Abstract
Photorefractive keratectomy (PRK) remodels corneal stroma to compensate refractive errors. The removal of epithelium and the ablation of stroma provoke the disruption of corneal nerves and a release of several peptides from tears, epithelium, stroma and nerves. A myriad of cytokines, growth factors, and matrix metalloproteases participate in the process of corneal wound healing. Their balance will determine if reepithelization and stromal remodeling are appropriate. The final aim is to achieve corneal transparency for restoring corneal function, and a proper visual quality. Therefore, wound-healing response is critical for a successful refractive surgery. Our goal is to provide an overview into how corneal wounding develops following PRK. We will also review the influence of intraoperative application of mitomycin C, bandage contact lenses, anti-inflammatory and other drugs in preventing corneal haze and post-PRK pain.
Keywords: Photorefractive keratectomy, Cornea, Wound healing, Contact lenses
Resumen
La queratectomía fotorrefractiva (PRK) remodela el estroma de la córnea para compensar los errores refractivos. La eliminación del epitelio y la ablación del estroma provoca la alteración de los nervios corneales y la liberación de diversos péptidos de la lágrima, epitelio, estroma y nervios. Innumerables citoquinas, factores de crecimiento y metaloproteasas de la matriz participan en el proceso de regeneración y cicatrización corneal. Su equilibrio determinará si la re-epitelización y la remodelación del estroma son adecuados. El objetivo final es el logro de la transparencia corneal para restablecer la función de la córnea, así como la calidad visual adecuada. Por tanto, la respuesta de regeneración y cicatrización corneal es esencial para el éxito de la cirugía refractiva. Nuestro objetivo es aportar una visión general sobre el modo en que se desarrolla dicho proceso tras la PRK. Revisaremos también la influencia de la aplicación intraoperatoria de mitomicina C, lentes de contacto terapéuticas, y otros fármacos para prevenir el haze y el dolor tras la PRK.
Palabras clave: Queratectomía fotorrefractiva, Córnea, Curación de heridas, Lentes de contacto
The ablation surgery of the corneal surface for the correction of refractive errors began with the development of the excimer laser. The acronym laser means “Light Amplification by the Stimulated Emission of Radiation”. Photorefractive keratectomy (PRK), developed by Trokel and colleagues in 1983, uses an excimer laser that emits ultraviolet light of 193 nanometers (nm), a combination of Argon and Fluor (ArF) to remodel the corneal.1–6 It was not until 1996 when the Food and Drug Administration (FDA) aproved PRK as a refractive surgery technique.7 In PRK the excimer laser acts on the anterior corneal stroma,2,8,9 producing a stromal remodeling, and, consequently, inducing a change in corneal refraction.10,11 It corrects mild to moderate myopia, hyperopia and astigmatism, with high level of safety and efficacy.3,11–20 However, the use of PRK has been reduced over the past years by the introduction of the Laser In Situ Keratomileusis (LASIK).12,21 Although LASIK provides less postoperative pain, less inflammation, and faster corneal wound healing and visual recovery,8,17,19,22–25 PRK may be a useful alternative in post-radial keratotomy,26–28 post-penetrating keratoplasty,29 in thin corneas, irregular topographies, alterations of the basal membrane, treatment of some LASIK flap complications or residual refractive errors after LASIK.11,12,19,30–32 It is also indicated in military pilots, professional athletes, or patients that have a high risk for traumatic postoperative flap dislocation.12,31 In addition to the above-mentioned advantages, the PRK has gained popularity with the recent wave front guided laser ablation, which reduces postoperative high order aberrations (HOA), improving the optical quality.30
The visual quality might not be optimal if some complications take place, like subepithelial corneal haze, epithelial hypertrophy, regression of refractive error, deposition of subepithelial extracellular matrix or fibrosis. Other adverse effects include postoperative pain, abnormal corneal nerve regeneration, and night vision symptoms like halos and glare.3,10,11,14,18,22,33–40
The purpose of this review is to explain the main cellular changes and complications that occur in different corneal layers after PRK, and to explain how they affect the visual quality. We discuss the role of mytomicin C and bandage contact lenses in corneal regeneration, and the role of different drugs in postoperative corneal pain management.
Corneal Wound Healing
Corneal wound healing is a complex process that, in normal conditions, culminates in the restoration of the tissue, without scar formation or vascularization. The aim is to maintain transparency to recover a proper visual function. After epithelial injury, the corneal healing starts with the removal of necrotic cells.41 Fibronectin provides a transient matrix for the adhesion of migrating cells, until an epithelial monolayer covers the injured area.42 Fibronectin also stimulates the production of plasminogen activator (PAA), and by a cascade of events, cell-subepithelial matrix adhesions break down.42 In the next step, limbal stem cells undergo mitosis to reestablish lost cells, and with the anchoring of hemidesmosomes to the underlying stroma, the epithelial regeneration process completes.41 Stromal wound healing depends on epithelial cells, and on their interaction with keratocytes.43 Following stromal injury, released cytokines induce the apoptosis of keratocytes under the wound, and stimulate the proliferation and migration of neighboring keratocytes.44 These active keratocytes synthesize matrix metalloproteases (MMP) to remodel the stroma. At later stages, a number of them take the repair phenotype, the so-called myofibroblasts,45 and produce collagen and extracellular matrix (ECM), until the basement membrane prevents the inflow of cytokines in the stroma, and myofibroblast, presumably, commit apoptosis (Fig. 1).46,47
Figure 1.
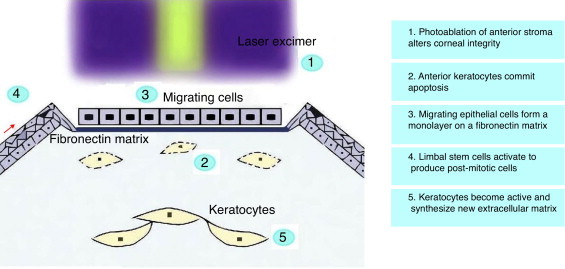
Corneal alterations and first steps of wound healing following PRK.
Epithelial Wound Healing Following PRK
The corneal epithelium is formed by superficial, wing and basal cells.48,49 In order to facilitate the stromal ablation in PRK, the corneal epithelium is removed. The absence of the epithelium will condition corneal repair. Corneal epithelial cells are the first cells involved in the corneal regeneration process after PRK.50 Epithelial cells proliferate and migrate from the limbus and the basal epithelial layer to reestablish corneal layers.8,51 Corneal regeneration after PRK can be better understood using current, non-invasive, confocal microscopy. It has been used on animals and on humans for corneal cellular structure visualization in real time.2,5,10,22,25,40,48 Esquenazi et al.22 proved using a new generation high-resolution in vivo confocal microscope that environmental conditions influenced the regeneration of the corneal epithelium. They showed that the number of the superficial cells was reduced in desiccating environments compared with normal conditions, and the number of basal epithelial cells was increased. Histological studies conducted in animals and in humans, have found that corneal epithelium is thicker after PRK,2,52 caused by an elongation of the basal epithelial cells and an increased number of superficial cell layers.25 The corneal flattening in myopic PRK may result in postoperative epithelial thickening due to the lack of mechanical influences of the upper eyelid that polishes the corneal surface with blinking.2 Epithelial hyperplasia in PRK is associated with deep stromal ablation depths and with small ablation zones (4.00–4.5 mm) because there is a marked curvature change in the edges of the ablated area. When ablation zones are large (6.00 mm), they have less demarcated contours, and thus, the change in epithelial thickness is minimal.53–56 Table 1 shows the variation of central corneal thickness with different surgery techniques published in the scientific literature. Erie2 proved that, after PRK, the central epithelial thickness returned to preoperative levels at 1 month. However, it continued to progressively increase during the first year, being 21% thicker at that time. This result is similar to the 22% thickness increase seen in LASIK by Erie et al.57 However, the time required for thickness stabilization differs between the two techniques, due to the complex interaction of epithelial cells and activated keratocytes in PRK.2 According to Patel et al.,25 central corneal epithelium in LASIK increased 24% during the first year after surgery and remained stable during the next 7 years. In PRK, corneal thickness continued to increase at 1 month, 1 year and 7 years (442±39 μm, 464±44 μm, 471±45 μm; respectively).25 Recently, Ivarsen et al.52 have concluded that in PRK and LASIK, the epithelial thickness increases 15%–20% after surgery, but the epithelial changes in LASIK occur during the first week and remain unaltered during the following 3 years. It has been suggested that epithelial hyperplasia can induce a reduction of postoperative refractive effect. Erie showed myopic regression significantly associated with epithelial thickness increase.2 Nevertheless, Ivarsen et al.52 did not found any correlation between changes in epithelial thickness and changes in refraction after PRK or LASIK, probably because of the small size of their sample.
Table 1.
Variation of Central Corneal Thickness, Mean±SD or Range (μm).
| Study | Technique | Preop | 1 week | 1 month | 3 months | 6 months | 1 year | 2 years | 3 years | 5 years | 7 years |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ivarsen et al. (2009)52 | LASIK | 529±49 | 460±47 | 477±43 | |||||||
| PRK | 522±32 | 423±35 | 463±32 | ||||||||
| Wallau and Campos (2008)159 | LASIK | 543±25 | 473±28 | 473±27 | 473±27 | ||||||
| PRK with MMCa | 545±26 | 456±30 | 458±30 | 460±29 | |||||||
| Patel et al. (2007)25 | LASIK | 514±37 (437–576) |
452±38 (411–563) |
456±42 (386–576) |
456±39 (402–564) |
462±37 (411–577) |
456±30 (388–524) |
455±31 (371–509) |
|||
| PRK | 493±36 (427–560) |
442±40 (388–526) |
464±44 (396–534) |
469±44 (411–544) |
472±59 (393–573) |
468±48 (406–555) |
471±45 (400–557) |
||||
| Kozak et al. (2003)56 | LASIK | 549±37 (515–620) |
467±29 | 474±30 | 481±23 | ||||||
| PRK | 552±34 (504–602) |
473±39 | 477±35 | 482±35 |
LASIK, laser in situ keratomileusis; PRK, photorefractive keratectomy; MMC, mitomycin C.
0.002%, 1 min.
Stromal Wound Healing Following PRK
Stroma occupies approximately the 90% of corneal thickness,58 and it can be subdivided into three continuous layers: anterior, middle and posterior.49 The corneal stroma is built up from collagen fibers, ground substance, keratocytes and nerve fibers.5,49 Keratocytes – corneal stromal cells – play a major role in maintaining corneal transparency, and synthesizing the components of the extracellular matrix (ECM).58 Active keratocytes produce collagen and proteoglycans to form the ECM after stromal injury. The human stromal cornea contains collagen type I, V and VI.59,60 Type I is predominant (75%), followed by type VI (approximately, 17%).60 Type III collagen appears in inflammatory events or during wound healing. Proteoglycans participate in collagen fibrillogenesis and matrix assembly.61 After corneal injury, newly produced collagen fibers tend to have larger diameters, as they contain high levels of dermatan sulphate (a type of proteoglycan) that lasts up to 6 months.62
Stromal keratocytes are normally quiescent or inactive, and are the second cells involved in the process of corneal regeneration, just after corneal epithelial cells. After PRK, keratocytes underlying the wound disappear by apoptosis due to a stress exposure.2,24,37,40,50,63 During the first 24 h after injury, macrophages, monocytes, T cells and polymorphonuclear cells infiltrate the area and remove damaged cells.44,64 Metallonoproteinases (MMPs) and the plasminogen activator system remove the affected extracellular matrix.9,65–67 The MMPs are proteolytic enzymes secreted by active keratocytes or fibroblasts, and degrade complex molecules of the extracellular matrix. Although nine types of MMPs exist, in the cornea only four MMPs are important, being MMP-1 the most relevant.67 MMP-8 concentration has been observed to be significantly elevated in the second day after PRK (P=.001).68 The remaining keratocytes, adjacent to wound borders, are activated in response to various cytokines released by cells in upper layers, such as interleukin (IL)-1, and growth factors like tumor necrosis factor (TNF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), epithelial growth factor (EGF), and transforming growth factor (TGF).2,8,14,22,24,35,51,58,69–74 These growth factors are normal components of the tear and corneal cells, produced by the lacrimal gland,35 and regulate a variety of processes involved in homeostasis and corneal wound healing, including migration, mitosis and cell differentiation.75 Particularly, transformation growth factor beta (TGF-beta) seems to transform active keratocytes into myofibroblasts that appear at later stages of stromal healing. Myofibroblasts can be identified through the expression of α-smooth muscle actin (SMA).45
PRK produces oxygen free radicals, secondary to the exposure of ultraviolet radiation, thermal increase, and polymorphonuclear cell infiltration.76,77 Free oxygen radicals may interact with lipid components, nucleotic acids, and sulphur contained in enzymes,72,78 and particularly with reactive oxygen species (ROE) that are considered to produce the most reactive and cytotoxic damage. In fact, they have been described as a partial cause of keratocyte apoptosis.77 Among the antioxidant enzymes that protect the cornea from radicals, superoxide dismutase (SOD), glutathione peroxidase (Gpx) and catalase are the most relevants.75,78 Ascorbic acid and dl-alpha-tocopherol (Vitamin E) also prevents from the effects of free radicals.78 Corneal epithelial ascorbic acid absorbs ultraviolet radiation, protecting keratocytes, but high or altered corneal ascorbate levels in the human cornea after PRK, may produce accelerated keratocyte death.5 In rabbit corneas, decreased activity of SOD and Gpx enzymes has been proved after refractive surgery.78 For this reason, additional antioxidant enzymes seem to be involved in reducing corneal oxidative stress following PRK. 1-cys peroxiredoxin (1-cys Prx) may be an important enzyme involved in the differentiation, migration and proliferation of epithelial cells. 1-cysPrx increases 4 h after PRK and remains in high levels until 7 days after PRK.75
The density of keratocytes varies across the stroma. It is estimated that in the anterior stroma the density is 5%–10% greater than in middle and posterior stroma.79,80 It has been documented that a corneal stroma rich in keratocytes prevents the epithelial corneal infection or, at least, minimizes the extension of the infection.2,5,10 After PRK, the anterior keratocyte population drastically diminishes, and the distribution and shape is greatly altered.2,10,34,40,81,82 In confocal microscopy, high reflectance, hyperplasticity, hypertrophy and a decrease in the contrast of the anterior stromal keratocytes can be observed.34,63,83 Human histological studies confirm that the decrease of anterior stromal keratocytes in humans and animal respond similarly.84,85 Table 2 shows the variation of anterior, posterior and total keratocyte density in the different studies published in the scientific literature. Erie et al.5 confirmed that after 5 years of PRK there were evidences of keratocyte density loss in middle and posterior stroma. They observed a reduction of 20%–24% in the posterior stroma (P<.05) although they claimed that this loss was not completely evident. Keratocyte density in the anterior 10% of the stroma continues to decrease 5% per year between 1 and 3 years after PRK.2 Erie2 reported a progressive decline in anterior stromal keratocytes, becoming significant at 36 months after PRK (P=.02). In contrast, middle and posterior keratocyte densities remained unchanged between 1 and 3 years after PRK.2 In another study, Erie et al.10 proved that the keratocyte density in the anterior 10% of the stroma, decreased at 6, 12, 24 and 36 months (41%, 40%, 43%, 45%; respectively) after PRK, compared to pre-PRK. In a posterior longer-term study, Erie et al.5 demonstrated a similar decreasing pace in anterior keratocyte density: 40%, 42%, 45%, and 47% at 6 months, 2 years, 3 years, and 5 years (P<.001). Amoozadeh et al.40 found a reduction in keratocyte density 6 months after surgery, but the loss was similar for LASIK and PRK interventions: in anterior stroma, 34.7% versus 31.13% (P>.05) and posterior stroma 0.31% versus 0.02%, (P>.05), respectively. However, other studies have seen differences between PRK and LASIK, probably associated with the more superficial ablation in PRK.5,25,40 The consequences of keratocyte density loss after PRK are still unknown, but the visual acuity and corneal clarity seem to be preserved.5
Table 2.
