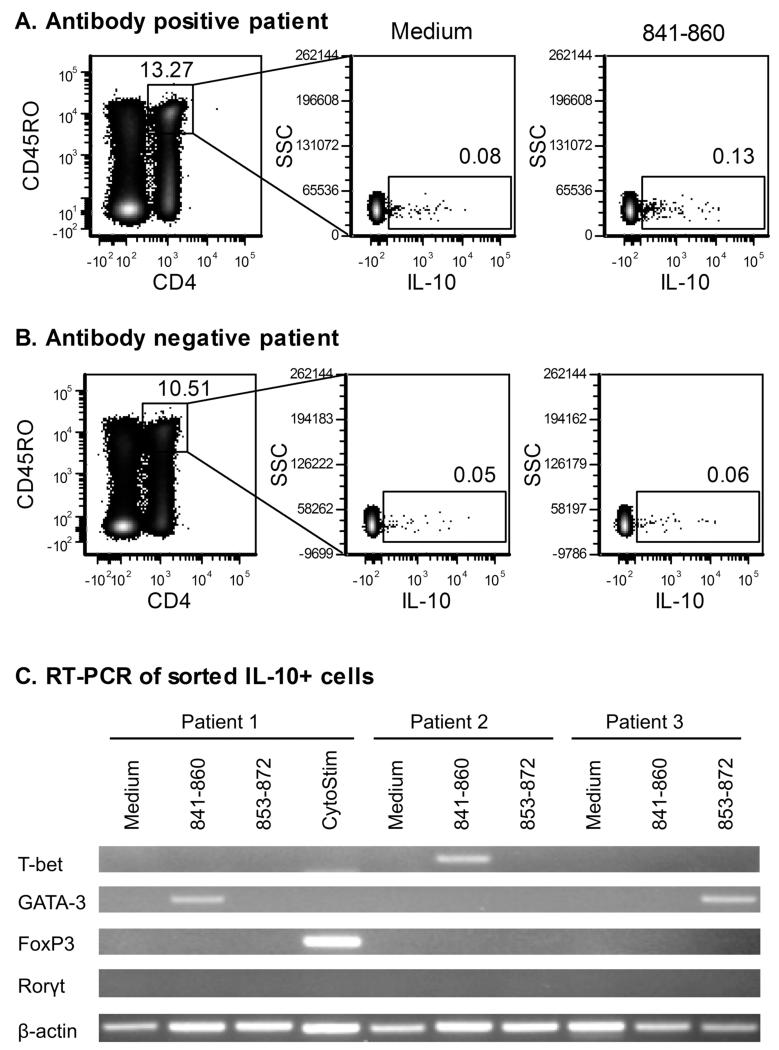
Phenotyping of IL-10 secreting T-cells
IL-10 is secreted by many T-cell subsets, depending on the context of antigen stimulation, so additional phenotypic analysis of T-cells secreting IL-10 in response to IA-2 peptide stimulation is required to understand the potential role of these cells in disease. Cells secreting IL-10 in response to peptides 841-860 and 853-872 were labelled using a cytokine secretion assay (Miltenyi Biotech), which captures the cytokine on the cell surface as it is released. Flow cytometric analysis of peptide-stimulated cells detected low numbers of IL-10 responding cells above background in IA-2 antibody-positive patients, consistent with the data obtained by ELISPOT assay (Fig 5A and 5B). Live CD4+ CD45RO+ T-cells from three patients were subsequently flow sorted and analysed for expression of transcription factors associated with differentiation of distinct T-cell subsets by RT-PCR. T-cells responding to 841-860 and 853-872 peptides were found to express T-bet or GATA-3 (Fig 5C), transcription factors associated with Th1 or Th2 lineage, but not FoxP3 (Treg) or Rorγt (Th17). In contrast, T-cells secreting IL-10 in response to stimulation with CytoStim, which activates T-cells by crosslinking of TCR, showed predominantly FoxP3 expression, consistent with regulatory T-cells being dominant within the general IL-10 responsive memory T-cell population.
FIGURE 5.
