Abstract
Purpose
To evaluate long-term keratoconus stability after corneal crosslinking (CXL) with riboflavin.
Methods
In this prospective study, 57 eyes of 55 patients with progressive keratoconus, consecutively treated with ultraviolet A (UVA) – riboflavin CXL, were examined with the corneal topographer Pentacam, the biometer IOLMaster and the analyzer of corneal biomechanics Ocular Response Analyzer before and during a 24 months follow-up after CXL.
Results
Twenty-four months after CXL, there was a significant improvement in best corrected visual acuity (BCVA) (P < 0.01), a significant decrease in corneal thinnest point (CTP), keratometry readings at the keratoconus apex (K max), and corneal volume (CV) (P < 0.01), and a significant increase in axial eye length (AL) (P = 0.01). No significant changes in anterior chamber volume (ACV) and depth (ACD), (P = 0.8), corneal hysteresis (CH) (P = 0.16) and corneal resistance factor (CRF) (P = 0.06) were found. However, in the subgroup of patients with decreased K max readings 24 months after treatment, both CH and CRF showed a significant reduction (P < 0.01).
Conclusion
In the first month after the procedure, CXL induces a reduction in corneal volume. During the 24 months follow-up the cornea tends to recover its original volume with a persistence of the CXL efficacy.
Keywords: Corneal crosslinking, Keratoconus, Axial eye length, Keratometry readings, Corneal thinnest point
Resumen
Objetivo
Evaluar la estabilidad del queratocono a largo plazo tras cross-linking corneal (CXL) con riboflavina.
Métodos
En este estudio prospectivo, se examinaron 57 ojos de 55 pacientes con queratocono progresivo, tratados consecutivamente con UVA-CXL con riboflavina utilizando el topógrafo corneal Pentacam, el biómetro IOLMaster y el analizar de la biomecánica corneal “Ocular Response Analyzer” preoperatoriamente y a los 24 meses de haberse realizado el CXL.
Resultados
A los veinticuatro meses del CXL, se produjo una mejora considerable de la agudeza visual mejor corregida (BCVA) (p < 0.01), un importante decremento del punto más fino de la córnea (CTP), de las medidas queratométricas en el vértice del queratocono (K max), y del volumen de la córnea (VC) (p < 0.01), y un incremento significativo de la longitud axial del ojo (LA) (p = 0.01). No se produjeron cambios significativos en el volumen de la cámara anterior (VCA) ni en la profundidad de la misma (PCA), (p = 0.8), histéresis de la córnea (HC) (p = 0.16) y factor de resistencia de la córnea (FRC) (p = 0.06). Sin embargo, en el subgrupo de pacientes con disminución de los valores de K max, a los 24 meses del tratamiento, tanto la HC como la FRC mostraron una reducción considerable (p < 0.01).
Conclusión
Durante el primer mes tras la intervención, el CXL induce una reducción del volumen de la córnea. Durante los 24 meses de seguimiento, la córnea tiende a recuperar su volumen original, persistiendo la eficacia del CXL.
Palabras clave: Cross-linking corneal, Queratocono, Longitud axial del ojo, Mediciones del queratómetro, Grosor corneal más delagado
Introduction
Keratoconus is a corneal ectasia resulting from non-inflammatory, progressive thinning of the corneal stroma.1 Initial management is based on refractive correction with spectacles and contact lenses or intrastromal corneal ring segments, but further ectatic progression may lead to corneal transplantation in 10–20% of the patients.2 Corneal collagen crosslinking (CXL) with riboflavin and ultraviolet A (UVA) has been used in several in vitro studies3–10 in the attempt to enhance corneal biomechanical resistance by an increase in collagen fiber diameters.11,12 As the main keratoconus characteristic is a reduced biomechanical corneal strength,13 CXL was recently proposed to halt its progression.12 In this study we report the clinical results of CXL treatment in patients with a progressive keratoconus.