Variation of Keratocyte Density After Surgery, Mean±SD (cell/mm2).
| Study | Technique | Preop | 3 months | 6 months | 1 year | 2 years | 3 years | 5 years | |
|---|---|---|---|---|---|---|---|---|---|
| Einollahi et al. (2011)63 | PRK with MD | AA | 902±107 | 704±119 | 643±134 | ||||
| PS | 653±72 | 622±53 | 609±60 | ||||||
| PRK with AAD | AS | 943±100 | 734.3±103.7 | 696.7±129.6 | |||||
| PS | 665±69 | 617±70 | 621±72 | ||||||
| Amoozadeh et al. (2009)40 | LASIK | AS | 1058±95 | 690±55 | |||||
| PS | 708±40 | 699±57 | |||||||
| PRK | AS | 1027±80 | 707±63 | ||||||
| PS | 719±51 | 719±45 | |||||||
| Midena et al. (2007)82 | PRK with MMCa | AS | 449±58 | 305±59 | |||||
| PS | 363±53 | 392±53 | |||||||
| PRK+corticosteroid | AS | 473±58 | 317±66 | ||||||
| PS | 365±53 | 392±46 | |||||||
| Erie et al. (2006)5 | LASIKb | FT | 31.108±4984 | 28.337±2863 | 27.533±2757 | 27.491±2693 | 26.320±1973 | 22.982±1829 | |
| PRKb | FT | 26.220±2897 | 24.756±3302 | 23.301±3564 | 23.776±3596 | 23.459±3394 | 21.017±4534 | ||
| PS | 23.524±5003 | 24.775±4234 | 22.564±4065 | 23.049±4362 | 22.337±3894 | 17.935±6668 |
PRK, photorefractive keratectomy; MD, mechanical debridement; AAD, alcohol-assisted debridement; LASIK, laser in situ keratomileusis; MMC, mitomycin C; AA, anterior stroma; PS, posterior stroma; FT, full-thickness.
0.02%, 2 min.
Results in volumetric values (cell/mm3).
After the initial depletion of anterior stromal keratocytes, an increase in the keratocyte density is observed over time, probably secondary to mitosis, cellular migration, or reproduction of keratocytes and myofibroblasts.5,9,10,34,51,86 Following apoptotic keratocyte loss, the first morphological changes of remaining keratocytes that can be histologically observed, are an increase in cell size and an increase in the size and the number of nucleoli, rough endoplasmatic reticula, mitochondria, free ribosomes and Golgi complexes, indicating an active state.87 These keratocytes quickly repopulate the anterior stroma, and return to similar preoperative levels.2,5,10,34 Several studies using confocal microscopy have analyzed the keratocyte density after PRK. Corbett et al.88 found that at 2 days after PRK the anterior keratocyte density was increased 50%, 100% at 1 month, and returned to preoperative levels at 6 months. Frueh et al.85 concluded that the anterior keratocyte density increased 15% at 1 and 4 months after PRK, and returned to preoperative levels 1 year after PRK. Similarly, Erie et al. found an increase of 20% in the anterior stroma at 3 months after PRK.2,89 According to the results of Corbett et al.88 and Erie et al.,10 anterior keratocytes proliferation begins 1 month after PRK, with a pick at 3 months, and return to preoperative levels at 6 months.
Corneal Haze
Corneal haze reduces corneal transparency at variable degrees.90,91 Subepithelial haze occurs in all patients 1 month after PRK, reaching the greatest intensity at 3–6 months, and gradually decreases from then on.2,8,34,92 Yet, some authors affirm that it begins to decrease at 12–24 months after PRK.8,92 Corneal haze is more common after correction of high myopia (>−6.00 D), and it is rarely seen after correction of <−6.00 D of myopia or <+4.00 D of hyperopia.43,71,91 Besides the ablation depth, the severity of corneal haze is correlated with excessive ocular UV-B radiation, duration of the epithelial defect, postoperative steroid treatment, male sex and with certain population with brown iris.2,16,19,21,24,28,71,81,86,93–95 PRK presents higher corneal haze incidence than LASIK, probably because of the destruction of the basement membrane.8,45,52 In the presence of damaged epithelial cells and basement membrane, cytokines and growth factors can easily flow from epithelium to anterior stroma.45,96 Cytokines released from epithelial cells activate keratocytes, as mentioned in a previous section, which synthesize large diameter collagen fibrils.8,11,33,73,81 Abnormally deposited extracellular matrix implies the development of corneal opacity.69 Moreover, active keratocytes present a high reflectance that also contributes to the decrease in corneal transparency. In addition, subepithelial vacuolation, deposit materials like proteoglycans, hyaluronic acid and collagen Type IV are involved in the formation of the corneal haze in advanced stages.33,77 Plasminogen activator–plasmin system degrades the damaged ECM, and extended low levels beyond the third day after PRK causes corneal haze formation.97 Guerriero et al.58 affirm that the loss of collagen type IV is related to the activation of keratocytes in vivo and in vitro, and Winkler et al.33 and Mohrenfels et al.98 emphasize on the role of type IV collagen in the development of corneal cloudiness. Secondary ultraviolet B (UV-B) exposure, originating from sun or solarium is a causal factor for aforementioned abnormal proteoglycan deposition and associated augmented corneal thickness.99
On the other hand, myofibroblast, derivatives of TGF-beta responding keratocytes, are thought to be the first biological event for corneal haze formation.51,93,94 Myofibroblasts play an essential role in the recovery of the corneal integrity after penetrating injury, mainly in advanced stages.22 They secrete extracellular matrix, contract wounds and have the ability to generate adhesion structures with the surrounding substrate.71 TGF-beta also induces the expression of connective tissue growth factor (CTGF), which mediates collagen synthesis, and along with myofibroblasts regulates the corneal wound healing, and may promote scar formation.100 After PRK, myofibroblasts appear as a pathological response to injury,71 and their decreased transparency roots in the low intracellular content of crystalline.101 Irregular surface has also been related to high incidence of corneal haze,94,102 and higher irregularity is seen with increasing dioptric corrections in PRK.103 Interestingly, surface irregularity is positively correlated with myofibroblast density in the anterior stroma.43 In normal corneal wound healing, complete regeneration of the basal membrane after PRK occurs within 6–8 weeks in rabbits,104 which limits the access of growth factors to the stroma69 and, consequently, myofibroblasts commit apoptosis46 modulated by IL-1.47 Therefore, the presence of myofibroblast, and subsequent corneal haze, is largely dependent upon the restoration of the basement membrane.43,105
Corneal haze has been traditionally measured in the slit-lamp, and graded with diverse scales, like Hanna's scale. The new technology leads us to use automated instruments for corneal haze measurement. In vivo confocal microscopy is a reliable tool, as far as standardized methods are used.106 It is the most widely used objective method in clinical setting for haze measurement. In the last years, alternative techniques have come out. Confocal imaging of second harmonic-generated (SHG) signals has been shown to be sensitive in measuring corneal fibrosis after refractive surgery.107 Recently, the densiometry program of Pentacam Scheimpflug imaging system (Oculus Optikgeräte GmbH) has been proved to be a useful method for measuring corneal haze.108
Visual Disturbances of Corneal Haze
The corneal haze produces a reduction of low contrast visual acuity and night vision symptoms that, in the vast majority of situations, improve with time.67 It is possible to see corneal haze formation after PRK by means of confocal microscopy, observed as a decrease in the contrast of the image and an increase in reflectivity.81 Böhnke et al.81 using a Tandem scanning confocal microscopy, correlated corneal haze and anterior stromal reflectivity. However, the tandem scanning confocal microscopy is not able to detect acellular regions of the anterior stroma early after PRK when epithelium and sub-basal plexus are not formed.10 Although corneal haze in humans is less pronunced than in animal models, if corneal haze persists and affects significantly to the corneal transparency, it causes light scatter.4,94,109 For this reason, corneal haze may be described and analyzed through back light scattering (backscatter).81,110 It also causes irregular astigmatisms,2,34,93 and subsequent loss of corrected distance visual acuity (CDVA).86
The regression of the refractive error may be produced by epithelial irregularity, alterations in the keratocyte density or subepithelial deposits. Myopic regression occurs in 78% of eyes in the first 12 months after PRK.2 Table 3 shows the mean spherical equivalent changes reported in different scientific studies. In the first week after PRK, epithelial irregularity causes a reduction in visual quality.88 During the first month, altered keratocytes decrease contrast sensitivity, mainly in high frequencies, and cause glare. During the next 2 months, subepithelial deposits produce a decrease in contrast sensitivity, especially in low frequencies.4,88 Ginis et al.4 reported that subepithelial deposits are the first factor that contributes to the development of corneal scatter. The visual quality is affected temporarily, although there is evidence that in some cases it persists for more than 1 year.43,91 In order to avoid a decrease in the visual quality, all postoperative efforts must go oriented to control the subepithelial matter.88 The corneal epithelium does not seem to contribute significantly to the refractive change after PRK, although some studies suggest that epithelial thickening may produce myopic regression,2 even 5 years after PRK.90 Moller-Pedersen et al.55 and Cua and Pepose92 suggested that new keratocytes growth in central cornea or postoperative corneal scarring is likely to be the main causes of myopic regression in ablations of 6 mm. In agreement with this hypothesis, Moller-Pedersen et al.55 demonstrated that hyperopic changes were the direct result of a stromal thinning. Erie2 found an increase of 12 μm of epithelial thickness at 12 months after PRK that was associated with a myopic regression of −0.41 diopters but no correlation was found between stromal thickening and myopic regression; however, the combined effect of epithelial and stromal thickening was correlated with myopic regression.
Table 3.
Mean Spherical Equivalent After Surgery, Mean±SD or Range (Diopters).
| Study | Technique | Preop | 1 month | 6 months | 1 year | 2 years | 3 years | 5 years | 7 years |
|---|---|---|---|---|---|---|---|---|---|
| Einollahi et al. (2011)63 | PRK+MD | −2.42±0.75 (−4.13 to −1.13) |
−0.34±1.00 | ||||||
| PRK+AAD | −2.38±0.72 (−4.00 to −1.25) |
−0.28±0.91 | |||||||
| Wallau and Campos (2008)159 | LASIK | −3.99±1.20 (−1.46 to −6.96) |
0.49±0.52 (−0.50 to 1.50) |
||||||
| PRK+MMCa | −3.85±1.12 (−1.95 to −6.40) |
0.61±0.61 (−0.50 to 2.88) |
|||||||
| Ghirlando et al. (2007)3 | PRK | − 4.37±1.35 | −0.37±0.61 | −0.27±0.31 | |||||
| LASEK | −3.95±1.29 | +0.22±0.79 | −0.17±0.35 | ||||||
| Nassaralla et al. (2007)28 | PRK+MMCb | −2.72±0.76 (−1.50 to −4.00) |
−0.08±0.38 (−0.75 to −0.75) |
−0.18±0.35 (−0.75 to −0.50 |
|||||
| Patel et al. (2007)25 | LASIK | −6.5±2.5 (−11.0 to −2.0) |
−0.1±0.5 (−1.0 to+1.0) |
−0.2±0.5 (−1.25 to+0.75) |
− 0.2±0.4 (−1.0 to+0.62) |
−0.2±0.4 (−1.0 to+0.75) |
−0.2±0.5 (−1.37 to+0.37) |
−0.4±0.5 (−1.25 to+0.25) |
|
| PRK | −3.7±1.4 (−5.75 to −1.25) |
−0.1±0.3 (−0.5 to+1.0) |
−0.3±0.3 (−0.87 to plano) |
−0.4±0.4 (−1.25 to+0.25) |
−0.3±0.2 (−0.75 to plano) |
−0.6±0.4 (−1.25 to plano) |
−0.5±0.4 (−1.0 to plano) |
||
| Lee et al. (2005)18 | PRK | −5.17±1.53 (−2.00 to −9.13) |
0.24±0.61 | −0.46±1.01 | |||||
| tPRK | −5.11±.51 (−1.87 to −9.50) |
0.59±0.78 | 0.18±0.91 | ||||||
| LASEK | −5.26±2.58 (−1.50 to −9.50) |
0.13±0.62 | −0.82±1.18 | ||||||
| Kozak et al. (2003)56 | LASIK | −6.00 | −0.48±0.30 (−0.16 to −1.10) |
||||||
| PRK | −6.00 | −0.67±0.35 (−0.21 to −1.21) |
PRK, photorefractive keratectomy; MD, mechanical debridement; AAD, alcohol-assisted debridement; LASIK, laser in situ keratomileusis; MMC, mitomycin C; LASEK, laser-assisted subepithelial keratectomy; tPRK, trans-PRK.
(0.002%, 1 min).
(0.02%, 2 min).
Regeneration of Corneal Innervation
The cornea is the most innervated tissue of the human body,7 and these sensory nerves are derived from the ophthalmic branch of the trigeminal nerve fibers.2,111 Corneal sensory nerves penetrate the limbus and form nerve bundles in the anterior third of the stroma. Once there, they run perpendicularly to cross Bowman's membrane, and form the sub-basal nerve plexus as a network between the basal epithelial cells and Bowman's layer (Fig. 2).49,111 Corneal nerve fibers, if visualized using confocal microscopy in normal conditions, show high reflectivity across the corneal stroma with a rectilinear pattern. Subepithelial nerve fibers, on the other hand, are thinner than stromal nerve fibers. Corneal fibers are considered primarily nociceptive (70%), followed by mechanosensitive fibers (20%).112 In PRK, photoablation severs nerves of the subbasal plexus and anterior stroma.2,83 It has been suggested that axotomy of corneal nerves might cause the decrease in keratocyte density after PRK,113,114 corneal nerves directly innervate keratocytes and provide trophic support in normal conditions.115
Figure 2.
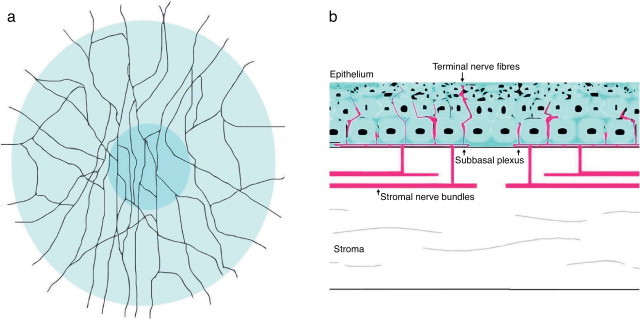
Schematic representation of corneal stromal nerves and subbasal plexus in human cornea. (A) Frontal view. (B) Cross section.
Animal studies have proved that the regeneration of the corneal nerves after PRK occurs as a biphasic process. In the first stage, a subbasal plexus originates from the cut end of subepithelial plexus, and the fine neurites run centrally with migrating cells.116,117 In the next phase, this transient plexus degenerates, and stromal originated nerves take place.117 Subbasal nervous plexus can have a significant influence on the regulation of epithelial healing.14 Substances like chemokines, proteases and neuropeptides are released after corneal injury,8,24,25,50,51,118 and it is postulated that neuropeptides like substance P (SP) and calcitonin-gene related peptide (CGRP) contribute to corneal wound healing.112 Corneal nerves also influence the production of collagen type VII, necessary for the anchoring of the epithelium to the stroma.119 Conversely, injured epithelial cells release nerve growth factor (NGF) that stimulates nerve regeneration.