Phenotype of autoantigen-specific IL-10 T-cells. Following overnight stimulation with medium alone, IA-2 peptide or CytoStim, IL-10 secreting cells were labelled using the IL-10 secretion assay detection kit (Miltenyi Biotec) and co-stained with a live/dead cell dye and antibodies to CD4 and CD45RO. Dot plots showing IL-10 secreting, CD4+, CD45RO+ T-cells detected after incubation with medium or 841-860 peptide for an antibody positive (A) and antibody negative (B) patient are shown. Percentages of cells within each of the gates are indicated on the plots. Live CD4+ CD45ROhi cells were flow sorted and analysed by RT-PCR for expression of transcription factors associated with differentiation of distinct T-cell subsets. PCR products were visualized on a 3% agarose gel to provide information on the phenotype of IL-10 secreting T-cells for each of the experimental conditions (C).
Discussion
The observation that appearance of autoantibodies in early life is associated with expression of high diabetes risk HLA alleles (35) and the associations of autoantibodies to insulin, GAD and IA-2 with specific alleles in the class II region of the HLA in new onset Type 1 diabetes (18-19; 36-37) support the view that HLA genes mediate their effects on disease susceptibility by influencing immune responses to islet autoantigens. Because HLA associations of individual autoantibody specificities do not precisely mimic HLA associations with disease, it has been argued that immune responses detected by autoantibody assays are unlikely to be directly involved in disease pathogenesis (19). However, the HLA region contains several loci conferring susceptibility to Type 1 diabetes, with independent associations detected at HLA-DQ, HLA-DR, HLA-B and HLA-A loci (38). This may reflect multiple effects of HLA genes on autoimmune responses to different islet autoantigens with the overall genetic susceptibility representing the combination of these effects. The progressive nature of appearance of antibodies to islet autoantigens (12; 39) and the fact that highest risk for progression to Type 1 diabetes is conferred by the presence of autoantibodies to multiple autoantigens rather than any single antibody specificity (15; 40) is consistent with spreading of responses to different immune targets being a crucial element of disease progression, and each of these responses is likely to have a different requirement for optimal antigen presentation on HLA molecules. Knowledge of how each of the products of diabetes-associated HLA genes regulates T- and B-cell responses to specific determinants on autoantigens is required to fully understand the molecular basis of HLA-mediated susceptibility to Type 1 diabetes. The results of the current study provide, for the first time, evidence of HLA-DR4 associations with T-cell responses to individual peptides derived from autoantigen and demonstrate a 3-way relationship between HLA gene expression, T-cell responses to specific IA-2 peptides and the presence of autoantibodies to IA-2 epitopes which support a role for T-B collaboration in the disease.
Previous studies have demonstrated that autoantibodies to epitopes in the JM domain appearing in early pre-diabetes are found predominantly in individuals expressing HLA-DR4 (41). The results of the current study show that this HLA-DR4 association is still evident at the time of clinical onset and the data also show a strong HLA-DR4 association to autoantibodies to another defined epitope within the central region (831-860) of the IA-2 PTP domain. Antibodies to the JM and central region epitopes were found at similar frequency in HLA-DR4 patients expressing either HLA-DQ7 or HLA-DQ8, indicating that the effects of the MHC class II region on the IA-2 antibody responses are conferred primarily by HLA-DR4 gene products, rather than those of linked DQ alleles. The presence of antibodies to other epitopes in the PTP domain showed no significant HLA-DR4 association, indicating stronger effects of the HLA region on autoantibody responses to JM and central region epitopes than those to other regions of IA-2. Hence, the use of assays that discriminate autoantibody responses to individual epitopes on islet antigens may be crucial to understand the relationships of B-cell, T-cells and HLA-genes in the autoimmune responses to islet autoantigens in Type 1 diabetes.