Methods
Fifty-seven eyes of 55 patients (39 men) with a mean age of 22.5 ± 5 years (range from 11 to 35) with progressive keratoconus, documented by refraction and corneal topography in the last 6 months, and defined as a recent keratometric increase and/or pachymetric decrease, were evaluated. In particular, keratoconus was considered to be progressive if, during a 12 months’ follow-up, there was an increase ≥1 diopter (D) in the steepest simulated keratometry value derived from computerized videokeratography or in the steepest meridian measured by manual keratometry, or an increase in astigmatism as determined by manifest subjective refraction ≥1.0 D, with subjective deterioration in vision.
Patients with a history of previous corneal surgery, systemic autoimmune diseases, diabetes, evidence of severe dry eye, corneal scars and corneal thickness less than 400 μm were excluded from the study. After the nature and aim of the study had been fully explained, a written informed consent was obtained from each patient. The study and data collection were carried out in adherence to the tenets of the Declaration of Helsinki.
All patients underwent a complete preoperative ophthalmologic examination including: measurement of best-corrected visual acuity (BCVA) in decimal scale; slit lamp and fundus examination; corneal evaluation, including corneal volume measurements, by a Scheimpflug imaging device (Oculus Pentacam, Optikgerate GmbH, Wetzlar, Germany); axial length (AL) measurement with an IOLMaster (Carl Zeiss Meditec, Jena, Germany); and evaluation of the biomechanical properties of the cornea, corneal hysteresis (CH) and corneal resistance factor (CRF), with the Ocular Response Analyzer (Reichert Technologies, Depew, USA).
All patients were treated with UVA–riboflavin CXL under aseptic conditions using topical preoperative anesthesia with oxybuprocaine hydrochloride 0.4% (Novesina, Novartis Farma) eye drops. Before the procedure, 2% pilocarpine (Pilocarpina, Farmigea) eye drops were instilled in the eye to be treated. Each patient was draped, the ocular surface was rinsed with Balanced saline solution (BSS) and a lid speculum was inserted. To ensure that the riboflavin penetrated the stroma and to achieve a high level of UVA absorption, the epithelial tissue was removed in a 9.0 mm diameter area. To allow sufficient saturation of the stroma, Riboflavin 0.1% in 20% dextran solution (Ricrolin, Sooft) was applied to the cornea 30 min before the irradiation, every 3 min. A calibrated UV power meter (UV LIGHT meter model yk-34uv, Lutron electronic, Coopersburg, USA) was used to control the desired levels of irradiance before each treatment. After that, an 8.0 mm diameter of the central cornea was irradiated with the Vega C.B.M. X-linker (C.S.O. Firenze) using standard parameters (UVA 365 nm, 3 mW/cm2). During the 30 min irradiation, drops of riboflavin solution were applied to the cornea every 2–3 min to maintain the necessary concentration of riboflavin and to prevent corneal drying. The treatment centration was controlled by the surgeon. At the end of the procedure, the eye surface was washed with BSS, and cyclopentolate (Ciclolux 10 mg/mL, Allergan) and ofloxacin (Exocin 3 mg/mL, Allergan) eye drops were administered and afterwards a soft bandage contact lens was applied. Ofloxacin drops and artificial tears were given 4 times a day until complete reepithelialization. After the contact lens was removed, steroid eye drops (Dexamono 1 mg/mL, Thea) were used in a tapered dose over 4 weeks and artificial tears were continued 4 times a day for the same period. Statistical analysis was performed using the paired Student's t test. P < 0.01 was considered to be statistically significant.
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers/animals were followed during this research.
Results
BCVA, corneal thinnest point (CTP), keratometry readings at the keratoconus apex (K max), AL, corneal volume (CV), anterior chamber volume (ACV), anterior chamber depth (ACD), and CH and CRF before treatment and during the follow-up are shown in Table 1. In Figs. 1–3 the differences in CTP (Fig. 1), K max (Fig. 2) and AL (Fig. 3) have been divided in 3 groups: stable, increased, and decreased.
Table 1.