Approximately, at 8 weeks after PRK, sub-epithelial nerve fibers are visible on the edges. Erie,2 using tandem scanning confocal microscope, visualized subbasal nerve fiber bundles in 17% of the corneas at 1 month after PRK. However, he noted that the density of these nerve fibers was 98% less than preoperatively.2 After about 3 months of the surgery, no branched nerve fibers can be visualized in the center of the zone of ablation. Changes in subepithelial plexus and stromal trunks begin to appear 2–4 months postoperatively.8 At 6–8 months after the intervention of PRK subepithelial nerve regeneration is almost complete,8,111,120 although changes in the structure of the corneal nerves can be appreciated by confocal microscopy up to 12 months postoperatively.120 However, nerve density continues to improve until 12 months after surgery, and returns to the preoperative values at 2 years.121 According to Moilanen et al.122 in 71% of cases the central branching postoperatively was comparable to control subjects at 5 years (P=.56). Erie2 proved that subbasal nerve density was reduced at 3, 6 and 12 months (87%, 75%, 60%, respectively) after PRK, and returned to preoperative levels at 24 and 36 months postoperatively. Subsequently, Erie et al.83 in a prospective 5-year longitudinal clinical trial, proved with confocal microscopy that the recovery of subbasal nerve density in central cornea was faster in PRK than in LASIK. The authors observed that subbasal corneal density was reduced by 59% at 1 year after PRK (2764±1321 μm/mm2) compared to preoperatively (6786±1948 μm/mm2; P<.001). Sub-basal nervous plexus was almost recovered 2 years after PRK (6242±1763 μm/mm2), and remained unchanged at 3 years (6358±2447 μm/mm2) and 5 years (5903±3086 μm/mm2).83 In LASIK, they observed that subbasal corneal density was reduced by 34% at 3 years (P<.001),83 with values at 5 years postoperatively comparable to those obtained preoperatively (5903±3086 μm/mm2).83 It is worth to note that in this study, the corneal flap was created using a mechanical microkeratome.83 As the new technology allows making corneal flaps with laser instead of with a mechanical microkeratome, it is possible that studies in the near future report a faster corneal nerve recovery after LASIK.
When the process of corneal nerve regeneration finalizes, morphological abnormalities are often observed.8,83,122 According to Erie,2 in the first 6 months after PRK the central subbasal nerves are organized in horizontal or oblique orientation. However, between 6 and 12 months, the subbasal nerve orientation rotes and comes to vertical orientation. In dry eye conditions, Esquenazi et al.22 observed active keratocytes, and they expressed nerve growth factor (NGF). NGF stimulates the proliferation of basal epithelial cells in normal conditions. Active keratocytes provoke an overexpression of NGF, which leads to abnormal findings in corneal nerves, such as hypertrophy.22 They also found higher nerve tortuosity, higher number of nerve beads, and the presence of nerve sprouts in desiccating environment group,22 which means there is a high metabolic activity to repair the alterations in the corneal epithelium.
Corneal Pain and Sensitivity
Photoablation severs corneal nerves, disrupting the lacrimal functional unit (LFU). LFU is constituted by the lachrymal gland, ocular surface and innervation. It regulates tear secretion, and affects its composition.123 Thereby, photoablation produces transitory dry eye, deterioration of corneal barrier function and alteration in corneal sensitivity.83,111,124 A reduction of the tear flow after PRK has been proved using Schirmer test.124 According to Erie et al.,83 LASIK presents higher prevalence of postoperative dry eye, altered corneal epithelium and tear film than PRK. Dry eye has been associated with low corneal sensitivity.125,126 Different devices are available to measure corneal sensitivity, as Cochet–Bonnet esthesiometry or non-contact gas esthesiometer. Cochet–Bonnet esthesiometer only stimulates mechanosensory fibers, whereas non-contact gas esthesiometer measures activation thresholds of nociceptors using controlled chemical, thermal and mechanical pulses. Non-contact gas esthesiometer is, therefore, a more sensitive device for measuring alterations in corneal sensitivity. Still, Coche–Bonnet esthesiometry is more widely used, and controversy remains about the time course of the corneal sensitivity recovery after PRK with this device. Kauffmann et al.120 affirm that the recovery of corneal sensitivity usually starts at 4–6 weeks, completing approximately within 6–12 months following PRK. However, Erie et al.83 claim that the recovery of corneal sensitivity is completed from 3 months to 1 year after PRK. Hypoesthesia is often expected until 3 months after surgery, due to the loss of corneal nerves.8 On the other hand, Gallar et al.127 measured corneal mechanical and chemical sensitivity following PRK with non-contact gas esthesiometer, and found that both types of sensitivities were reduced even 5 years postoperatively, achieving normal values in 10 years. Despite the diminished corneal sensitivity, intense pain is usually present hours after PRK.128 Gallar et al.129 attributed corneal pain and discomfort sensations to the altered functionality of corneal nerves. They recorded spontaneous activity and modified responsiveness in corneal fibers of cats that underwent PRK.129 Experimental evidences support the idea that ongoing activity evokes spontaneous pain sensations.130,131
Acceleration of Corneal Regeneration Process, Reduction of Corneal Haze and Corneal Pain Management
Nowadays there are several alternatives to speed up the process of epithelial regeneration, like epithelial removal techniques, amniotic membrane, or bandage contact lenses. In PRK, agents like mytomicin-C (MMC) or fluoroquinones that reduce the corneal haze formation are used, and drugs to reduce the corneal pain and inflammation are also prescribed.
Epithelial Removal Techniques
In PRK, previous to the impact of laser energy over the cornea, the corneal epithelium has to be removed. The removal of the corneal epithelium is carried out mainly with epithelial mechanical scraping using chemical agents like diluted ethanol solution,9 through a rotary brush or using the laser itself – known as transepithelial ablation (Fig. 3).18,21,22,39,63,128,132,133 The epithelial scraping has postoperative adverse effects like pain, myopic regression or corneal haze. Some modification in PRK technique can alter the wound healing response with the aim of minimizing the adverse effects.25 The exposition to agents such as ethanol can produce an increase in the inflammatory response and more damage to the anterior stromal keratocytes that could increase the haze formation.21,94 Yet, controversy remains in the scientific literature because other authors affirm that alcohol-assisted epithelial removal produces less inflammation, favoring epithelial regeneration and preventing corneal haze or keratoyce apoptosis.9,63 Esquenazi et al.22 proved that the epithelial scraping might be associated with an increase in the number of reflective structures in the stroma, mainly in corneas with ocular dryness after PRK. The laser-scrape epithelial removal decreases the degree of keratocyte apoptosis, producing a less pronounced loss of superficial keratocytes.2 However, the irrigation with cold balanced salt solution (BSS) may alter the keratocyte apoptosis in the retroablation zone.2 The time necessary for mechanical debridement is greater than the time required for laser or alcohol scrape techniques, even for expert surgeons.18 Mechanical debridement is related to stromal dehydration and disappearance of anterior stromal keratocytes.18,63 This loss provokes an increase of cells in the underlying stroma, causing stromal hyperplasia and haze formation.134 Einollahi et al.63 found faster mean epithelial healing time in the alcohol-assisted group than in the mechanical group (3.0±0.3 versus 3.2±0.4 days, P=.001). They observed greater anterior retroablation stromal keratocyte density in the mechanical group than in the alcohol-assisted groups at 3 months (704.3±119.9 cells/mm2 versus 743.3±103.7 cells/mm2, P=.05) and at 6 months (643.8±134.4 cells/mm2 versus 696.7±129.6 cells/mm2, P=.02).63 In the same study, Bahram et al. did not found statistically significant differences in middle and posterior keratocyte density between the mechanical and alcohol-assisted groups.63 They also proved that mechanical and alcohol-assisted epithelial debridement after PRK present similar visual and refractive outcomes in patients with mild myopia,63 in agreement with the results of Goreishi et al.135 They reported similar safety and efficacy with alcohol-assisted and mechanical debridement in a 1250 eye sample, but anterior keratocyte density was not assessed in this study.135
Figure 3.

TransPRK (transepithelial photorefractive keratectomy) on the Schwind Amaris 1050 RS laser platform (with permission of Schwind Eye-Tech-Solutions).
Laser-assisted subepithelial keratomileusis (LASEK) was developed in order to reduce corneal pain and haze formation associated with PRK, and to accelerate visual recovery. Epithelial delamination with diluted alcohol showed in an electron microscope study that was able to leave a smooth surface, ideal for LASEK intervention.136 It seems that a regular surface before laser application helps corneal healing and prevents haze.137 Chen et al.138 contrasted these findings in a later study, and showed a high variability in morphological changes after diluted alcohol treatment, dependent upon concentration and time. Cell viability was affected when alcohol exceeded its concentration by 25% or 25-s exposure.138 Yet, these studies have been conducted in vitro, and the complex interactions of tear film and corneal surface were not considered. In vivo studies do not show any difference between LASEK and PRK.139 Lee et al.18 evaluated epithelial healing, postoperative pain and visual outcomes using epithelial mechanical (conventional PRK), transepithelial PRK and 20% diluted alcohol laser-assisted subepithelial keratomileusis (LASEK) with flap repositioning. After 6 months, they found little differences in clinical outcomes between the 3 techniques, noting a slight overcorrection in the transepithelial PRK and slight undercorrection in LASEK. Corneal pain and subepithelial haze results were similar.18 Subsequently, Ghanem et al.139 proved in a prospective randomized double-masked study that the reepithelialization was faster in a PRK group compared with a butterfly LASEK group, even though epithelial semi-discs were repositioned intraoperatively in LASEK group. (4.35±0.48 days versus. 4.75±0.72 days, P=.002). They also found lower pain level in PRK group, but pain scores and ocular discomfort were not statistically different from butterfly LASEK (3.31±4.09 versus. 4.43±4.27; P=.18).139
It has been proven in animal studies that transepithelial ablation produces a uniform surface for corneal regeneration, and prevents keratocyte apoptosis,36 reducing the risk of corneal haze.21,72 Wang et al.140 presented promising preliminary results of SCHWIND-ESIRIS excimer laser for transepithelial ablation, but the flawed design of the study makes difficult to assess the real value of this technique. Later, Aslanides et al.21 proved in humans that transepithelial ablation was safer than the epithelial mechanical scraping using chemical agents as alcohol, as it provides a faster epithelial healing, less postoperative pain and less corneal haze at 1 week (P=.07), and at 1, 3, and 6 months after surgery (P<.05). In addition, they observed an improvement of 3 Snellen lines in visual acuity on day 3 in the modified transepithelial PRK (all-surface laser ablation) group compared to conventional alcohol-assisted PRK group (0.4 versus 0.2; P<.05).21 Transepithelial ablation also resulted in better corrected distance visual acuity (DCVA) than conventional alcohol-assisted PRK (33% versus 13%, respectively, P>.05),21 although differences in higher order aberrations were not statistically significant.21
Amniotic Membrane Transplantation
Apart from the above mentioned techniques, amniotic membrane transplantation reduces the inflammation after PRK, prevents polymorphonuclear cell infiltration, produces less peroxidation, avoids keratocyte apoptosis and stimulates corneal epithelialization.37,77 It is usually combined with PRK to treat corneal dystrophies, corneal degenerations, scars, keratopathies,141,142 or even to treat corneal haze secondary to PRK.143 The amniotic membrane restricts the influx of polymorphonuclear cells (PMC) to the patch.144,145 PMCs adhere to the amniotic membrane and eventually commit apoptosis.146 This is a physiological way of suppressing corneal inflammation.147 In addition, amniotic membrane has intrinsic keratocyte growth factors, EGF and neurotrophins that promote epithelization.148,149 It also suppresses TGF-beta1, collagen III and fibronectin.150 Taken together, amniotic membrane has a potent anti-scarring effect that reduces corneal haze formation, as demonstrated in animal studies.144,151
Agents to Enhance Wound Healing
The wound healing response may be altered by the prophylactic application of a topical solution of mitomycin-C (MMC) immediately after the laser ablation,27 in order to avoid or minimize myofibroblast activation.8,25,40,71,86,90,133,152–154 MMC is an antineoplastic antibiotic agent of the family of anti-tumor quinolones and derived from Streptomyces caespitosus. It is a potent DNA crosslinker: it inhibits the replication of deoxyribonucleic acid (DNA).28,32,91,93,155–159 Thereby, MMC inhibit cell mitosis, including epithelial and stromal cells.8,34,86,93,133,155,157,160,161 Mitomycin-C decreases corneal haze compared to corticosteroid treatment,82 and, consequently, improves visual acuity.152 Its use is specially indicated in high myopia (≥−6.00 D) and deeper ablation depths (≥75 μm).82,86,90,133,155,160 Wallau and Campos162 obtained better UCVA and BSCVA with the combination of PRK with MMC, than with LASIK (P=.027 and P<.001, respectively) at 3, 6 and 12 months after surgery. Goreishi et al.163 reported an incidence of 4% of corneal haze at 1 year postoperatively with intraoperative application of 0.02% MMC, in a sample with a mean refractive error of −5D. Fazel et al.164 found that two-step administration of 0.02% MMC (45 s, followed by 15 s) further decreased corneal haze formation in high myopia, compared to a single dose of 45 s. The benefits of MMC have also been described once the haze has been established, where mechanical epithelial scraping and instillation of MMC restores corneal transparency.165
Although the application of the mitomycin C is helpful for corneal recovery, it is necessary to control the doses and the time of exposure.20 According to Thornton et al.133 the concentration is a more important factor than the duration of MMC exposure in corneal haze prevention. Rajan et al.34 analyzed the effects of MMC after correction of −9.00 diopters by PRK in 3 groups of human corneas: without MMC application, with MMC (0.2 mg/mL) application for 1 min and with MMC (0.2 mg/mL) application for 2 min. The 2 min MMC group (0.2 mg/mL) had thinner epithelium than the 1 min and without MMC application groups (P<.0001). The application of the intraoperative MMC lasts between 10 s and 120 s, depending on the surgeon.11 According to Khoury et al.156 the application of intraoperative MMC vary from 12 s to 5 min. Shojaei et al.153 affirm that short-time MMC exposure prevents low-grade haze in low ablation depths. The MMC doses oscillate between 0.002% and 0.06%.156 The intraoperative application of 0.02% MMC solution is the most recommended, as it produces less corneal haze, and provides better uncorrected visual acuity (UCVA) and best spectacle-corrected visual acuity (BSCVA).124,166,167 Still, Ramjoo et al.90 found similar refractive and haze outcomes with 0.01% and 0.02% MMC for mild myopia, recommending the use of 0.01%. The lowest dose available is recommended to avoid side effects. Rajan et al.34 observed a delay in keratocyte regeneration after MMC application (P<.0005). Midena et al.82 proved by means of confocal microscopy that the application of 0.02% MMC produced a considerable decrease of anterior stromal keratocytes, but there is no evidence of this decline in the posterior stromal keratocytes. Subsequently, Thornton et al.133 observed a keratocyte loss in the anterior stroma 1 month and 6 months after PRK with standard MMC concentrations (0.02%). Razmjoo et al.90 did not found significant reduction in keratocyte density after application of 0.02% mitomycin C (MMC). The dose of MMC is associated with the grade of refractive error. Thornton et al.133 believe that for high myopia corrections (>−6.00 D) standard concentration of topical MMC (0.02%) may be used, whereas for moderate myopia (−3.00 to −5.90 D) low dose of MMC (0.002%) may be considered, although it seems that intermediate dose of MMC (0.02%) is more effective than 0.002% for moderate myopia.