T-cell responses to individual IA-2 peptides were also found to be HLA-DR4-associated, a novel finding because previous demonstrations of HLA associations with T-cell responses have required pooling of data from experiments with multiple peptides from different islet antigens (20). These associations were restricted to T-cell responses to two overlapping peptides within the central region of the IA-2 PTP domain. The strongest association was found for peptide 841-860, originally identified as a T-cell epitope in diabetes by defining the specificity of T-cell lines derived from patients (27) and not previously shown to have links with HLA-DR4. The second peptide, 853-872, was identified as a naturally processed T-cell epitope by elution of peptides from HLA-DR4 molecules isolated from IA-2-loaded B-cells (20). T-cell responses to several other peptides eluted from DR4 molecules in the latter study did not show such associations in our experiments. Whilst the majority of DR4 patients in our study were of DRB1*0401 subtype, a proportion (14/34) expressed other DR4 subtypes (DRB1*0402, 0404, 0405, 0408, or 0409), which differ at 1-3 amino acids within the region of HLA-DR4 molecule representing the peptide binding site. The frequency and magnitude of T-cell responses to the 841-860 and 853-872 peptides were not significantly different between patients with DR*0401 and other DR4 subtypes, suggesting that individual peptide presentation by various HLA-DR4 subtypes can tolerate small differences in the HLA-DR4 beta chain sequence. Much larger studies are required to determine the contribution of the individual DR4 subtypes to IA-2 peptide presentation. T-cell responses to the 841-860 and 853-872 peptides showed associations with antibodies to the JM domain and the central PTP domain epitope, respectively, which themselves are DR4-associated. Hence the study establishes direct links between HLA-DR4 expression, T-cell responses to specific IA-2 determinants and autoantibody responses to specific IA-2 epitopes, consistent with co-operation between T- and B-cells at the level of antigen presentation. Antibodies to specific epitopes may therefore be effective surrogate markers of underlying T-cell responses and may prove valuable for monitoring of autoimmune responses or for selection of candidates for antigen-specific immune intervention.
Studies on the natural history of the B-cell responses to islet antigens in early pre-diabetes have shown that the JM domain of IA-2 is an early autoantibody target, with antibodies to epitopes in the JM domain found almost exclusively in individuals expressing HLA-DR4 (17; 41). In several individuals in whom early antibody responses are restricted to the JM domain, autoantibody reactivity subsequently spread to epitopes in the PTP domain of IA-2. Our finding that T-cell responses to two overlapping determinants in the 841-872 region of the PTP domain of IA-2 are linked to antibodies to the JM and PTP domains may be relevant to our understanding of this determinant spreading. T-cell responses to the 841-860 peptide were associated with antibodies to both JM and PTP domain epitopes. Although the JM and PTP determinants are separated by more than 200 residues in the primary structure of IA-2, these regions may be closely aligned in the 3-dimensional structure, such that B-cell recognition of JM domain epitopes may facilitate presentation of determinants within the PTP domain. Evidence for close structural relationships between the JM and central PTP domain epitopes comes from competitive binding studies of IA-2 antibodies in which mouse monoclonal antibodies directed to JM domain epitopes were shown to block IA-2 binding of the human monoclonal IA-2 autoantibody M13 (42), now known to bind epitopes in the 831-860 region of IA-2 (23).
In common with several other studies of T-cell responses in Type 1 diabetes (28; 43-45), T-cells were detected responding to peptides derived from islet autoantigens by secretion of both pro-inflammatory (IFN-γ) and anti-inflammatory (IL-10) cytokines. T-cell responses with more than 15 responders per 106 PBMC were only detected to the 831-850, 841-860, 853-872 and 955-976 IA-2 peptides in IL-10 and IFN-γ assays. Although IFN-γ T-cell responses were detected at similar frequency to those in the IL-10 assay, statistically significant associations of T-cell reactivity with HLA-DR4 or IA-2 autoantibodies were only observed in the IL-10 assay. A major question is whether these IL-10 responses represent pathogenic or protective immune responses. Type 1 diabetes has been considered a Th1 mediated disease, with T-cell responses to IA-2 and proinsulin peptides skewed towards production of IFN-γ (28). IL-10 clearly has anti-inflammatory properties and detection of T-cells secreting IL-10 in response to peptides from islet antigens has been interpreted as indicative of immune regulation (28). However, it is now recognised that IL-10 is not produced exclusively by Th2 or T regulatory cells but is also secreted, depending on the context of antigen exposure, by virtually all CD4+ helper subsets, potentially as a mechanism to self-regulate and prevent bystander damage (46). Modification of our cytokine secretion assay to allow capture and fluorescent labelling of IL-10 on the surface of responding T-cells allowed purification of T-cells responding to the 841-860 and 853-872 peptides for phenotypic analysis by flow cytometry and RT-PCR. The results demonstrated that the peptide-responsive CD4+ CD45ROhi T-cells express transcription factors associated with Th2 (GATA-3) or Th1 (Tbet) differentiation, whereas FoxP3, typical of regulatory phenotype is not expressed. These results suggest that the conditions used for re-stimulation of memory T-cells with peptide in vitro may not be sufficient for induction of cytokines normally associated with the parental phenotype. Similar results have been reported by the Lanzavecchia group, where Th17 memory cells that were found to produce IL-10 when re-stimulated in vitro in the absence of cytokines that promote the Th17 phenotype (47). It is clear that IL-10 secretion by T-cells per se cannot be regarded as a reliable marker of immune regulation and that careful phenotyping of peripheral blood T-cells producing IL-10 on islet antigen stimulation is essential to understand their role in the pathogenesis of Type 1 diabetes. The detection of T-cells responding to the 841-860 and 853-872 peptides with Th2 and Th1 phenotypic markers suggest that the responding cells may have roles both in supporting antibody production and in driving inflammatory responses.