Comparison in best corrected visual acuity (BCVA) in decimals, Sphere and Cylinder, corneal thinnest point (CTP), keratometry readings at the keratoconus apex (K max), axial eye length (AL), corneal volume (CV), anterior chamber volume (ACV), anterior chamber depth (ACD), corneal hysteresis (CH) and corneal resistance factor (CRF) before treatment and during the 24 months follow-up (mean ± SD, and range).
| Pre op. | 1 Month | 3 Months | 6 Months | 12 Months | 24 Months | |
|---|---|---|---|---|---|---|
| BCVA | 0.56 ± 0.26 0.1 to 1 |
0.52 ± 0.24 0.1 to 1 P = 0.02a |
0.59 ± 0.25 0.1 to 1 P = 0.05a |
0.61 ± 0.26 0.1 to 1 P = 0.6a |
0.69 ± 0.25 0.3 to 1 P = 0.003a |
0.81 ± 0.24 0.2 to 1 P < 0.01a P < 0.01b |
| Sph (D) | 0.07 ± 2.24 −6 to +5 |
0.13 ± 2.29 −6 to +4.5 P = 0.1a |
−0.01 ± 2.64 −6 to +6 P = 0.4a |
−0.23 ± 2.19 −6 to +4.5 P = 0.9a |
0.66 ± 2.15 −4 to +6 P = 0.06a |
0.14 ± 2.12 −5 to +5 P = 0.08aP = 0.7b |
| Cyl (D) | −3.18 ± 1.54 −8 to −0.5 |
−3.02 ± 1.38 −6 to −0.5 P = 0.7a |
−3.26 ± 1.71 −8 to −0.5 P = 0.8a |
−3.07 ± 1.68 −8 to 0 P = 0.1a |
−3.44 ± 1.26 −6 to −1 P = 0.19a |
−2.8 ± 2.05 −8 to +1 P < 0.01a P < 0.3b |
| CTP (μm) | 458.4 ± 30.5 400 to 517 |
418.2 ± 38.6 348 to 502 P < 0.01a |
415.3 ± 33.3 358 to 482 P = 0.06a |
422.9 ± 44.7 340 to 576 P = 0.17a |
441.1 ± 31.2 366 to 499 P = 0.38a |
436.2 ± 38.1 332 to 516 P= 0.45a P < 0.01b P < 0.01c |
| K max (D) | 58.04 ± 4.92 47.4 to 70.6 |
58.77 ± 4.79 47.6 to 72.5 P < 0.01a |
58.27 ± 4.85 47 to 72.2 P < 0.01a |
56.75 ± 5.2 46.7 to 73.2 P = 0.01a |
56.31 ± 5.71 48.7 to 73.1 P = 0.09a |
55.82 ± 5.37 46.7 to 73.7 P < 0.01a P < 0.01b |
| AL (mm) | 23.92 ± 0.96 22.2 to 26.22 |
24.09 ± 0.92 22.31 to 26.25 P < 0.01a |
23.99 ± 0.99 22.14 to 26.32 P = 0.79a |