The cytotoxicity of MMC increases with cumulative doses,161 and when MMC is combined with ethanol, which increments the apoptosis of keratocytes.20 Few complications have been associated with its use with the exception of a decrease in the short term of the keratocyte density.71,155,157 However, some complications have been documented at the time of instillation or after some weeks. Although unusual, scleral ulceration, non-healing conjunctivas and complications associated with high MMC doses (0.04%) or prolonged postoperative topical use may appear,86 because high doses of MMC suppress cellular RNA replication and protein synthesis.93 As MMC is applied in the stromal bed, it seems that it might penetrate into the anterior chamber, because cytotoxic effects on the ciliary body epithelium have been reported.11,161 There is controversy in the scientific literature, but MMC does not seem to cause any alteration in the ciliary body or intraocular pressure (IOP) after PRK.161 Kymionis et al.161 investigated the effects of MMC after PRK in 40 eyes of 20 rabbits. They applied 0.02% MMC for 2 min in one eye, and balanced salt solution (BSS) for 2 min in the contralateral eye. After 3 months, they did not found differences in the morphology of the ciliary body, and tonometric measurements remained stable (P=.075).
The endothelium is the inner layer of the cornea. Endothelial cells have a hexagonal or polygonal shape,48 and they are homogeneously distributed, without signs of polymegatism and pleomorphism in normal conditions. Endothelial cells are not able to regenerate,40,157 and a reduction in the number of cells is seen with age. After PRK, endothelial structure, shape and density remain unaltered.81,85,168 Table 4 shows the variation of endothelial cells in the different studies published in the scientific literature. Polymegatism or pleomorphism, if present, may be secondary to still unknown corneal metabolism.169 There is also controversy about the toxic effect of MMC in the overall morphology of the endothelium.8,11,86,153 Morales et al.158 proved that intraoperative 0.02% MMC during 30 s after PRK induced corneal endothelial cell loss at 1 month and 3 months (P=.0006, P=.002; respectively). Diakonis et al.11 applied Mitomycin C (MMC) for 15 s and the density of endothelial cells was not affected. Zare et al.170 obtained similar results when 0.02% MMC was applied for 45 s. Subsequently, Shojaei et al.153 found significant differences of mean endothelial cell densities in the MMC group and in the control group at 6 months after surgery (2878.79±283.04 cells/mm2 versus 2826.19±286.25 cells/mm2, P=.25). Undoubtedly, after the application of MMC the DNA of endothelial cells gets damaged.171 It remains to be determined the long-term effects of such event. According to Wilson,71 long-term studies (more than 10 years) are necessary to determine the adverse effects of MMC.
Table 4.
Variation of Endothelial cell density After Surgery, Mean±SD or Range (cell/mm2).
| Study | Technique | Preoperatively | 1 month | 3 months | 6 months | 12 months |
|---|---|---|---|---|---|---|
| Shojaei et al. (2013)153 | PRK with MMC | 2879±298 | 2849±296 | 2878±283 | ||
| PRK with BSS | 2819±303 | 2825±283 | 2826±286 | |||
| Einollahi et al. (2011)63 | PRK with MD | 3102±281 (2498–3823) |
2996±259 | 2795±764 | ||
| PRK with AAD | 3125±299 (2610–4276) |
3011±240 | 2946±240 | |||
| Amoozadeh et al. (2009)40 | LASIK | 3022±224 | 3030±186 | |||
| PRK | 2983±293 | 3025±404 | ||||
| Wallau and Campos (2008)159 | LASIK | 2709±242 | 2667±277 | |||
| PRK with MMCb | 2709±246 | 2686±253 | ||||
| Diakonis et al. (2007)11 | PRK+MMCe | 2757±117 | 2736±144 | 2729±131 | 2716±136 | 2721±113 |
| Epi-LASIK | 2769±158 | 2727±179 | 2741±177 | 2758±176 | 2760±102 | |
| Nassaralla et al. (2007)28 | PRK with MMCa,c | 2150±180 (1800–2650) |
2100±205 (1680–2540) |
2200±210 (1680–2500) |
||
| Morales et al. (2006)158 | PRK+MMCd | 2835±395 | 2416±291 | 2357±404 | ||
| PRK+BSS | 2779±492 | 2711±555 | 2746±526 |
PRK, photorefractive keratectomy; MMC, mitomycin C; BSS, balanced saline solution; MD, mechanical debridement; AAD, alcohol-assisted debridement; LASIK, laser in situ keratomileusis; Epi-LASIK, epipolis laser in situ keratomileusis.
After radial keratotomy.
0.002%, 1 min
0.02%, 2 min.
0.02%, 30 s.
0.02%, 15 s.
New generation quinolones, instead of preventing corneal haze, are used as prophylactic antibiotics to avoid corneal infections after refractive surgery.172 They also enhance the rate of corneal recovery. Fourth generation fluoroquinolones like gatifloxacin (Zymar, Allergan, Irvine, California) and moxifloxacin (Vigamox, Alcon Laboratories, Fort Worth, Texas) have been demonstrated to mediate faster corneal healing,172 without evident differences between both of them in terms of visual outcomes.118
Bandage Contact Lenses
After PRK, the corneal surface needs between 2 and 4 days to regenerate,2 and the vision may fluctuate for several weeks to months. If epithelial regeneration delays, the subepithelial haze increases; for this reason, an appropriate corneal reepithelization is crucial.8,105 Reepithelization is the first step during corneal regeneration after PRK.51 If the reepithelialization is facilitated with the appropriate contact lenses, visual acuity improves.30,173 Although therapeutic contact lenses have been used for more than 40 years, PRK has increased their popularity.23,30 One of the major disadvantages of PRK is the pain and discomfort during 1–3 days after intervention.7,15,174 To ease off the postoperative pain and discomfort, and to promote epithelial healing, bandage contact lenses are fitted for 3–5 days after surgery.12,23,30,31 Other techniques and medications has been proposed in order to reduce corneal pain like occlusive pressure patching, but the bandage contact lenses are still the gold standard.173 Bandage contact lenses are used to protect the epithelium from the eyelid, to reduce the haze formation,31,173 to enhance epithelial healing, to control the sensation of pain, and to prevent epithelial erosions.12,23,30,128 Faster reepithelialization produces a reduction of discomfort, facilitates visual recovery, and restores the corneal barrier to prevent infections.12
Because of the prolonged use of therapeutic contact lenses, and to assure the proper corneal metabolism, a high oxygen permeability (Dk/t) contact lens are used.23,30,31,173 Silicone hydrogel contact lenses have a Dk/t coefficient 5–10-fold greater than conventional hydrogel lenses.12 For this reason, silicone hydrogel bandage contact lenses are widely fitted,7,12,30,31 and are the ones approved by the FDA for prolonged use after PRK. Currently, a variety of contact lenses are used as therapeutic soft contact lenses after PRK like Lotrafilcon A (Focus Night & Day, Ciba Vision), Lotrafilcon B (O 2 Optix, Ciba Vision), Senofilcon A (Acuvue Oasys, Vistakon Inc.), Balafilcon A, Omafilcon A (Proclear, Cooper Vision) and Senofilcon A.12,23,30,31 Lotrafilcon B is approved by FDA for 6 days of continuous wear and Senofilcon A for 1 week of continuous wear, while Lotrafilcon A is approved for 30 days of continuous wear and therapeutic use.12,23 The therapeutic efficacy of the Lotrafilcon A after PRK has been intensively studied,12,23,30,31 and reduction of discomfort and faster corneal reepithelialization in 48 h have been described.12,23 Edwards et al.31 proved that Lotrafilcon A showed better best spectacle-correction visual acuity (BSCVA) than Omafilcon A, without statistically significant differences in contrast sensitivity or uncorrected visual acuity (UVA). Omafilcon A reduced the BSCVA in 40.4% of patients at 1 month, whereas Lotrafilcon A reduced the BSCVA in 18.6% of the patients (P=.002). The corneal pain was greater with Omafilcon A than with Lotrafilcon A at 1 day (P=.000) and 4 days postoperatively (P=.027).31 In contrast, an increase in corneal infiltrates with Lotrafilcon A was observed compared to Omafilcon A, and there was not a statistically significant difference in reepithelialization.31 The authors suggested that corneal infiltrates might be a consequence of Lotraficon A's rigidity due to its reduced water content (24%) versus 59% of Omafilcon A.31 Subsequently, Razmjoo et al.30 in a comparative study, found that the 58.3% of the eyes with Senofilcon A and 41.7% of the eyes with Lotrafilcon A completed the reepithelialization at day 5 (P>.05). Although there were not statistically significant differences in the rate of corneal reepithelialization between both contact lenses (P>.05), and the postoperative pain and discomfort index was significantly lower in Senofilcon A group (P<.05).30 They also compared the visual acuity between Senofilcon A and Lotrafilcon A after PRK, and proved that in both groups the UCVA was worse at 3 days than at day 1. However, the UCVA improved at day 5, with 97.7% reaching UCVA of 20/40. A feasible explanation is that, at day 3, the epithelial healing process is located in the center of the cornea.30 As only 44 patients were included, in future studies a larger size sample would be recommendable.
Bandage contact lenses also minimize corneal haze. Edwards et al.31 showed a minimum tendency to a high level of corneal haze with Omafilcon A compared with Lotrafilcon A (P=.0064). However, all efforts are made to minimize the corneal haze intraoperatively, using cold balanced saline (BSS) and MMC. Application of BSS in the stromal body reduces the corneal pain and corneal haze;128 yet, the application of mitomycin-C (MMC) is more widely used.
Although bandage contact lenses have various advantages, the presence of silicone may produce irritation, increased protein and lipid deposits, and reduced wettability because of its hydrophibicity.31 A plasma treatment is given to enhance the hydrophilicity of Lotrafilcon A surface, but this technique is not completely effective.31 Bacterial keratitis and subepithelial infiltrates have been described with bandage contact lenses after PRK.17 The risk of infectious keratitis of soft contact lenses fitted for approximately 3 days is low, and antibiotics are prescribed to further minimize the risk.175
Corticosteroids and Non-steroidal Anti-inflammatory Agents (NSAIDs) Therapy
It is necessary to distinguish between corneal haze that appears in the first weeks or months after PRK and pathological corneal haze that appears as a result of myofibroblasts.71 If the corneal haze persists over time, it may cause a corneal opacity and the thickening of the tissue that would result in a regression of the refractive error, decreased visual acuity and irregular astigmatism.19,34,67,71 Clinically significant corneal haze occurs in 0.5%–5% of the cases.109 Corneal haze that most commonly occurs after PRK is not clinically significant, and is not attributed to myofibroblasts.71,121 According to Wilson,71 in human corneas that develop late corneal haze after PRK, the resolution of the opacity is slow, and the restauration of the refractive correction is produced between 1 and 3 years postoperatively. It has been postulated that the extinction of corneal haze can be influenced by the disappearance of myofibroblasts, reabsortion of abnormal extracellular matrix (ECM) and restoration of normal corneal structure.71
After surgery, a variety of drugs are prescribed to avoid corneal haze, for instance, corticosteroids – antiinflammatories to avoid the pain and inflammation-, plasmin inhibitors, growth factors or antimetabolites.13,176 Topical therapy after PRK prevents complications like keratitis, infections or corneal haze.177 The most common treatment after PRK to avoid the corneal inflammation is the application of corticosteroids.109,158 Corticosteroids are not recommended for long periods because of their side effects, like intraocular pressure (IOP) rise and the risk of cataracts.109,178,179 Javadi et al.180 reported a rise in the IOP using 0.1% betamethasone at 2 weeks post-PRK in a minority of patients. Furthermore, corticosteroids delay epithelial healing.179 When corneal haze appears 2–3 months after PRK, the clinical observations confirm that haze is “corticosteroid-responsive” in 10%–15% of patients.71 Researchers disagree about the benefit of corticosteroids to reduce the corneal haze after PRK.2,177 According to Wilson,71 the topical administration of 1% prednisolone acetate (Pred Forte) quickly removes the corneal opacity and produces a change in refractive error. In the remaining 85% or 90% of cases, the corticosteroids do not exert any change.71 Corticosteroids could be replaced by non-steroidal anti-inflammatory agents (NSAIDs), tranilast, cysteine or antioxidants like Vitamine E.109,177 NSAIDs are effective in reducing corneal pain, postoperative photophobia and inflammation.7,128 The inflammatory response is mediated by prostaglandins synthesized from arachidonic acid by cyclooxygenase 1 (COX-1) or cyclooxygenase 2 (COX-2).128 The antiinflammatory and analgesic properties of the nonsteroidal anti-inflammatory drugs (NSAIDs) are achieved by the inhibition of COXs activity.7,128
The use of certain steroidal and non-steroidal anti-inflammatory drugs (NSAID) delay reepithelialization and increase the risk of haze formation,7 although the results are still contradictory. Vetrugno et al.177 proved that 0.1% fluorometholone acetate administered in the first day after PRK reduced corneal haze and myopic regression, particularly in high myopic patients. NSAIDs like diclofenac and ketorolac have shown reduction in the pain sensation,7,128 but also a significant delay in corneal reepithelialization after PRK.181 Nepafenac (Nevanac; Alcon Laboratories Inc., Ft Worth, Tex) is a new topical NSAID with greater corneal permeability that has been approved for the treatment of inflammation after surgery.7,181 Jalali et al.13 found that 0.1% Nepafenac did not increase haze formation, neither hamper corneal epithelial healing, but they did not found statistically significant differences in corneal reepithelization between nepafenac and non-nepafenac groups (P=.61). Caldwell et al.,181 in a randomized double-masked study, demonstrated that 0.1% nepafenac was safe for corneal reepithelialization, and reduced the postoperative pain at day 1 (0.76 versus 1.68) and day 2 (1.26 versus 2.23) compared with the placebo group (P<.0005). Other NSAIDs for corneal pain reduction are also available, like Bromfenac, Flurbiprofen sodium and Indomethacin.7
Despite the presence of complications is low, non-steroidal anti-inflammatory drugs (NSAIDs) may produce conjunctival hyperemia, transient burning, stinging, superficial punctate keratitis, epithelial defects, subepithelial infiltrates, corneal melting and perforation.7,17 However, Caldwell et al.,181 in a randomized double-masked study, proved that 0.1% nepafenac did not had adverse effects. Postoperative oral analgesics, like NSAIDs, are able to produce gastrointestinal, cardiovascular, respiratory and central nervous system complications.17,181 Another treatment that is widespread for the inhibition of inflammation and for treatment of dry eye is the Cyclosporine A, with doses from 0.05% to 2.00%.109 Nien et al.109 used Cyclosporine A 0.05% and prednisolone acetate 0.01% to compare the effect in corneal haze prevention in rabbit corneas following PRK. They concluded that Cyclosporine A did not have any effect, whereas prednisolone acetate was effective in reducing short-term corneal haze, but did not prevent corneal fibrosis.109
Alternative Therapies for Corneal Haze Prevention
The use of drugs does not completely suppress corneal haze formation after PRK. Research has focused on new therapies that could prevent corneal haze, like genetic evaluation of type IV collages synthesis.33 Lumican and keratocan genes have also been evaluated for management of subepithelial persistent corneal haze after PRK, but without a consistent finding.182 It has been postulated that vitamin E, probulcol or heparin may inhibit collagen type IV synthesis, but they have not been approved for topic use because of their adverse effects.33 Vitamin E and amino acids play an important role in corneal reepithelialization and in the prevention of corneal haze and keratocyte apoptosis, especially in high myopia.9,77 A preliminary clinical trial concluded that oral supplementation with vitamin A and vitamin E accelerated the reepithelization, and reduced corneal haze formation,183 but it seems that the topical administration of vitamin A alone do not have any effect.184 Alternative treatments to MMC that prevent corneal haze formation, but produce less damage to keratocyte are bevacizumab and rapamycin.185 Subconjunctival injection of PRM-151 could presumably prevent corneal haze, as it inhibits the pro-fibrotic myofibroblast differentation.186 Trichostatin A, similarly, prevents myofibroblast formation by inhibiting TGF-beta1.187 As cytokines and growth factors control the synthesis of collagen type IV, they might be also useful treatments for corneal haze prevention.33 PRK increases the release of leukocytes, TGF-β1, TNF-α and PDGF-BB in human tears during the first days of wound healing.14,50 TGF is a cytokine released by the lacrimal gland, corneal epithelium and conjunctival cells.179 Three forms of TGF-β exist (TGF-β1, TGF-β2 and TGF-β3) and each one is involved in the wound healing process in a different way. TGF-β1 is increased in early epithelial healing, and exerts an influence in the subepithelial fibrosis formation and activation of keratocytes after PRK.73 Bühren et al.179 proved that the application of anti-TGF-β in felines reduced the differentiation in vitro of keratocytes into myofibroblast, and corneal haze diminished. They suggested that this reduction in differentiation improved optical quality. The combination of the nerve growth factor (NGF) and decosahexanoic acid stimulated the regeneration of basal epithelial cells in rabbits after PRK,22 which is imperative for a proper wound healing. Medduri et al.35 studied the effect of basic fibroblast growth factor (b-FGF) in circumstances of delayed healing after PRK.9 50 patients were enrolled in b-FGF eye drop treatment group and 50 patients in saline drops (placebo) group. They observed greater corneal epithelial healing in the b-FGF group than in the placebo group at 4 days (98% versus 72%, respectively) and 5 days after surgery (100% versus 92%, respectively).35
Artificial tears are the most widely used solution for corneal lubrication. However, they do not have biological components that promote corneal regeneration. In fact, they contain stabilizers, preservatives, or other additives that may induce toxic or allergic reactions.51 Blood derivates as plasma rich in growth factors are an alternative to artificial tears, and have not possibility of rejection.51 Anitua et al.51 proved that plasma rich in growth factors obtained from patient's blood enhanced corneal healing, and reduced the formation of corneal haze. The difference between the plasma rich in growth factors group (PRGF-Endoret treatment) and control group was negligible at day 3. They attributed it to the increase of proliferative cells (Ki-67 þ) in the control group. They suggested that the increase in proliferative cells could be associated with epithelial hyperplasia observed at day 3 and 7 after PRK in control group.51 They also found that the epithelium of the PRGF-Endoret group was formed by 5–6 layers.51
Corneal Nerve Regeneration and Neuropathic Corneal Pain Management
The regeneration of the corneal nerves after PRK is associated with the improvement of cellular integrity.22 To date, few therapeutic treatments have been developed for nerve regeneration. Javaloy et al.188 investigated the benefits of topical platelet-rich plasma, but subbasal nerve density did not improve after 3 months of treatment compared to controls (P=.66). Studies in animal models have demonstrated more encouraging results. Esquenazi et al.22 studied the outcomes of the combination of nerve growth factor (NGF) and docosahexaenoic acid (DHA) in rabbits in promoting corneal nerve regeneration. They observed that this combination increased corneal nerve regeneration, as well as epithelial proliferation and decreased rose bengal staining compared to the application of NGF or DHA alone. Cortina et al.189 showed similar results with pigment epithelial-derived factor (PEDF) plus docosahexaenoic acid (DHA). Moreover, this combination proved to enhance corneal sensitivity. Recent evidences suggest that peripheral nervous system regeneration and inflammatory processes share common pathways, and some degree of inflammation is required for neuroregeneration.190 Therefore, cyclosporine A and corticosteroid treatments could interfere in a proper nervous recovery.