Studies on T-cell responses in human Type 1 diabetes suggest a diversity of specificity (reviewed in (48)) and phenotype that may indeed be expected following spreading of immune responses during a prolonged period of inflammation that may precede disease onset. Diabetes-specific autoreactive CD4+ T-cells in peripheral blood are found within the CD45RO+ memory population, but interpretation of T-cell data can be complicated by the detection of naïve T-cell responses that are irrelevant to disease (49). T-cell responses to the 841-860 and 853-872 peptides are of particular interest because of their association with a major disease susceptibility allele, their link to autoantibodies in the JM domain that feature early in the autoimmune response to IA-2 and their localisation of within the PTP domain that suggest involvement in spreading of autoimmunity from JM domain epitopes. Understanding how the T- and B-cell responses to the major autoantigens in Type 1 diabetes diversify over time, and which antibody targets are critical for spreading of the destructive T-cell response, is crucial for the targeting of antigen-specific immunotherapy to determinants most relevant to disease progression.
Acknowledgments
We thank the patients and their families for agreeing to participate in the study, and clinical and nursing staff within the collaborating centres for facilitating recruitment.
This study was funded by research grants from the JDRF (grant reference 1-2008-525) and Diabetes UK (grant reference 11/0004297). Flow cytometry was supported by the National Institute for Health Research Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London.
Footnotes
Disclosures
The authors declare that they have no conflict of interest.
References
- 1.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol. Rev. 2011;91:79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 2.Miller B, Appel M, O’Neil J, Wicker L. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J. Immunol. 1988;140:52–58. [PubMed] [Google Scholar]
- 3.Singal DP, Blajchman MA. Histocompatibility (HL-A) antigens, lymphocytotoxic antibodies and tissue antibodies in patients with diabetes mellitus. Diabetes. 1973;22:429–432. doi: 10.2337/diab.22.6.429. [DOI] [PubMed] [Google Scholar]
- 4.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin. Exp. Immunol. 2009;155:173–181. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nerup J, Platz P, Andersen OO, Christy M, Lyngsoe J, Poulsen JE, Ryder LP, Nielsen LS, Thomsen M, Svejgaard A. HL-A antigens and diabetes mellitus. Lancet. 1974;2:864–866. doi: 10.1016/s0140-6736(74)91201-x. [DOI] [PubMed] [Google Scholar]
- 6.Bingley PJ, Christie MR, Bonifacio E, Bonfanti R, Shattock M, Fonte MT, Bottazzo GF, Gale EA. Combined analysis of autoantibodies improves prediction of IDDM in islet cell antibody-positive relatives. Diabetes. 1994;43:1304–1310. doi: 10.2337/diab.43.11.1304. [DOI] [PubMed] [Google Scholar]
- 7.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, Raskin P, Rodriguez H, Schatz DA, Wherrett D, Wilson DM, Lachin JM, Skyler JS. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N. Engl. J. Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serreze DV, Chapman HD, Varnum DS, Hanson MS, Reifsnyder PC, Richard SD, Fleming SA, Leiter EH, Shultz LD. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: Analysis of a new ‘speed congenic’ stock of NOD.Ig mu (null) mice. J. Exp. Med. 1996;184:2049–2053. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noorchashm H, Noorchashm N, Kern J, Rostami SY, Barker CF, Naji A. B-cells are required for the initiation of insulitis and sialitis in nonobese diabetic mice. Diabetes. 1997;46:941–946. doi: 10.2337/diab.46.6.941. [DOI] [PubMed] [Google Scholar]