24.03 ± 0.99 22.29 to 26.43 P = 0.51a |
23.79 ± 0.96 22.3 to 25.35 P = 0.37a |
24.09 ± 0.96 22.31 to 26.23 P = 0.06a P = 0.01b P = 0.04c |
| CV (mm3) | 56.9 ± 3 51.1 to 64.5 |
54.7 ± 3.2 48.8 to 62.6 P < 0.01a |
55.7 ± 3.4 49.6 to 63.1 P < 0.01a |
55.8 ± 3.5 43.3 to 62.2 P = 0.8a |
57.4 ± 3.2 50.2 to 63.7 P = 0.5a |
56.6 ± 3 50.4 to 64.1 P = 0.4a P < 0.01b P < 0.01c |
| ACV (mm3) | 226.9 ± 33.9 155 to 297 |
230.6 ± 38.3 155 to 313 P = 0.1a |
235.5 ± 32.7 175 to 298 P = 0.6a |
230.9 ± 37.1 161 to 304 P = 0.5a |
223 ± 34.7 152 to 289 P = 0.6a |
226.8 ± 37.8 145 to 291 P = 0.8a P = 0.3b |
| ACD (mm) | 3.99 ± 0.3 3.43 to 4.72 |
4.0 ± 0.33 3.35 to 4.79 P = 0.96a |
4.03 ± 0.31 3.41 to 4.79 P = 0.01a |
3.99 ± 0.32 3.41to 4.72 P = 0.44a |
3.94 ± 0.32 3.41 to 4.52 P = 0.71a |
3.94 ± 0.35 3.2 to 4.70 P = 0.59a P < 0.01b |
| CH (mmHg) | 8.0 ± 1.2 5.4 to 10.4 |
7.5 ± 1.1 5to 10.1 P = 0.07a |
7.4 ± 0.9 5.8 to 9.2 P = 0.3a |
7.2 ± 1.3 5.4 to 9.1 P = 0.2a |
7.7 ± 1.1 5.5 to 9.5 P = 0.02a |
7.8 ± 1.3 5.9 to 11 P = 0.9a P = 0.16b |
| CRF (mmHg) | 6.6 ± 1.4 3.9 to 9.5 |
6.9 ± 1.2 4.6 to 9.0 P = 0.01a |
6.2 ± 1.2 3.8 to 8.3 P = 0.01a |
6.1 ± 1.8 3.8 to 9.2 P = 0.3a |
6.4 ± 1.2 4.3 to 8.1 P = 0.4a |
6.3 ± 1.3 3.7 to 8.8 P = 0.8a P = 0.06b |
T test evaluation with immediately previous follow-up.
T test evaluation between pre-operative and 24 months follow-up.
T test evaluation between 1 month and 24 months follow-up.
Figure 1.
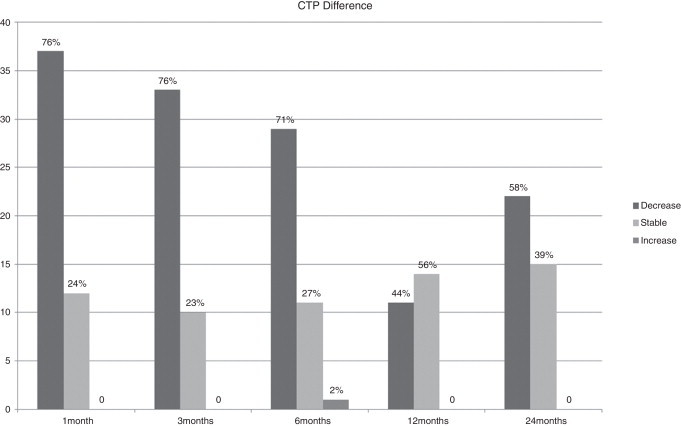
Percentage of eyes with stability, increase or decrease in corneal thinnest point (CTP) values.
Figure 2.
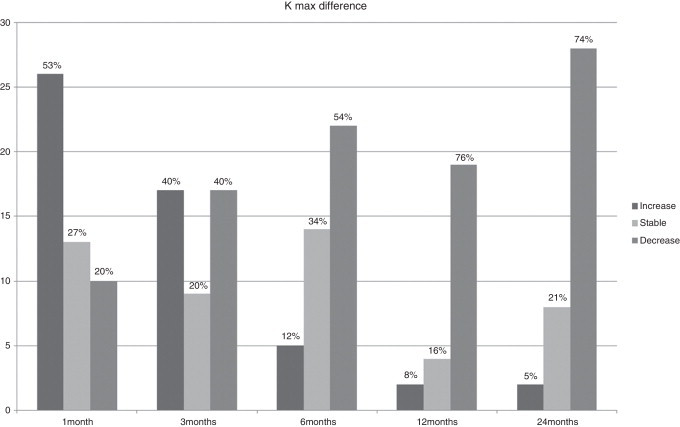
Percentage of eyes with stability, increase or decrease in keratometry at keratoconus apex (K max) values.