When corneal nerve regeneration process fails, corneal neuropathic pain might take place. The up- and down-regulation of ion channels in axotomized nerves can change the excitability of fibers,191 and produce spontaneous discharges and altered sensibility to exogenous stimuli.192 This would result in corneal pain non-treatable with aforementioned drugs. Anticonvulsants, opiates and topical local anesthetics can manage corneal neuropathic pain. The anticonvulsant Gabapentin (Neurontin) is an analog of gamma-aminobutyric acid (GABA),193 and its reliability in treating corneal pain is conflicting, mainly because of a lack of studies.7 Lichtinger et al.193 compared in a prospective randomized, double-blind, placebo-controlled study the efficacy of Gabapentin in the reduction of the corneal pain. They administrated gabapentin capsules (300 mg) in 20 patients and additional 20 patients received identical placebo capsules. They demonstrated that gabapentin reduced corneal pain during the first 24 h (P=.003), at postoperative day 1 (P=.002), between 24 and 48 h (P=.024), at postoperative day 2 (P=.018) and between 48 and 72 h (P=.001). Faktorovich et al.17 trying to prove the efficacy of topical opioid in the treatment of pain, concluded in a double-blind randomized prospective study that the administration of 0.5% morphine drops was an effective and safe method to control of post-PRK pain, and did not hamper epithelial healing or refractive outcomes. Topical local anesthetics include tetracaine, proparacaine, lidocaine and bupivacaine can be also used. Topical tetracaine has been documented to be successful in pain control management and does not produce delayed corneal healing times.7,39 However, it produces keratocyte toxicity and keratitis.7,17,39 Topical anesthetics should be used cautiously and for short-term treatments. Antidepressants are prescribed for neuropathic pain management elsewhere in the body. To date, no study has been published evaluating the effect of antidepressants for treating post-PRK corneal pain.
Conclusions
Photorefractive keratectomy disrupts corneal structure affecting epithelium, Bowman's membrane, and anterior stroma. Corneal nerves are severed, which alters corneal integrity and function temporarily. The subsequent corneal wound healing is a complex process that is regulated by a variety of factors. A balance of peptides will determine the final outcome, and the presence of postoperative complications. Corneal wound healing process can be managed with several drugs to enhance regeneration and prevent corneal haze and pain after PRK. Further research in this field is required to completely understand post-PRK corneal regeneration in order to prevent complications, and provide outstanding visual outcomes.
Directions for Future Research
This review clearly states that corneal regeneration after PRK is not completely understood. The ongoing research in new drugs development, more efficient surgical techniques, and new imaging technologies are trying to answer some of the unresolved questions. Still, future research should be oriented to elucidate the following aspects:
-
•
The long-term effects of keratocyte death and MMC application.
-
•
Although corneal haze has been correlated to several factors, its origin is still unknown.
-
•
The beneficial role of corticosteroid administration in corneal haze prevention.
-
•
The causal factors of myopic regression.
-
•
More studies using non-contact gas esthesiometer will help to better assess the time course of corneal sensitivity recovery.
Acknowledgements
Authors would like to thank Manuel Alejandro Amaya Alcaraz for the collaboration given in the revision of this article.
Footnotes
The authors have not proprietary or commercial interest in the medical devices that are involved in this manuscript.
References
- 1.L’Esperance F.A., Jr., Taylor D.M., Del Pero R.A., Roberts A., Gigstad J., Stokes M.T. Human excimer laser corneal surgery: preliminary report. Trans Am Ophthalmol Soc. 1988;86:208–275. [PMC free article] [PubMed] [Google Scholar]
- 2.Erie J.C. Corneal wound healing after photorefractive keratectomy: a 3-year confocal microscopy study. Trans Am Ophthalmol Soc. 2003;101:293–333. [PMC free article] [PubMed] [Google Scholar]
- 3.Ghirlando A., Gambato C., Midena E. LASEK and photorefractive keratectomy for myopia: clinical and confocal microscopy comparison. J Refract Surg. 2007;23:694–702. doi: 10.3928/1081-597X-20070901-08. [DOI] [PubMed] [Google Scholar]
- 4.Ginis H., Pentari I., de Brouwere D., Bouzoukis D., Naoumidi I., Pallikaris I. Narrow angle light scatter in rabbit corneas after excimer laser surface ablation. Ophthalmic Physiol Opt. 2009;29:357–362. doi: 10.1111/j.1475-1313.2009.00649.x. [DOI] [PubMed] [Google Scholar]
- 5.Erie J.C., Patel S.V., McLaren J.W., Hodge D.O., Bourne W.M. Corneal keratocyte deficits after photorefractive keratectomy and laser in situ keratomileusis. Am J Ophthalmol. 2006;141:799–809. doi: 10.1016/j.ajo.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Trokel S.L., Srinivasan R., Braren B. Excimer laser surgery of the cornea. Am J Ophthalmol. 1983;96:710–715. doi: 10.1016/s0002-9394(14)71911-7. [DOI] [PubMed] [Google Scholar]
- 7.Woreta F.A., Gupta A., Hochstetler B., Bower K.S. Management of post-photorefractive keratectomy pain. Surv Ophthalmol. 2013;58:529–535. doi: 10.1016/j.survophthal.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Alio J.L., Javaloy J. Corneal inflammation following corneal photoablative refractive surgery with excimer laser. Surv Ophthalmol. 2013;58:11–25. doi: 10.1016/j.survophthal.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Meduri A., Scorolli L., Scalinci S.Z., Grenga P.L., Lupo S., Rechichi M. Effect of the combination of basic fibroblast growth factor and cysteine on corneal epithelial healing after photorefractive keratectomy in patients affected by myopia. Indian J Ophthalmol. 2014;62:424–428. doi: 10.4103/0301-4738.119420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erie J.C., Patel S.V., McLaren J.W., Hodge D.O., Bourne W.M. Keratocyte density in the human cornea after photorefractive keratectomy. Arch Ophthalmol. 2003;121:770–776. doi: 10.1001/archopht.121.6.770. [DOI] [PubMed] [Google Scholar]
- 11.Diakonis V.F., Pallikaris A., Kymionis G.D., Markomanolakis M.M. Alterations in endothelial cell density after photorefractive keratectomy with adjuvant mitomycin. Am J Ophthalmol. 2007;144:99–103. doi: 10.1016/j.ajo.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 12.Engle A.T., Laurent J.M., Schallhorn S.C., Toman S.D., Newacheck J.S., Tanzer D.J. Masked comparison of silicone hydrogel lotrafilcon A and etafilcon A extended-wear bandage contact lenses after photorefractive keratectomy. J Cataract Refract Surg. 2005;31:681–686. doi: 10.1016/j.jcrs.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Jalali S., Yuen L.H., Boxer Wachler B.S. Effect of nepafenac sodium 0.1% on delayed corneal epithelial healing and haze after photorefractive keratectomy retrospective comparative study. J Cataract Refract Surg. 2008;34:1542–1545. doi: 10.1016/j.jcrs.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 14.Tuominen I.S., Tervo T.M., Teppo A.M., Valle T.U., Grönhagen-Riska C., Vesaluoma M.H. Human tear fluid PDGF-BB, TNF-alpha and TGF-beta1 vs corneal haze and regeneration of corneal epithelium and subbasal nerve plexus after PRK. Exp Eye Res. 2001;72:631–641. doi: 10.1006/exer.2001.0999. [DOI] [PubMed] [Google Scholar]
- 15.Kanitkar K.D., Camp J., Humble H., Shen D.J., Wang M.X. Pain after removal by ethanol-assisted mechanical versus transepithelial excimer laser debridement. J Refract Surg. 2000;16:519–522. doi: 10.3928/1081-597X-20000901-06. [DOI] [PubMed] [Google Scholar]
- 16.Gómez S., Herreras J.M., Merayo J., García M., Argüeso P., Cuevas J. Effect of hyaluronic acid on corneal haze in a photorefractive keratectomy experimental model. J Refract Surg. 2001;17:549–554. doi: 10.3928/1081-597X-20010901-08. [DOI] [PubMed] [Google Scholar]
- 17.Faktorovich E.G., Basbaum A.I. Effect of topical 0.5% morphine on postoperative pain after photorefractive keratectomy. J Refract Surg. 2010;26:934–941. doi: 10.3928/1081597X-20100212-06. [DOI] [PubMed] [Google Scholar]
- 18.Lee H.K., Lee K.S., Kim J.K., Kim H.C., Seo K.R., Kim E.K. Epithelial healing and clinical outcomes in excimer laser photorefractive surgery following three epithelial removal techniques: mechanical, alcohol, and excimer laser. Am J Ophthalmol. 2005;139:56–63. doi: 10.1016/j.ajo.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 19.Gamaly T.O., El Danasoury A., El Maghraby A. A prospective, randomized, contralateral eye comparison of epithelial laser in situ keratomileusis and photorefractive keratectomy in eyes prone to haze. J Refract Surg. 2007;23(9 Suppl.):S1015–S1020. doi: 10.3928/1081-597X-20071102-07. [DOI] [PubMed] [Google Scholar]
- 20.Netto M.V., Barreto J., Jr., Santo R., Bechara S., Kara-Jose N., Wilson S.E. Synergistic effect of ethanol and mitomycin C on corneal stroma. J Refract Surg. 2008;24:626–632. doi: 10.3928/1081597X-20080601-13. [DOI] [PubMed] [Google Scholar]
- 21.Aslanides I.M., Padroni S., Arba Mosquera S., Ioannides A., Mukherjee A. Comparison of single-step reverse transepithelial all-surface laser ablation (ASLA) to alcohol-assisted photorefractive keratectomy. Clin Ophthalmol. 2012;6:973–980. doi: 10.2147/OPTH.S32374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esquenazi S., Bazan H.E., Bui V., He J., Kim D.B., Bazan N.G. Topical combination of NGF and DHA increases rabbit cornea nerve regeneration after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2005;46:3121–3127. doi: 10.1167/iovs.05-0241. [DOI] [PubMed] [Google Scholar]
- 23.Grentzelos M.A., Plainis S., Astyrakakis N.I., Diakonis V.F., Kymionis G.D., Kallinikos P. Efficacy of 2 types of silicone hydrogel bandage contact lenses after photorefractive keratectomy. J Cataract Refract Surg. 2009;35:2103–2108. doi: 10.1016/j.jcrs.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Mohan R.R., Hutcheon A.E., Choi R., Hong J., Lee J., Mohan R.R. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- 25.Patel S.V., Erie J.C., McLaren J.W., Bourne W.M. Confocal microscopy changes in epithelial and stromal thickness up to 7 years after LASIK and photorefractive keratectomy for myopia. J Refract Surg. 2007;23:385–392. doi: 10.3928/1081-597X-20070401-11. [DOI] [PubMed] [Google Scholar]
- 26.Ghanem R.C., Ghanem V.C., de Souza D.C., Kara-José N., Ghanem E.A. Customized topography-guided photorefractive keratectomy with the MEL-70 platform and mitomycin C to correct hyperopia after radial keratotomy. J Refract Surg. 2008;24:911–922. doi: 10.3928/1081597X-20081101-10. [DOI] [PubMed] [Google Scholar]
- 27.Virasch V.V., Majmudar P.A., Epstein R.J., Vaidya N.S., Dennis R.F. Reduced application time for prophylactic mitomycin C in photorefractive keratectomy. Ophthalmology. 2010;117:885–889. doi: 10.1016/j.ophtha.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 28.Nassaralla B.A., McLeod S.D., Nassaralla J.J., Jr. Prophylactic mitomycin C to inhibit corneal haze after photorefractive keratectomy for residual myopia following radial keratotomy. J Refract Surg. 2007;23:226–232. doi: 10.3928/1081-597X-20070301-04. [DOI] [PubMed] [Google Scholar]
- 29.Barreto J., Jr., Netto M.V., Reis A., Nakano M., Alves M.R., Bechara S.J. Topography-guided (NIDEK customized aspheric treatment zone) photorefractive keratectomy with mitomycin C after penetrating keratoplasty for keratoconus: case report. J Refract Surg. 2009;25(1 Suppl.):S131–S135. doi: 10.3928/1081597X-20090115-10. [DOI] [PubMed] [Google Scholar]
- 30.Razmjoo H., Abdi E., Atashkadi S., Reza A.M., Reza P.A., Akbari M. Comparative study of two silicone hydrogel contact lenses used as bandage contact lenses after photorefractive keratectomy. Int J Prev Med. 2012;3:718–722. [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards J.D., Bower K.S., Sediq D.A., Burka J.M., Stutzman R.D., Vanroekel C.R. Effects of lotrafilcon A and omafilcon A bandage contact lenses on visual outcomes after photorefractive keratectomy. J Cataract Refract Surg. 2008;34:1288–1294. doi: 10.1016/j.jcrs.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 32.Srinivasan S., Drake A., Herzig S. Photorefractive keratectomy with 0.02% mitomycin C for treatment of residual refractive errors after LASIK. J Refract Surg. 2008;24:S64–S67. doi: 10.3928/1081597X-20080101-12. [DOI] [PubMed] [Google Scholar]
- 33.Winkler von Mohrenfels C., Reischl U., Lohmann C.P. Corneal haze after photorefractive keratectomy for myopia: role of collagen IV mRNA typing as a predictor of haze. J Cataract Refract Surg. 2002;28:1446–1451. doi: 10.1016/s0886-3350(02)01273-7. [DOI] [PubMed] [Google Scholar]
- 34.Rajan M.S., O’Brart D.P., Patmore A., Marshall J. Cellular effects of mitomycin-C on human corneas after photorefractive keratectomy. J Cataract Refract Surg. 2006;32:1741–1747. doi: 10.1016/j.jcrs.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Meduri A., Aragona P., Grenga P.L., Roszkowska A.M. Effect of basic fibroblast growth factor on corneal epithelial healing after photorefractive keratectomy. J Refract Surg. 2012;28:220–223. doi: 10.3928/1081597X-20120103-02. [DOI] [PubMed] [Google Scholar]