- 10.Akashi T, Nagafuchi S, Anzai K, Kondo S, Kitamura D, Wakana S, Ono J, Kikuchi M, Niho Y, Watanabe T. Direct evidence for the contribution of B cells to the progression of insulitis and the development of diabetes in non-obese diabetic mice. Int. Immunol. 1997;9:1159–1164. doi: 10.1093/intimm/9.8.1159. [DOI] [PubMed] [Google Scholar]
- 11.Noorchashm H, Lieu YK, Noorchashm N, Rostami SY, Greeley SAS, Schlachterman A, Song HK, Noto LE, Jevnikar AM, Barker CF, Naji A. I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet beta cells of Nonobese Diabetic Mice. J. Immunol. 1999;163:743–750. [PubMed] [Google Scholar]
- 12.Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes. 1999;48:460–468. doi: 10.2337/diabetes.48.3.460. [DOI] [PubMed] [Google Scholar]
- 13.Gorus FK, Goubert P, Semakula C, Vandewalle CL, De Schepper J, Scheen A, Christie MR, Pipeleers DG. IA-2-autoantibodies complement GAD65-autoantibodies in new-onset IDDM patients and help predict impending diabetes in their siblings. The Belgian Diabetes Registry. Diabetologia. 1997;40:95–99. doi: 10.1007/s001250050648. [DOI] [PubMed] [Google Scholar]
- 14.Bingley PJ, Bonifacio E, Gale EA. Antibodies to glutamic acid decarboxylase as predictors of insulin-dependent diabetes mellitus. Lancet. 1994;344:266–267. doi: 10.1016/s0140-6736(94)93033-3. [DOI] [PubMed] [Google Scholar]
- 15.Christie MR, Roll U, Payton MA, Hatfield EC, Ziegler AG. Validity of screening for individuals at risk for type I diabetes by combined analysis of antibodies to recombinant proteins. Diabetes Care. 1997;20:965–970. doi: 10.2337/diacare.20.6.965. [DOI] [PubMed] [Google Scholar]
- 16.Christie MR, Genovese S, Cassidy D, Bosi E, Brown TJ, Lai M, Bonifacio E, Bottazzo GF. Antibodies to islet 37k antigen, but not to glutamate decarboxylase, discriminate rapid progression to IDDM in endocrine autoimmunity. Diabetes. 1994;43:1254–1259. doi: 10.2337/diab.43.10.1254. [DOI] [PubMed] [Google Scholar]
- 17.Naserke HE, Ziegler AG, Lampasona V, Bonifacio E. Early development and spreading of autoantibodies to epitopes of IA-2 and their association with progression to type 1 diabetes. J. Immunol. 1998;161:6963–6969. [PubMed] [Google Scholar]
- 18.Genovese S, Bonfanti R, Bazzigaluppi E, Lampasona V, Benazzi E, Bosi E, Chiumello G, Bonifacio E. Association of IA-2 autoantibodies with HLA DR4 phenotypes in IDDM. Diabetologia. 1996;39:1223–1226. doi: 10.1007/BF02658510. [DOI] [PubMed] [Google Scholar]
- 19.Howson JM, Stevens H, Smyth DJ, Walker NM, Chandler KA, Bingley PJ, Todd JA. Evidence that HLA class I and II associations with type 1 diabetes, autoantibodies to GAD and autoantibodies to IA-2, are distinct. Diabetes. 2011;60:2635–2644. doi: 10.2337/db11-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peakman M, Stevens EJ, Lohmann T, Narendran P, Dromey J, Alexander A, Tomlinson AJ, Trucco M, Gorga JC, Chicz RM. Naturally processed and presented epitopes of the islet cell autoantigen IA-2 eluted from HLA-DR4. J. Clin. Invest. 1999;104:1449–1457. doi: 10.1172/JCI7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bunce M, O’Neill CM, Barnardo MC, Krausa P, Browning MJ, Morris PJ, Welsh KI. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 & DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP) Tissue Antigens. 1995;46:355–367. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 22.Zetterquist H, Olerup O. Identification of the HLA-DRB1*04, -DRB1*07, and -DRB1*09 alleles by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours. Human Immunol. 1992;34:64–74. doi: 10.1016/0198-8859(92)90086-3. [DOI] [PubMed] [Google Scholar]