Figure 3.
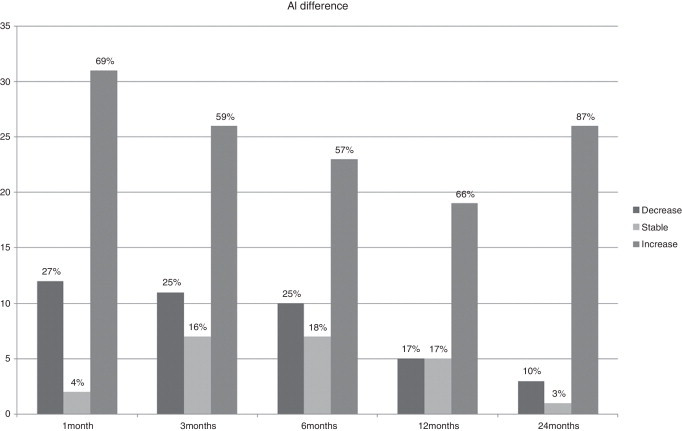
Percentage of eyes with stability, increase or decrease in axial eye length (AL) values.
Table 2 shows the changes in CH and CRF before and 24 months after treatment in patients with stability and decrease in K max readings, respectively.
Table 2.
Changes in corneal hysteresis (CH) and corneal resistance factor (CRF) in the group of patients with stability (GROUP 1) and in the one with decrease (GROUP 2) in keratometry readings at the keratoconus apex (K max) before treatment and at 24 months follow-up (mean ± SD, and range).
| GROUP 1 |
GROUP 2 |
|||
|---|---|---|---|---|
| Pre op. | 24 Months | Pre op. | 24 Months | |
| CH (mmHg) | 8.1 ± 1.6 6.1 to 10.4 |
8.7 ± 1.6 6.9 to 11.0 P = 0.08 |
8.2 ± 1.0 6.5 to 9.6 |
7.6 ± 1.1 5.9 to 9.4 P < 0.01 |
| CRF (mmHg) | 6.5 ± 1.7 4.3 to 8.8 |
6.8 ± 1.6 5.0 to 8.8 P = 0.87 |
6.8 ± 1.0 4.6 to 8.3 |
6.1 ± 1.2 3.7 to 8.0 P < 0.01 |
These results indicate that 24 months after CXL, there was a significant improvement in BCVA (P < 0.01), a significant decrease in CTP, K max, and CV (P < 0.01),and a significant increase in AL (P = 0.01). Concerning the AL difference, we divided the patients into two groups: pediatric patients (6 eyes) and adult patients (24 eyes) to check if the increase in the axial length could be due to an ocular growth. In both cases the Wilcoxon rank test showed a significant increase (P < 0.05).
No significant changes in ACV and ACD, (P = 0.8), CH (P = 0.16) and CRF (P = 0.06) were found. However, in the subgroup of patients with decreased K max readings 24 months after treatment, both CH and CRF showed a significant reduction (P < 0.01).
If however, we observe the subgroup of patients, in which a reduction of CH and CRF was found at 24 months follow-up, the mean difference in K max value was −1.21 ± 2.67 D (range +1.4 to −7.2 D); the mean difference in CTP was −26.63 ± 25.83 μm (range +16 to −56 μm); the mean difference in CV was −0.91 ± 0.71 μm3 (range +0.4 to −1.8 μm3), confirming the previous results.
Discussion
Several studies have been published reporting the clinical data after CXL, but only few of them presented a long follow-up and some of them evaluated a small number of patients.14–18
Raiskup-Wolf et al.14 found in 66 eyes that in most of them there was an improvement in BCVA, astigmatism and K readings with a non-significant change in central corneal thickness (CCT) at 1 year and an increase after 24 months. Vinciguerra et al.,15 in 40 eyes of 40 pediatric patients with progressive keratoconus found a significant improvement in uncorrected visual acuity (UCVA) and BCVA with a statistically significant reduction in mean spherical equivalent and a flattening effect of CXL on the keratoconic cornea. Moreover, they found no statistically significant difference in pupil center pachymetry, thinnest point, corneal volume, anterior chamber volume and anterior chamber depth compared to preoperative data.