- 36.Buzzonetti L., Petrocelli G., Laborante A., Mazzilli E., Gaspari M., Valente P. A new transepithelial phototherapeutic keratectomy mode using the NIDEK CXIII excimer laser. J Refract Surg. 2009;25(1 Suppl.):S122–S124. doi: 10.3928/1081597X-20090115-08. [DOI] [PubMed] [Google Scholar]
- 37.Kim T.H., Lee D.Y., Rho J.H., Rho S.H., Yoo K.W., Ahn H.B. Application of newly developed amniotic membrane ointment for photorefractive keratectomy in rabbits. Ophthalmic Res. 2006;38:58–61. doi: 10.1159/000090328. [DOI] [PubMed] [Google Scholar]
- 38.Chung S.A., Kim E.K., Ryu I.H., Kim J.K., Lee H.K. Effectiveness of cultured human keratinocyte onlays on epithelial healing and clinical outcome after photorefractive keratectomy. J Refract Surg. 2008;24:826–832. doi: 10.3928/1081597X-20081001-10. [DOI] [PubMed] [Google Scholar]
- 39.Magone M.T., Engle A.T., Easter T.H., Stanley P.F., Howells J., Pasternak J.F. Flap-off epi-LASIK versus automated epithelial brush in PRK: a prospective comparison study of pain and reepitheliazation times. J Refract Surg. 2012;28:682–689. doi: 10.3928/1081597X-20120921-02. [DOI] [PubMed] [Google Scholar]
- 40.Amoozadeh J., Aliakbari S., Behesht-Nejad A.H., Seyedian M.A., Rezvan B., Hashemi H. Confocal microscopy of corneal stroma and endothelium after LASIK and PRK. J Refract Surg. 2009;25(10 Suppl.):S963–S967. doi: 10.3928/1081597X-20090915-12. [DOI] [PubMed] [Google Scholar]
- 41.Dua H.S., Gomes J.A., Singh A. Corneal epithelial wound healing. Br J Ophthalmol. 1994;78:401–408. doi: 10.1136/bjo.78.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujikawa L.S., Foster C.S., Harrist T.J., Lanigan J.M., Colvin R.B. Fibronectin in healing rabbit corneal wounds. Lab Invest. 1981;45:120–129. [PubMed] [Google Scholar]
- 43.Netto M.V., Mohan R.R., Sinha S., Sharma A., Dupps W., Wilson S.E. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. 2006;82:788–797. doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martínez-García M.C., Merayo-Llovés J., Blanco-Mezquita T., Mar-Sardaña S. Wound healing following refractive surgery in hens. Exp Eye Res. 2006;83:728–735. doi: 10.1016/j.exer.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 45.Chaurasia S.S., Kaur H., de Medeiros F.W., Smith S.D., Wilson S.E. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp Eye Res. 2009;89:133–139. doi: 10.1016/j.exer.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson S.E., Chaurasia S.S., Madeiros F.W. Apoptosis in the initiation. Modulation and termination of the corneal wound healing response. Exp Eye Res. 2007;85:305–311. doi: 10.1016/j.exer.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barbosa F.L., Chaurasia S.S., Kaur H., de Medeiros F.W., Agrawal V., Wilson S.E. Stromal interleukin-1 expression in the cornea after haze-associated injury. Exp Eye Res. 2010;91:456–461. doi: 10.1016/j.exer.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tavakoli M., Hossain P., Malik R.A. Clinical applications of corneal confocal microscopy. Clin Ophthalmol. 2008;2:435–445. doi: 10.2147/opth.s1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Efron N. Contact lens-induced changes in the anterior eye as observed in vivo with the confocal microscope. Prog Retin Eye Res. 2007;26:398–436. doi: 10.1016/j.preteyeres.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Gan L., Hamberg-Nyström H., Fagerholm P., Van Setten G. Cellular proliferation and leukocyte infiltration in the rabbit cornea after photorefractive keratectomy. Acta Ophthalmol Scand. 2001;79:488–492. doi: 10.1034/j.1600-0420.2001.790512.x. [DOI] [PubMed] [Google Scholar]
- 51.Anitua E., Muruzabal F., Alcalde I., Merayo-Lloves J., Orive G. Plasma rich in growth factors (PRGF-Endoret) stimulates corneal wound healing and reduces haze formation after PRK surgery. Exp Eye Res. 2013;115:153–161. doi: 10.1016/j.exer.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Ivarsen A., Fledelius W., Hjortdal J.Ø. Three-year changes in epithelial and stromal thickness after PRK or LASIK for high myopia. Invest Ophthalmol Vis Sci. 2009;50:2061–2066. doi: 10.1167/iovs.08-2853. [DOI] [PubMed] [Google Scholar]
- 53.Gauthier C.A., Epstein D., Holden B.A., Tengroth B., Fagerholm P., Hamberg-Nyström H. Epithelial alterations following photorefractive keratectomy for myopia. J Refract Surg. 1995;11:113–118. doi: 10.3928/1081-597X-19950301-11. [DOI] [PubMed] [Google Scholar]
- 54.Hamberg-Nyström H., Gauthier C.A., Holden B.A., Epstein D., Fagerholm P., Tengroth B. A comparative study of epithelial hypertrophy after PRK: summit versus VISX in the same patient. Acta Ophthalmol Scand. 1996;74:228–231. doi: 10.1111/j.1600-0420.1996.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 55.Moller-Pedersen T., Cavanagh H.D., Petroll W.M., Jester J.V. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: a 1-year confocal microscopic study. Ophthalmology. 2000;107:1235–1245. doi: 10.1016/s0161-6420(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 56.Kozak I., Hornak M., Juhas T., Shah A., Rawlings E.F. Changes in central corneal thickness after laser in situ keratomileusis and photorefractive keratectomy. J Refract Surg. 2003;19:149–153. doi: 10.3928/1081-597X-20030301-10. [DOI] [PubMed] [Google Scholar]
- 57.Erie J.C., Patel S.V., McLaren J.W., Ramirez M., Hodge D.O., Maguire L.J. Effect of myopic laser in situ keratomileusis on epithelial and stromal thickness: a confocal microscopy study. Ophthalmology. 2002;109:1447–1452. doi: 10.1016/s0161-6420(02)01106-5. [DOI] [PubMed] [Google Scholar]
- 58.Guerriero E., Chen J., Sado Y., Mohan R.R., Wilson S.E., Funderburgh J.L. Loss of alpha3 (IV) collagen expression associated with corneal keratocyte activation. Invest Ophthalmol Vis Sci. 2007;48:627–635. doi: 10.1167/iovs.06-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee R.E., Davison P.F. The collagens of the developing bovine cornea. Exp Eye Res. 1984;39:639–652. doi: 10.1016/0014-4835(84)90063-0. [DOI] [PubMed] [Google Scholar]
- 60.Zimmermann D.R., Trueb B., Winterhalter K.H., Witmer R., Fischer R.W. Type VI collagen is a major component of the human cornea. FEBS Lett. 1986;197:55–58. doi: 10.1016/0014-5793(86)80297-6. [DOI] [PubMed] [Google Scholar]
- 61.Michelacci Y.M. Collagens and proteoglycans of the corneal extracellular matrix. Braz J Med Biol Res. 2003;36:1037–1046. doi: 10.1590/s0100-879x2003000800009. [DOI] [PubMed] [Google Scholar]
- 62.Mutoh T., Nishio M., Matsumoto Y., Arai K., Chikuda M. Photorefractive keratectomy: measuring the matrix metalloproteinase activity and chondroitin sulfate concentration in tear fluid. Clin Ophthalmol. 2010;4:1015–1018. doi: 10.2147/opth.s12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Einollahi B., Baradaran-Rafii A., Rezaei-Kanavi M., Eslani M., Parchegani M.R., Zare M. Mechanical versus alcohol-assisted epithelial debridement during photorefractive keratectomy: a confocal microscopic clinical trial. J Refract Surg. 2011;27:887–893. doi: 10.3928/1081597X-20110823-02. [DOI] [PubMed] [Google Scholar]
- 64.Dupps W.J., Jr., Wilson S.E. Biomechanics and wound healing in the cornea. Exp Eye Res. 2006;83:709–720. doi: 10.1016/j.exer.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berman M., Leary R., Gage J. Evidence for a role of the plasminogen activator–plasmin system in corneal ulceration. Invest Ophthalmol Vis Sci. 1980;19:1204–1221. [PubMed] [Google Scholar]
- 66.Ye H.Q., Maeda M., Yu F.S., Azar D.T. Differential expression of MT1-MMP (MMP-14) and collagenase III (MMP-13) genes in normal and wounded rat corneas. Invest Ophthalmol Vis Sci. 2000;41:2894–2899. [PubMed] [Google Scholar]
- 67.Corbett M.C., O’Brart D.P., Patmore A.L., Marshall J. Effect of collagenase inhibitors on corneal haze after PRK. Exp Eye Res. 2001;72:253–259. doi: 10.1006/exer.2000.0959. [DOI] [PubMed] [Google Scholar]
- 68.Holopainen J.M., Moilanen J.A., Sorsa T., Kivelä-Rajamäki M., Tervahartiala T., Vesaluoma M.H. Activation of matrix metalloproteinase-8 by membrane type 1-MMP and their expression in human tears after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2003;44:2550–2556. doi: 10.1167/iovs.02-1190. [DOI] [PubMed] [Google Scholar]
- 69.Torricelli A.A., Singh V., Agrawal V., Santhiago M.R., Wilson S.E. Transmission electron microscopy analysis of epithelial basement membrane repair in rabbit corneas with haze. Invest Ophthalmol Vis Sci. 2013;54:4026–4033. doi: 10.1167/iovs.13-12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davies B.W., Panday V., Caldwell M., Scribbick F., Reilly C.D. Effect of topical immunomodulatory interleukin 1 receptor antagonist therapy on corneal healing in New Zealand white rabbits (Oryctolagus cunniculus) after photorefractive keratectomy. Arch Ophthalmol. 2011;129:909–913. doi: 10.1001/archophthalmol.2011.168. [DOI] [PubMed] [Google Scholar]
- 71.Wilson S.E. Corneal myofibroblast biology and pathobiology: generation, persistence, and transparency. Exp Eye Res. 2012;99:78–88. doi: 10.1016/j.exer.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bilgihan K., Adiguzel U., Sezer C., Akyol G., Hasanreisoglu B. Effects of topical vitamin E on keratocyte apoptosis after traditional photorefractive keratectomy. Ophthalmologica. 2001;215:192–196. doi: 10.1159/000050857. [DOI] [PubMed] [Google Scholar]
- 73.Lee J.B., Choe C.M., Kim H.S., Seo K.Y., Seong G.J., Kim E.K. Comparison of TGF-beta1 in tears following laser subepithelial keratomileusis and photorefractive keratectomy. J Refract Surg. 2002;18:130–134. doi: 10.3928/1081-597X-20020301-05. [DOI] [PubMed] [Google Scholar]
- 74.Kaur H., Chaurasia S.S., Agrawal V., Wilson S.E. Expression of PDGF receptor-alpha in corneal myofibroblasts in situ. Exp Eye Res. 2009;89:432–434. doi: 10.1016/j.exer.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tchah H., Kim M.J., Kim T.I., Choi H.J., Kim J.Y., Kim M.J. Regulation of 1-cys peroxiredoxin expression in the process of stromal wound healing after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2005;46:2396–2403. doi: 10.1167/iovs.05-0107. [DOI] [PubMed] [Google Scholar]
- 76.Hayashi S., Ishimoto S., Wu G.S., Wee W.R., Rao N.A., McDonnell P.J. Oxygen free radical damage in the cornea after excimer laser therapy. Br J Ophthalmol. 1997;81:141–144. doi: 10.1136/bjo.81.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brancato R., Fiore T., Papucci L., Schiavone N., Formigli L., Orlandini S.Z. Concomitant effect of topical ubiquinone Q10 and vitamin E to prevent keratocyte apoptosis after excimer laser photoablation in rabbits. J Refract Surg. 2002;18:135–139. doi: 10.3928/1081-597X-20020301-06. [DOI] [PubMed] [Google Scholar]
- 78.Bilgihan A., Bilgihan K., Yis O., Sezer C., Akyol G., Hasanreisoglu B. Effects of topical vitamin E on corneal superoxide dismutase, glutathione peroxidase activities and polymorphonuclear leucocyte infiltration after photorefractive keratectomy. Acta Ophthalmol Scand. 2003;81:177–180. doi: 10.1034/j.1600-0420.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 79.Patel S., McLaren J., Hodge D., Bourne W. Normal human keratocyte density and corneal thickness measurements by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci. 2001;42:333–339. [PubMed] [Google Scholar]
- 80.McLaren J.W., Nau C.B., Patel S.V., Bourne W.M. How precisely can we determine keratocyte density by confocal microscopy? Invest Ophthalmol Vis Sci. 2001;42:S281. [PubMed] [Google Scholar]
- 81.Böhnke M., Thaer A., Schipper I. Confocal microscopy reveals persisting stromal changes after myopic photorefractive keratectomy in zero haze corneas. Br J Ophthalmol. 1998;82:1393–1400. doi: 10.1136/bjo.82.12.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Midena E., Gambato C., Miotto S., Cortese M., Salvi R., Ghirlando A. Long-term effects on corneal keratocytes of mitomycin C during photorefractive keratectomy: a randomized contralateral eye confocal microscopy study. J Refract Surg. 2007;23(9 Suppl.):S1011–S1014. doi: 10.3928/1081-597X-20071102-06. [DOI] [PubMed] [Google Scholar]
- 83.Erie J.C., McLaren J.W., Hodge D.O., Bourne W.M. Recovery of corneal subbasal nerve density after PRK and LASIK. Am J Ophthalmol. 2005;140:1059–1064. doi: 10.1016/j.ajo.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 84.Ambrósio R., Jr., Kalina R., Mohan R.R., Mohan R.R., Possin D.D., Huang J. Early wound healing response to epithelial scrape injury in the human cornea. Invest Ophthalmol Vis Sci. 2002;43 E-Abstract 4206. [Google Scholar]
- 85.Frueh B.E., Cadez R., Böhnke M. In vivo confocal microscopy after photorefractive keratectomy in humans. A prospective, long-term study. Arch Ophthalmol. 1998;116:1425–1431. doi: 10.1001/archopht.116.11.1425. [DOI] [PubMed] [Google Scholar]
- 86.Hofmeister E.M., Bishop F.M., Kaupp S.E., Schallhorn S.C. Randomized dose–response analysis of mitomycin-C to prevent haze after photorefractive keratectomy for high myopia. J Cataract Refract Surg. 2013;39:1358–1365. doi: 10.1016/j.jcrs.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 87.Fini E.M. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18:529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- 88.Corbett M.C., Prydal J.I., Verma S., Oliver K.M., Pande M., Marshall J. An in vivo investigation of the structures responsible for corneal haze after photorefractive keratectomy and their effect on visual function. Ophthalmology. 1996;103:1366–1380. doi: 10.1016/s0161-6420(96)30495-8. [DOI] [PubMed] [Google Scholar]