- 23.Weenink SM, Lo J, Stephenson CR, McKinney PA, Ananieva-Jordanova R, Rees Smith B, Furmaniak J, Tremble JM, Bodansky HJ, Christie MR. Autoantibodies and associated T-cell responses to determinants within the 831-860 region of the autoantigen IA-2 in Type 1 diabetes. J. Autoimmun. 2009;33:147–154. doi: 10.1016/j.jaut.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc. Natl. Acad. Sci. U.S.A. 2007;104:17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatfield ECI, Hawkes CJ, Payton MA, Christie MR. Cross reactivity between IA-2 and phogrin/IA-2beta in binding of autoantibodies in IDDM. Diabetologia. 1997;40:1327–1333. doi: 10.1007/s001250050828. [DOI] [PubMed] [Google Scholar]
- 26.Dromey JA, Weenink SM, Peters GH, Endl J, Tighe PJ, Todd I, Christie MR. Mapping of epitopes for autoantibodies to the type 1 diabetes autoantigen IA-2 by peptide phage display and molecular modeling: overlap of antibody and T cell determinants. J. Immunol. 2004;172:4084–4090. doi: 10.4049/jimmunol.172.7.4084. [DOI] [PubMed] [Google Scholar]
- 27.Hawkes CJ, Schloot NC, Marks J, Willemen SJ, Drijfhout JW, Mayer EK, Christie MR, Roep BO. T-cell lines reactive to an immunodominant epitope of the tyrosine phosphatase-like autoantigen IA-2 in type 1 diabetes. Diabetes. 2000;49:356–366. doi: 10.2337/diabetes.49.3.356. [DOI] [PubMed] [Google Scholar]
- 28.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM, Roep BO, Peakman M. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J. Clin. Invest. 2004;113:451–463. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lighvani AA, Frucht DM, Jankovic D, Yamane H, Aliberti J, Hissong DD, Nguyen BV, Gadina M, Sher A, Paul WE, O’Shea JJ. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc. Natl. Acad. Sci. U.S.A. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu HR, Chang JC, Chen RF, Chuang H, Hong KC, Wang L, Yang KD. Different antigens trigger different Th1/Th2 reactions in neonatal mononuclear cells (MNCs) relating to T-bet/GATA-3 expression. J. Leukoc. Biol. 2003;74:952–958. doi: 10.1189/jlb.0902474. [DOI] [PubMed] [Google Scholar]
- 31.Ayyoub M, Deknuydt F, Raimbaud I, Dousset C, Leveque L, Bioley G, Valmori D. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc. Natl. Acad. Sci. U.S.A. 2009;106:8635–8640. doi: 10.1073/pnas.0900621106. D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S, Sakaguchi S. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int. Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 33.Lachenbruch PA. Analysis of data with excess zeros. Statistical Methods in Medical Research. 2002;11:297–302. doi: 10.1191/0962280202sm289ra. [DOI] [PubMed] [Google Scholar]
- 34.Hersperger AR, Pereyra F, Nason M, Demers K, Sheth P, Shin LY, Kovacs CM, Rodriguez B, Sieg SF, Teixeira-Johnson L, Gudonis D, Goepfert PA, Lederman MM, Frank I, Makedonas G, Kaul R, Walker BD, Betts MR. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog. 2010;6:e1000917. doi: 10.1371/journal.ppat.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziegler AG, Bonifacio E. Age-related islet autoantibody incidence in offspring of patients with type 1 diabetes. Diabetologia. 2012;55:1937–1943. doi: 10.1007/s00125-012-2472-x. [DOI] [PubMed] [Google Scholar]
- 36.Ziegler R, Alper CA, Awdeh ZL, Castano L, Brink SJ, Soeldner JS, Jackson RA, Eisenbarth GS. Specific association of HLA-DR4 with increased prevalence and level of insulin autoantibodies in first-degree relatives of patients with type I diabetes. Diabetes. 1991;40:709–714. doi: 10.2337/diab.40.6.709. [DOI] [PubMed] [Google Scholar]
- 37.Serjeantson SW, Kohonen-Corish MR, Rowley MJ, Mackay IR, Knowles W, Zimmet P. Antibodies to glutamic acid decarboxylase are associated with HLA-DR genotypes in both Australians and Asians with type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:996–1001. doi: 10.1007/BF00401432. [DOI] [PubMed] [Google Scholar]