Vinciguerra et al., in another paper,16 found similar results in 28 eyes with keratoconus, except for the total corneal volume, which decreased (P = 0.045) at 12 months, with a slight increase 24 months after treatment (P = 0.06 compared with the 12-month values) and for the corneal pachymetry at the thinnest point, which decreased from baseline to 1 year after CXL (P = 0.04) and increased to achieve recovery 24 months postoperatively (P = 0.71). Anterior chamber volume, and anterior and posterior elevation did not change significantly during the 24-months follow-up, but anterior chamber depth decreased 24 months postoperatively with a statistically significant difference. Caporossi et al.,17 in 44 patients with keratoconus between 10 and 40 years old, with a minimum follow-up of 48 months, found an improvement in UCVA and BCVA and a reduction in mean K readings with a hyperopic shift, without statistically significant differences in central corneal thickness, measured by US pachymetry, over the third month of follow-up. Goldich et al.,18 in 14 eyes treated with collagen crosslinking for progressive keratoconus, found a significant improvement in BCVA, in steepest-meridian keratometry, in the mean cylinder, with a significant elongation of the eyes, whereas no significant changes in mean simulated keratometry, minimal corneal thickness, endothelial cell density or foveal thickness were observed. Our data show that there was a significant improvement in BCVA, with a relative stability in ACV and in the values of sphere and cylinder, and a decrease of the K max values, in agreement with previous published papers. Concerning the corneal thickness19 and the corneal volume, our findings of a statistically significant decrease 1 month after treatment, that tend to increase during the 24 months follow-up, without reaching the preoperative values, could be explained by previous in vivo findings showing that, 6 months after CXL treatment, stromal keratocyte repopulation was complete and was accompanied by the disappearance of stromal edema.20,21 Another explanation could be the presence of corneal edema in the first months, causing artifacts in CCT measurements. Regarding the AL, 1 month after surgery, the treated eyes showed a statistically significant elongation compared to the preoperative data, which remained stable during the following months, similarly to that reported by Goldich et al.18 Our study shows an AL increase, associated with the stability of the ACV and ACD during the 24 months follow up. This is an important finding, as it supports the theory that this increase is due to an elongation of the posterior segment of the eye, unrelated to the treatment. When we divide the patients into two groups, adults and pediatrics, in both cases we found a significant increase. This finding does not prove that the elongation is unrelated to eye growth, as it has been shown that in some individuals the eye elongation can also be present also in adult age.22–24 On the other hand, the increase in AL, with corneal flattening, (decrease in K max values), could explain the stability in refraction during the entire follow-up examination.
Concerning the biomechanical parameters, our data on all the examined population, showing no statistical differences between preoperative and 24 months follow-up data, agree with the other papers dealing with this issue.25–29 However, when we analyze only the subgroup of eyes with improvement (decrease) in K max readings at 24 months follow-up, we observe a statistically significant reduction of CH and CRF (Table 2). This finding is confirmed when we analyze the data of patients with reduction in CH and CRF at 24 months follow-up, as in this subgroup of patients there is a mean reduction in K max readings, in CV and in CTP.
The correlation between CH and CRF with corneal thickness could explain this finding.28,29
Another explanation may be that CXL produces a stabilization of the keratoconus evolution with ultra-structural changes of the cornea without affecting the corneal viscoelasticity.