- 89.Erie J.C., Patel S.V., McLaren J.W., Maguire L.J., Ramirez M., Bourne W.M. Keratocyte density in vivo after photorefractive keratectomy in humans. Trans Am Ophthalmol Soc. 1999;97:221–236. [PMC free article] [PubMed] [Google Scholar]
- 90.Razmjoo H., Kooshanmehr M.R., Peyman A., Kor Z., Mohammadesmaeil E. Comparison of standard and low dose intraoperative mitomycin C in prevention of corneal haze after photorefractive keratectomy. Int J Prev Med. 2013;4:204–207. [PMC free article] [PubMed] [Google Scholar]
- 91.Netto M.V., Mohan R.R., Sinha S., Sharma A., Gupta P.C., Wilson S.E. Effect of prophylactic and therapeutic mitomycin C on corneal apoptosis, cellular proliferation, haze, and long-term keratocyte density in rabbits. J Refract Surg. 2006;22:562–574. doi: 10.3928/1081-597x-20060601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cua I.Y., Pepose J.S. Late corneal scarring after photorefractive keratectomy concurrent with development of systemic lupus erythematosus. J Refract Surg. 2002;18:750–752. doi: 10.3928/1081-597X-20021101-16. [DOI] [PubMed] [Google Scholar]
- 93.Kremer I., Ehrenberg M., Levinger S. Delayed epithelial healing following photorefractive keratectomy with mitomycin C treatment. Acta Ophthalmol. 2012;90:271–276. doi: 10.1111/j.1755-3768.2010.01894.x. [DOI] [PubMed] [Google Scholar]
- 94.de Medeiros F.W., Mohan R.R., Suto C., Sinhá S., Bonilha V.L., Chaurasia S.S. Haze development after photorefractive keratectomy: mechanical vs ethanol epithelial removal in rabbits. J Refract Surg. 2008;24:923–927. doi: 10.3928/1081597X-20081101-11. [DOI] [PubMed] [Google Scholar]
- 95.Resch M.D., Nagy Z.Z., Szentmáry N., Máthé M., Kovalszky I., Süveges I. Spatial distribution of keratin sulfate in the rabbit cornea following photorefractive keratectomy. J Refract Surg. 2005;21:485–493. doi: 10.3928/1081-597X-20050901-11. [DOI] [PubMed] [Google Scholar]
- 96.Meltendorf C., Burbach G.J., Bühren J., Bug R., Ohrloff C., Deller T. Corneal femtosecond laser keratotomy results in isolated stromal injury and favorable wound-healing response. Invest Ophthalmol Vis Sci. 2007;48:2068–2075. doi: 10.1167/iovs.06-1150. [DOI] [PubMed] [Google Scholar]
- 97.Csutak A., Tözsér J., Békési L., Hassan Z., Berta A., Silver D.M. Plasminogen activator activity in tears after excimer laser photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2000;41:3743–3747. [PubMed] [Google Scholar]
- 98.von Mohrenfels C.W., Reischl U., Gabler B., Lohmann C.P. Corneal haze after photorefractive keratectomy. Role of individual collagen type IV synthesis on postoperative corneal opacity. Ophthalmologe. 2002;99:532–537. doi: 10.1007/s00347-001-0559-8. [DOI] [PubMed] [Google Scholar]
- 99.Nagy Z.Z., Hiscott P., Seitz B., Shlötzer-Schrehardt U., Simon M., Jr., Süveges I. Ultraviolet-B enhances stromal response to 193-nm excimer laser treatment. Ophthalmology. 1997;104:375–380. doi: 10.1016/s0161-6420(97)30305-4. [DOI] [PubMed] [Google Scholar]
- 100.Blalock T.D., Duncan M.R., Varela J.C., Goldstein M.H., Tuli S.S., Grotendorst G.R. Connective tissue growth factor expression and action in human corneal fibroblast cultures and rat corneas after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2003;44:1879–1887. doi: 10.1167/iovs.02-0860. [DOI] [PubMed] [Google Scholar]
- 101.Jester J.V., Petroll W.M., Cavanagh H.D. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- 102.Vinciguerra P., Azzolini M., Airaghi P., Radice P., De Molfetta V. Effect of decreasing surface and interface irregularities after photorefractive keratectomy and laser in situ keratomileusis on optical and functional outcomes. J Refract Surg. 1998;14:S199–S203. doi: 10.3928/1081-597X-19980401-12. [DOI] [PubMed] [Google Scholar]
- 103.Taylor S.M., Fields C.R., Barker F.M., Sanzo C.J. Effect of depth upon the smoothness of excimer laser corneal ablation. Optom Vis Sci. 1994;71:104–108. doi: 10.1097/00006324-199402000-00009. [DOI] [PubMed] [Google Scholar]
- 104.Javier J.A., Lee J.B., Oliveira H.B., Chang J.H., Azar D.T. Basement membrane and collagen deposition after laser subepithelial keratomileusis and photorefractive keratectomy in the leghorn chick eye. Arch Ophthalmol. 2006;124:703–709. doi: 10.1001/archopht.124.5.703. [DOI] [PubMed] [Google Scholar]
- 105.Nakamura K., Kurosaka D., Bissen-Miyajima H., Tsubota K. Intact corneal epithelium is essential for the prevention of stromal haze after laser assisted in situ keratomileusis. Br J Ophthalmol. 2001;85:209–213. doi: 10.1136/bjo.85.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McLaren J.W., Bourne W.M., Patel S.V. Standardization of corneal haze measurement in confocal microscopy. Invest Ophthalmol Vis Sci. 2010;51:5610–5616. doi: 10.1167/iovs.10-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Farid M., Morishige N., Lam L., Wahlert A., Steinert R.F., Jester J.V. Detection of corneal fibrosis by imaging second harmonic-generated signals in rabbit corneas treated with mitomycin C after excimer laser surface ablation. Invest Ophthalmol Vis Sci. 2008;49:4377–4383. doi: 10.1167/iovs.08-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takacs A.I., Mihaltz K., Nagy Z.Z. Corneal density with the Pentacam after photorefractive keratectomy. J Refract Surg. 2011;27:269–277. doi: 10.3928/1081597X-20100618-02. [DOI] [PubMed] [Google Scholar]
- 109.Nien C.J., Flynn K.J., Chang M., Brown D., Jester J.V. Reducing peak corneal haze after photorefractive keratectomy in rabbits: prednisolone acetate 1.00% versus cyclosporine A 0.05% J Cataract Refract Surg. 2011;37:937–944. doi: 10.1016/j.jcrs.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Møller-Pedersen T., Cavanagh H.D., Petroll W.M., Jester J.V. Corneal haze development after PRK is regulated by volume of stromal tissue removal. Cornea. 1998;17:627–639. doi: 10.1097/00003226-199811000-00011. [DOI] [PubMed] [Google Scholar]
- 111.Nejima R., Miyata K., Tanabe T., Okamoto F., Hiraoka T., Kiuchi T. Corneal barrier function, tear film stability, and corneal sensation after photorefractive keratectomy and laser in situ keratomileusis. Am J Ophthalmol. 2005;139:64–71. doi: 10.1016/j.ajo.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 112.Belmonte C., Acosta M.C., Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78:513–525. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 113.Prydal J.I., Kerr Muir M.G., Dilly P.N., Corbett M.C., Verma S., Marshall J. Confocal microscopy using oblique sections for measurement of corneal epithelial thickness in conscious humans. Acta Ophthalmol Scand. 1997;75:624–628. doi: 10.1111/j.1600-0420.1997.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 114.Vesaluoma M., Perez-Santonja J., Petroll W.M., Linna T., Alió J., Tervo T. Corneal stromal changes induced by myopic LASIK. Invest Ophthalmol Vis Sci. 2000;41:369–376. [PubMed] [Google Scholar]
- 115.Müller L.J., Pels L., Vrensen G.F. Ultrastructural organization of human corneal nerves. Ophthalmol Vis Sci. 1996;37:476–488. [PubMed] [Google Scholar]
- 116.Tervo K., Latvala T.M., Tervo T.M. Recovery of corneal innervation following photorefractive keratoablation. Arch Ophthalmol. 1994;112:1466–1470. doi: 10.1001/archopht.1994.01090230080025. [DOI] [PubMed] [Google Scholar]
- 117.Rozsa A.J., Guss R.B., Beuerman R.W. Neural remodeling following experimental surgery of the rabbit cornea. Invest Ophthalmol Vis Sci. 1983;24:1033–1051. [PubMed] [Google Scholar]
- 118.Burka J.M., Bower K.S., Vanroekel R.C., Stutzman R.D., Kuzmowych C.P., Howard R.S. The effect of moxifloxacion and gatifloxacin on long-term visual outcomes following photorefractive keratectomy. J Refract Surg. 2007;23:414–417. doi: 10.3928/1081-597X-20070401-15. [DOI] [PubMed] [Google Scholar]
- 119.Baker K.S., Anderson S.C., Romanowski E.G., Thoft R.A., SundarRaj N. Trigeminal ganglion neurons affect corneal epithelial phenotype. Influence on type VII collagen expression in vitro. Invest Ophthalmol Vis Sci. 1993;34:137–144. [PubMed] [Google Scholar]
- 120.Kauffmann T., Bodanowitz S., Hesse L., Kroll P. Corneal reinnervation after photorefractive keratectomy and laser in situ keratomileusis: an in vivo study with a confocal videomicroscope. Ger J Ophthalmol. 1996;5:508–512. [PubMed] [Google Scholar]
- 121.Neira-Zalentein W., Moilanen J.A., Tuisku I.S., Holopainen J.M., Tervo T.M. Photorefractive keratectomy retreatment after LASIK. J Refract Surg. 2008;24:710–712. doi: 10.3928/1081597X-20080901-12. [DOI] [PubMed] [Google Scholar]
- 122.Moilanen J.A., Vesaluoma M.H., Müller L.J., Tervo T.M. Long-term corneal morphology after PRK by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2003;44:1064–1069. doi: 10.1167/iovs.02-0247. [DOI] [PubMed] [Google Scholar]
- 123.Stern M.E., Gao J., Siemasko K.F., Beuerman R.W., Pflugfelder S.C. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004;78:409–416. doi: 10.1016/j.exer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 124.Kymionis G.D., Tsiklis N.S., Ginis H., Diakonis V.F., Pallikaris I. Dry eye after photorefractive keratectomy with adjuvant mitomycin C. J Refract Surg. 2006;22:511–513. doi: 10.3928/1081-597X-20060501-16. [DOI] [PubMed] [Google Scholar]
- 125.Hoşal B.M., Ornek N., Zilelioğlu G., Elhan A.H. Morphology of corneal nerves and corneal sensation in dry eye: a preliminary study. Eye (Lond) 2005;19:1276–1279. doi: 10.1038/sj.eye.6701760. [DOI] [PubMed] [Google Scholar]
- 126.Bourcier T., Acosta M.C., Borderie V., Borrás F., Gallar J., Bury T. Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci. 2005;46:2341–2345. doi: 10.1167/iovs.04-1426. [DOI] [PubMed] [Google Scholar]
- 127.Gallar J., Moilanen J., Acosta C., Holopainen J., Belmonte C., Tervo T. Long-term corneal sensitivity after PRK determined by non-contact gas esthesiometry. Invest Ophthalmol Vis Sci. 2013;54 [E-Abstract 904] [Google Scholar]
- 128.Sher N.A., Golben M.R., Bond W., Trattler W.B., Tauber S., Voirin T.G. Topical bromfenac 0.09% vs. ketorolac 0.4% for the control of pain, photophobia, and discomfort following PRK. J Refract Surg. 2009;25:214–220. doi: 10.3928/1081597X-20090201-07. [DOI] [PubMed] [Google Scholar]
- 129.Gallar J., Acosta M.C., Gutiérrez A.R., Belmonte C. Impulse activity in corneal sensory nerve fibers after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2007;48:4033–4037. doi: 10.1167/iovs.07-0012. [DOI] [PubMed] [Google Scholar]
- 130.Campero M., Serra J., Marchettini P., Ochoa J.L. Ectopic impulse generation and autoexcitation in single myelinated afferent fibers in patients with peripheral neuropathy and positive sensory symptoms. Muscle Nerve. 1998;21:1661–1667. doi: 10.1002/(sici)1097-4598(199812)21:12<1661::aid-mus6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 131.Ji R.R., Kohno T., Moore K.A., Woolf C.J. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 132.Sia R.K., Ryan D.S., Stutzman R.D., Psolka M., Mines M.J., Wagner M.E. Alcohol versus brush PRK: visual outcomes and adverse effects. Lasers Surg Med. 2012;44:475–481. doi: 10.1002/lsm.22036. [DOI] [PubMed] [Google Scholar]
- 133.Thornton I., Xu M., Krueger R.R. Comparison of standard (0.02%) and low dose (0.002%) mitomycin C in the prevention of corneal haze following surface ablation for myopia. J Refract Surg. 2008;24:S68–S76. doi: 10.3928/1081597X-20080101-13. [DOI] [PubMed] [Google Scholar]
- 134.Hanna K.D., Pouliquen Y.M., Waring G.O., III, Savoldelli M., Fantes F., Thompson K.P. Corneal wound healing in monkeys after repeated excimer laser photorefractive keratectomy. Arch Ophthalmol. 1992;110:1286–1291. doi: 10.1001/archopht.1992.01080210104035. [DOI] [PubMed] [Google Scholar]
- 135.Ghoreishi M., Attarzadeh H., Tavakoli M., Moini H.A., Zandi A., Masjedi A. Alcohol-assisted versus mechanical epithelium removal in photorefractive keratectomy. J Ophthalmic Vis Res. 2010;5:223–227. [PMC free article] [PubMed] [Google Scholar]
- 136.Browning A.C., Shah S., Dua H.S., Maharajan S.V., Gray T., Bragheeth M.A. Alcohol debridement of the corneal epithelium in PRK and LASEK: an electron microscopic study. Invest Ophthalmol Vis Sci. 2003;44:510–513. doi: 10.1167/iovs.02-0488. [DOI] [PubMed] [Google Scholar]
- 137.Shah S., Sebai Sarhan A.R., Doyle S.J., Pillai C.T., Dua H.S. The epithelial flap for photorefractive keratectomy. Br J Ophthalmol. 2001;85:393–396. doi: 10.1136/bjo.85.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen C.C., Chang J.H., Lee J.B., Javier J., Azar D.T. Human corneal epithelial cell viability and morphology after dilute alcohol exposure. Invest Ophthalmol Vis Sci. 2002;43:2593–2602. [PubMed] [Google Scholar]
- 139.Ghanem V.C., Souza G.C., Souza D.C., Viese J.M., Weber S.L., Kara-José N. PRK and butterfly LASEK: prospective, randomized, contralateral eye comparison of epithelial healing and ocular discomfort. J Refract Surg. 2008;24:591–599. doi: 10.3928/1081597X-20080601-07. [DOI] [PubMed] [Google Scholar]
- 140.Wang D.M., Du Y., Chen G.S., Tang L.S., He J.F. Transepithelial photorefractive keratectomy mode using SCHWIND-ESIRIS excimer laser: initial clinical results. Int J Ophthalmol. 2012;5:334–337. doi: 10.3980/j.issn.2222-3959.2012.03.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mannan R., Pruthi A., Rampal U. Combined phototherapeutic keratectomy and amniotic membrane grafts for symptomatic bullous keratopathy. Cornea. 2010;29:1207–1208. doi: 10.1097/ICO.0b013e3181d25ffe. [DOI] [PubMed] [Google Scholar]