- 38.Nejentsev S, Howson JM, Walker NM, Szeszko J, Field SF, Stevens HE, Reynolds P, Hardy M, King E, Masters J, Hulme J, Maier LM, Smyth D, Bailey R, Cooper JD, Ribas G, Campbell RD, Clayton DG, Todd JA. Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature. 2007;450:887–892. doi: 10.1038/nature06406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barker JM, Barriga KJ, Yu L, Miao D, Erlich HA, Norris JM, Eisenbarth GS, Rewers M. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY) J. Clin. Endocrinol. Metab. 2004;89:3896–3902. doi: 10.1210/jc.2003-031887. [DOI] [PubMed] [Google Scholar]
- 40.Kulmala P, Savola K, Petersen JS, Vahasalo P, Karjalainen J, Lopponen T, Dyrberg T, Akerblom HK, Knip M, The Childhood Diabetes in Finland Study Group Prediction of insulin-dependent diabetes mellitus in siblings of children with diabetes. A population-based study. J. Clin. Invest. 1998;101:327–336. doi: 10.1172/JCI119879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bearzatto M, Naserke H, Piquer S, Koczwara K, Lampasona V, Williams A, Christie MR, Bingley PJ, Ziegler AG, Bonifacio E. Two distinctly HLA-associated contiguous linear epitopes uniquely expressed within the islet antigen 2 molecule are major autoantibody epitopes of the diabetes-specific tyrosine phosphatase-like protein autoantigens. J. Immunol. 2002;168:4202–4208. doi: 10.4049/jimmunol.168.8.4202. [DOI] [PubMed] [Google Scholar]
- 42.Ananieva-Jordanova R, Evans M, Nakamatsu T, Premawardhana LD, Sanders J, Powell M, Chen S, McGrath V, Belton C, Arnold C, Baker S, Betterle C, Zanchetta R, Smith BR, Furmaniak J. Isolation and characterisation of a human monoclonal autoantibody to the islet cell autoantigen IA-2. J. Autoimmun. 2005;24:337–345. doi: 10.1016/j.jaut.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Durinovic-Bello I, Schlosser M, Riedl M, Maisel N, Rosinger S, Kalbacher H, Deeg M, Ziegler M, Elliott J, Roep BO, Karges W, Boehm BO. Pro- and anti-inflammatory cytokine production by autoimmune T cells against preproinsulin in HLA-DRB1*04, DQ8 Type 1 diabetes. Diabetologia. 2004;47:439–450. doi: 10.1007/s00125-003-1315-1. [DOI] [PubMed] [Google Scholar]
- 44.Ott PA, Herzog BA, Quast S, Hofstetter HH, Boehm BO, Tary-Lehmann M, Durinovic-Bello I, Berner BR, Lehmann PV. Islet-cell antigen-reactive T cells show different expansion rates and Th1/Th2 differentiation in type 1 diabetic patients and healthy controls. Clin. Immunol. 2005;115:102–114. doi: 10.1016/j.clim.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Chujo D, Foucat E, Nguyen TS, Chaussabel D, Banchereau J, Ueno H. ZnT8-Specific CD4(+) T Cells Display Distinct Cytokine Expression Profiles between Type 1 Diabetes Patients and Healthy Adults. PLoS One. 2013;8:e55595. doi: 10.1371/journal.pone.0055595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 47.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1 beta. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 48.Di Lorenzo TP, Peakman M, Roep BO. Translational mini-review series on type 1 diabetes. Clin. Exp. Immunol. 2007;148:1–16. doi: 10.1111/j.1365-2249.2006.03244.x. Systematic analysis of T cell epitopes in autoimmune diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monti P, Scirpoli M, Rigamonti A, Mayr A, Jaeger A, Bonfanti R, Chiumello G, Ziegler AG, Bonifacio E. Evidence for in vivo primed and expanded autoreactive T cells as a specific feature of patients with type 1 diabetes. J. Immunol. 2007;179:5785–5792. doi: 10.4049/jimmunol.179.9.5785. [DOI] [PubMed] [Google Scholar]