One limitation of this study might be the lack of data regarding the endothelial cells. The reason why we did not evaluate the cell density was that the safety of this kind of treatment was not the aim of this study. However, a recently published paper, investigating the effects of CXL in eyes with progressive keratoconus, showed no significant difference in endothelial cell density at any follow-up time when compared with baseline data and, similarly, no significant differences with the control group.30
In conclusion, our data seem to confirm the effectiveness of the treatment that induces shrinkage of the corneal lamellae, with a reduction in corneal volume, in the first month after the procedure, and a tendency to recover to preoperative values during the following period, but with persistence of the efficacy of the treatment at 24 months follow-up.
Financial support
None.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Krachmer J.H., Feder R.S., Belin M.W. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 2.Tuft S.J., Moodaley L.C., Gregory W.M., Davison C.R., Buckley R.J. Prognostic factors for the progression of keratoconus. Ophthalmology. 1994;101:439–447. doi: 10.1016/s0161-6420(94)31313-3. [DOI] [PubMed] [Google Scholar]
- 3.Wollensak G., Spoerl E., Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29:1780–1785. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 4.Kohlhaas M., Spoerl E., Schilde T., Unger G., Wittig C., Pillunat L.E. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg. 2006;32:279–283. doi: 10.1016/j.jcrs.2005.12.092. [DOI] [PubMed] [Google Scholar]
- 5.Dupps W.J., Jr., Netto M.V., Herekar S., Krueger R.R. Surface wave elastometry of the cornea in porcine and human donor eyes. J Refract Surg. 2007;23:66–75. doi: 10.3928/1081-597x-20070101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rocha K.M., Ramos-Esteban J.C., Qian Y., Herekar S., Krueger R.R. Comparative study of riboflavin-UVA cross-linking and “flashlinking” using surface wave elastometry. J Refract Surg. 2008;24:S748–S751. doi: 10.3928/1081597X-20080901-20. [DOI] [PubMed] [Google Scholar]
- 7.Spoerl E., Wollensak G., Seiler T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res. 2004;29:35–40. doi: 10.1080/02713680490513182. [DOI] [PubMed] [Google Scholar]
- 8.Mazzotta C., Balestrazzi A., Traversi C. Treatment of progressive keratoconus by riboflavin-UVA-induced cross-linking of corneal collagen; ultrastructural analysis by Heidelberg Retinal Tomograph II in vivo confocal microscopy in humans. Cornea. 2007;26:390–397. doi: 10.1097/ICO.0b013e318030df5a. [DOI] [PubMed] [Google Scholar]
- 9.Wollensak G., Wilsch M., Spoerl E., Seiler T. Collagen fiber diameter in the rabbit cornea after collagen crosslinking by riboflavin/UVA. Cornea. 2004;23:503–507. doi: 10.1097/01.ico.0000105827.85025.7f. [DOI] [PubMed] [Google Scholar]
- 10.Spoerl E., Wollensak G., Dittert D.-D., Seiler T. Thermomechanical behaviour of collagen-cross-linked porcine cornea. Ophthalmologica. 2004;218:136–140. doi: 10.1159/000076150. [DOI] [PubMed] [Google Scholar]
- 11.Spoerl E., Huhle M., Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998;66:97–103. doi: 10.1006/exer.1997.0410. [DOI] [PubMed] [Google Scholar]
- 12.Wollensak G., Spoerl E., Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 13.Andreassen T.T., Simonsen A.H., Oxlund H. Biomechanical properties of keratoconus and normal corneas. Exp Eye Res. 1980;31:435–441. doi: 10.1016/s0014-4835(80)80027-3. [DOI] [PubMed] [Google Scholar]