- 142.Rathi V.M., Vyas S.P., Sangwan V.S. Phototherapeutic keratectomy. Indian J Ophthalmol. 2012;60:5–14. doi: 10.4103/0301-4738.91335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee H.K., Kim J.K., Kim E.K., Kim G.O., Lee I.S. Phototherapeutic keratectomy with amniotic membrane for severe subepithelial fibrosis following excimer laser refractive surgery. J Cataract Refract Surg. 2003;29:1430–1435. doi: 10.1016/s0886-3350(02)02042-4. [DOI] [PubMed] [Google Scholar]
- 144.Choi Y.S., Kim J.Y., Wee W.R., Lee J.H. Effect of the application of human amniotic membrane on rabbit corneal wound healing after excimer laser photorefractive keratectomy. Cornea. 1998;17:389–395. doi: 10.1097/00003226-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 145.Wang M.X., Gray T., Parks W.C., Prabhasawat P., Culbertson W., Forster R. Reduction in corneal haze and apoptosis by amniotic membrane matrix in excimer laser photoablation in rabbits. J Cataract Refract Surg. 2001;27:310–319. doi: 10.1016/s0886-3350(00)00467-3. [DOI] [PubMed] [Google Scholar]
- 146.Park W.C., Tseng S.C. Modulation of acute inflammation and keratocyte death by suturing, blood, and amniotic membrane in PRK. Invest Ophthalmol Vis Sci. 2000;41:2906–2914. [PubMed] [Google Scholar]
- 147.Savill J., Haslett C. Granulocyte clearance by apoptosis in the resolution of inflammation. Semin Cell Biol. 1995;6:385–393. doi: 10.1016/s1043-4682(05)80009-1. [DOI] [PubMed] [Google Scholar]
- 148.Khokhar S., Natung T., Sony P., Sharma N., Agarwal N., Vajpayee R.B. Amniotic membrane transplantation in refractory neurotrophic corneal ulcers: a randomized, controlled clinical trial. Cornea. 2005;24:654–660. doi: 10.1097/01.ico.0000153102.19776.80. [DOI] [PubMed] [Google Scholar]
- 149.Meller D., Pauklin M., Thomasen H., Westekemper H., Steuhl K.P. Amniotic membrane transplantation in the human eye. Dtsch Arztebl Int. 2011;108:243e8. doi: 10.3238/arztebl.2011.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhong Y., Zhai Z., Zhou Y., Ye W., Wang K. Effect of amniotic membrane on expressions of TGF-beta 1, collagens I, III and fibronectin in rabbit corneal healing after photorefractive keratectomy. Yan Ke Xue Bao. 2000;16:239–242. [PubMed] [Google Scholar]
- 151.Woo H.M., Kim M.S., Kweon O.K., Kim D.Y., Nam T.C., Kim J.H. Effects of amniotic membrane on epithelial wound healing and stromal remodelling after excimer laser keratectomy in rabbit cornea. Br J Ophthalmol. 2001;85:345–349. doi: 10.1136/bjo.85.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Farahi A., Hashemi H., Mehravaran S. The effects of mitomycin C on tear function after photorefractive keratectomy: a contralateral comparative study. J Refract Surg. 2013;29:260–264. doi: 10.3928/1081597X-20130318-05. [DOI] [PubMed] [Google Scholar]
- 153.Shojaei A., Ramezanzadeh M., Soleyman-Jahi S., Almasi-Nasrabadi M., Rezazadeh P., Eslani M. Short-time mitomycin-C application during photorefractive keratectomy in patients with low myopia. J Cataract Refract Surg. 2013;39:197–203. doi: 10.1016/j.jcrs.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 154.Dawson D.G., Grossniklaus H.E., McCarey B.E., Edelhauser H.F. Biomechanical and wound healing characteristics of corneas after excimer laser keratorefractive surgery: is there a difference between advanced surface ablation and subBowman's keratomileusis? J Refract Surg. 2008;24:S90–S96. doi: 10.3928/1081597X-20080101-16. [DOI] [PubMed] [Google Scholar]
- 155.Netto M.V., Chalita M.R., Krueger R.R. Corneal haze following PRK with mitomycin C as a retreatment versus prophylactic use in the contralateral eye. J Refract Surg. 2007;23:96–98. doi: 10.3928/1081-597X-20070101-16. [DOI] [PubMed] [Google Scholar]
- 156.Khoury J.M., Farah T., El-Haibi C.P., Noureddin B.N. Corneal light shield as a delivery system for standardized application of mitomycin C in excimer surface ablation. J Refract Surg. 2007;23:716–719. doi: 10.3928/1081-597X-20070901-11. [DOI] [PubMed] [Google Scholar]
- 157.Goldsberry D.H., Epstein R.J., Majmudar P.A., Epstein R.H., Dennis R.F., Holley G. Effect of mitomycin C on the corneal endothelium when used for corneal subepithelial haze prophylaxis following photorefractive keratectomy. J Refract Surg. 2007;23:724–727. doi: 10.3928/1081-597X-20070901-14. [DOI] [PubMed] [Google Scholar]
- 158.Morales A.J., Zadok D., Mora-Retana R., Martínez-Gama E., Robledo N.E., Chayet A.S. Intraoperative mitomycin and corneal endothelium after photorefractive keratectomy. Am J Ophthalmol. 2006;142:400–404. doi: 10.1016/j.ajo.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 159.Wallau A.D., Campos M. Photorefractive keratectomy with mitomycin C versus LASIK in custom surgeries for myopia: a bilateral prospective randomized clinical trial. J Refract Surg. 2008;24:326–336. doi: 10.3928/1081597X-20080401-03. [DOI] [PubMed] [Google Scholar]
- 160.Sia R.K., Ryan D.S., Edwards J.D., Stutzman R.D., Bower K.S. The U.S. Army Surface Ablation Study: comparison o PRK, MMC-PRK, and LASEK in moderate to high myopia. J Refract Surg. 2014;30:256–264. doi: 10.3928/1081597X-20140320-04. [DOI] [PubMed] [Google Scholar]
- 161.Kymionis G.D., Diakonis V.F., Charisis S., Pallikaris A.I., Bouzoukis D.I., Yoo S.H. Effects of topical mitomycin C on the ciliary body and intraocular pressure after PRK: an experimental study. J Refract Surg. 2008;24:633–638. doi: 10.3928/1081597X-20080601-14. [DOI] [PubMed] [Google Scholar]
- 162.Wallau A.D., Campos M. One-year outcomes of a bilateral randomised prospective clinical trial comparing PRK with mitomycin C and LASIK. Br J Ophthalmol. 2009;93:1634–1638. doi: 10.1136/bjo.2008.152579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ghoreishi M., Attarzadeh H., Zandi A., Moini H.A., Tavakoli M., Fesharaki H. Outcomes of photorefractive keratectomy with intraoperative mitomycin-C. J Ophthalmic Vis Res. 2009;4:142–146. [PMC free article] [PubMed] [Google Scholar]
- 164.Fazel F., Roshani L., Rezaei L. Two-step versus single application of mitomycin-C in photorefractive keratectomy for high myopia. J Ophthalmic Vis Res. 2012;7:17–23. [PMC free article] [PubMed] [Google Scholar]
- 165.Spadea L., Verrecchia V. Effectiveness of scraping and mitomycin C to treat haze after myopic photorefractive keratectomy. Open Ophthalmol J. 2011;5:63–65. doi: 10.2174/1874364101105010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Majmudar P.A., Forstot S.L., Dennis R.F., Nirankari V.S., Damiano R.E., Brenart R. Topical mitomycin-C for subepithelial fibrosis after refractive corneal surgery. Ophthalmology. 2000;107:89–94. doi: 10.1016/s0161-6420(99)00019-6. [DOI] [PubMed] [Google Scholar]
- 167.Xu H., Liu S., Xia X., Huang P., Wang P., Wu X. Mitomycin C reduces haze formation in rabbits after excimer laser photorefractive keratectomy. J Refract Surg. 2001;17:342–349. doi: 10.3928/1081-597X-20010501-08. [DOI] [PubMed] [Google Scholar]
- 168.Patel S.V., Bourne W.M. Corneal endothelial cell loss 9 years after excimer laser keratorefractive surgery. Arch Ophthalmol. 2009;127:1423–1427. doi: 10.1001/archophthalmol.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mardelli P.G., Piebenga L.W., Matta C.S., Hyde L.L., Gira J. Corneal endothelial status 12 to 55 months after excimer laser photorefractive keratectomy. Ophthalmology. 1995;102:544–549. doi: 10.1016/s0161-6420(95)30984-0. [DOI] [PubMed] [Google Scholar]
- 170.Zare M., Jafarinasab M.R., Feizi S., Zamani M. The effect of mitomycin-C on corneal endothelial cells after photorefractive keratectomy. J Ophthalmic Vis Res. 2011;6:8–12. [PMC free article] [PubMed] [Google Scholar]
- 171.Roh D.S., Funderburgh J.L. Impact on the corneal endothelium of mitomycin C during photorefractive keratectomy. J Refract Surg. 2009;25:894–897. doi: 10.3928/1081597X-20090617-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Burka J.M., Bower K.S., Vanroekel R.C., Stutzman R.D., Kuzmowych C.P., Howard R.S. The effect of fourth-generation fluoroquinolones gatifloxacin and moxifloxacin on epithelial healing following photorefractive keratectomy. Am J Ophthalmol. 2005;140:83–87. doi: 10.1016/j.ajo.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 173.Plaka A., Grentzelos M.A., Astyrakakis N.I., Kymionis G.D., Pallikaris I.G., Plainis S. Efficacy of two silicone-hydrogel contact lenses for bandage use after photorefractive keratectomy. Cont Lens Anterior Eye. 2013;36:243–246. doi: 10.1016/j.clae.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 174.Brilakis H.S., Deutsch T.A. Topical tetracaine with bandage soft contact lens pain control after photorefractive keratectomy. J Refract Surg. 2000;16:444–447. doi: 10.3928/1081-597X-20000701-07. [DOI] [PubMed] [Google Scholar]
- 175.Dantas P.E., Nishiwaki-Dantas M.C., Ojeda V.H., Holzchuh N., Mimica L.J. Microbiological study of disposable soft contact lenses after photorefractive keratectomy. CLAO J. 2000;26:26–29. [PubMed] [Google Scholar]
- 176.Cherry P.M. The treatment of pain following excimer laser photorefractive keratectomy: additive effect of local anesthetic drops, topical diclofenac, and bandage soft contact. Ophthalmic Surg Lasers. 1996;27(5 Suppl.):S477–S480. [PubMed] [Google Scholar]
- 177.Vetrugno M., Maino A., Quaranta G.M., Cardia L. The effect of early steroid treatment after PRK on clinical and refractive outcomes. Acta Ophthalmol Scand. 2001;79:23–27. doi: 10.1034/j.1600-0420.2001.079001023.x. [DOI] [PubMed] [Google Scholar]
- 178.Vigo L., Scandola E., Carones F. Scraping and mitomycin C to treat haze and regression after photorefractive keratectomy for myopia. J Refract Surg. 2003;19:449–454. doi: 10.3928/1081-597X-20030701-12. [DOI] [PubMed] [Google Scholar]
- 179.Bühren J., Nagy L., Swanton J.N., Kenner S., MacRae S., Phipps R.P. Optical effects of anti-TGFbeta treatment after photorefractive keratectomy in a cat model. Invest Ophthalmol Vis Sci. 2009;50:634–643. doi: 10.1167/iovs.08-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Javadi M.A., Mirbabaei-Ghafghazi F., Mirzade M., Yazdani S., Yaseri M. Steroid induced ocular hypertension following myopic photorefractive keratectomy. J Ophthalmic Vis Res. 2008;3:42–46. [PMC free article] [PubMed] [Google Scholar]
- 181.Caldwell M., Reilly C. Effects of topical nepafenac on corneal epithelial healing time and postoperative pain after PRK: a bilateral, prospective, randomized, masked trial. J Refract Surg. 2008;24:377–382. doi: 10.3928/1081597X-20080401-11. [DOI] [PubMed] [Google Scholar]
- 182.Szabó V., Balogh K., Süveges I., Rácz K., Hunyady L., Nagy Z.Z. The role of lumican and keratocan genes in persistent subepithelial corneal haze following excimer laser photorefractive keratectomy. Mol Vis. 2006;12:597–605. [PubMed] [Google Scholar]
- 183.Vetrugno M., Maino A., Cardia G., Quaranta G.M., Cardia L. A randomised, double masked, clinical trial of high dose vitamin A and vitamin E supplementation after photorefractive keratectomy. Br J Ophthalmol. 2001;85:537–539. doi: 10.1136/bjo.85.5.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chelala E., Dirani A., Fadlallah A., Fahd S. The role of topical vitamin A in promoting healing in surface refractive procedures: a prospective randomized controlled study. Clin Ophthalmol. 2013;7:1913–1918. doi: 10.2147/OPTH.S52280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lee K.S., Ko D.A., Kim E.S., Kim M.J., Tchah H., Kim J.Y. Bevacizumab and rapamycin can decrease corneal opacity and apoptotic keratocyte number following photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2012;53:7645–7653. doi: 10.1167/iovs.12-10494. [DOI] [PubMed] [Google Scholar]
- 186.Santhiago M.R., Singh V., Barbosa F.L., Agrawal V., Wilson S.E. Monocyte development inhibitor PRM-151 decreases corneal myofibroblast generation in rabbits. Exp Eye Res. 2011;93:786–789. doi: 10.1016/j.exer.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sharma A., Mehan M.M., Sinha S., Cowden J.W., Mohan R.R. Trichostatin a inhibits corneal haze in vitro and in vivo. Invest Ophthalmol Vis Sci. 2009;50:2695–2701. doi: 10.1167/iovs.08-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Javaloy J., Alió J.L., Rodriguez A.E., Vega A., Muñoz G. Effect of platelet-rich plasma in nerve regeneration after LASIK. J Refract Surg. 2013;29:213–219. doi: 10.3928/1081597X-20130129-04. [DOI] [PubMed] [Google Scholar]
- 189.Cortina M.S., He J., Li N., Bazan N.G., Bazan H.E. Recovery of corneal sensitivity, calcitonin gene-related peptide-positive nerves, and increased wound healing induced by pigment epithelial-derived factor plus docosahexaenoic acid after experimental surgery. Arch Ophthalmol. 2012;130:76–83. doi: 10.1001/archophthalmol.2011.287. [DOI] [PubMed] [Google Scholar]
- 190.Shaheen B.S., Bakir M., Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59:263–285. doi: 10.1016/j.survophthal.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain. 2006;7(Suppl. 1):S3–S12. doi: 10.1016/j.jpain.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 192.Rivera L., Gallar J., Pozo M.A., Belmonte C. Responses of nerve fibres of the rat saphenous nerve neuroma to mechanical and chemical stimulation: an in vitro study. J Physiol. 2000;527:305–313. doi: 10.1111/j.1469-7793.2000.t01-1-00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lichtinger A., Purcell T.L., Schanzlin D.J., Chayet A.S. Gabapentin for postoperative pain after photorefractive keratectomy: a prospective, randomized, double-blind, placebo-controlled trial. J Refract Surg. 2011;27:613–617. doi: 10.3928/1081597X-20110210-01. [DOI] [PubMed] [Google Scholar]