- 14.Raiskup-Wolf F., Hoyer A., Spoerl E., Pillunat L.E. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. 2008;34:796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 15.Vinciguerra P., Albè E., Frueh B.E., Trazza S., Epstein D. Two-year corneal cross-linking results in patients younger than 18 years with documented progressive keratoconus. Am J Ophthalmol. 2012;154:520–526. doi: 10.1016/j.ajo.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Vinciguerra P., Albè E., Trazza S., Seiler T., Epstein D. Intraoperative and postoperative effects of corneal collagen cross-linking on progressive keratoconus. Arch Ophthalmol. 2009;127:1258–1265. doi: 10.1001/archophthalmol.2009.205. [DOI] [PubMed] [Google Scholar]
- 17.Caporossi A., Mazzotta C., Baiocchi S., Caporossi T. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am J Ophthalmol. 2010;149:585–593. doi: 10.1016/j.ajo.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Goldich Y., Marcovich A.L., Barkana Y. Clinical and corneal biomechanical changes after collagen cross-linking with riboflavin and UV irradiation in patients with progressive keratoconus: results after 2 years of follow-up. Cornea. 2012;31:609–614. doi: 10.1097/ICO.0b013e318226bf4a. [DOI] [PubMed] [Google Scholar]
- 19.Rosa N., Lanza M., Borrelli M., Polito B., Filosa M.L., De Bernardo M. Comparison of central corneal thickness measured with Orbscan and Pentacam. J Refract Surg. 2007;23:895–899. doi: 10.3928/1081-597X-20071101-05. [DOI] [PubMed] [Google Scholar]
- 20.Ortiz D., Pinero D., Shabayek M.H., Arnalich-Montiel F., Alió J.L. Corneal biomechanical properties in normal, post-laser in situ keratomileusis, and keratoconic eyes. J Cataract Refract Surg. 2007;33:1371–1375. doi: 10.1016/j.jcrs.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Caporossi A., Baiocchi S., Mazzotta C., Traversi C., Caporossi T. Parasurgical therapy for keratoconus by riboflavin-ultraviolet type A rays induced cross-linking of corneal collagen: preliminary refractive results in an Italian study. J Cataract Refract Surg. 2006;32:837–845. doi: 10.1016/j.jcrs.2006.01.091. [DOI] [PubMed] [Google Scholar]
- 22.Fledelius H.C., Goldschmidt E. The highly myopic eye – oculometric considerations. Acta Clin Croat. 2012;51:123–126. [PubMed] [Google Scholar]
- 23.Rosa N., Lanza M., Capasso L., Lucci M., Polito B., Romano A. Anterior chamber depth measurement before and after photorefractive keratectomy: comparison between IOL master and Orbscan II. Ophthalmology. 2006;113:962–969. doi: 10.1016/j.ophtha.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 24.De Bernardo M., Rosa N. Diehl-Miller nomogram for intraocular lens power calculation. J Cataract Refract Surg. 2013;39:1791. doi: 10.1016/j.jcrs.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 25.Goldich Y., Barkana Y., Morad Y., Hartstein M., Avni I., Zadok D. Can we measure corneal biomechanical changes after collagen cross-linking in eyes with keratoconus?. a pilot study. Cornea. 2009;28:498–502. doi: 10.1097/ICO.0b013e318190734d. [DOI] [PubMed] [Google Scholar]
- 26.Vinciguerra P., Albè E., Mahmoud A.M., Trazza S., Hafezi F., Roberts C.J. Intra- and postoperative variation in ocular response analyzer parameters in keratoconic eyes after corneal cross-linking. J Refract Surg. 2010;26:669–676. doi: 10.3928/1081597X-20100331-01. [DOI] [PubMed] [Google Scholar]
- 27.Spoerl E., Terai N., Scholz F., Raiskup F., Pillunat L.E. Detection of biomechanical changes after corneal cross-linking using Ocular Response Analyzer software. J Refract Surg. 2011;27:452–457. doi: 10.3928/1081597X-20110106-01. [DOI] [PubMed] [Google Scholar]
- 28.Greenstein S.A., Fry K.L., Hersh P.S. In vivo biomechanical changes after corneal collagen cross-linking for keratoconus and corneal ectasia: 1-year analysis of a randomized, controlled, clinical trial. Cornea. 2012;31:21–25. doi: 10.1097/ICO.0b013e31821eea66. [DOI] [PubMed] [Google Scholar]
- 29.Gkika M., Labiris G., Giarmoukakis A., Koutsogianni A., Kozobolis V. Evaluation of corneal hysteresis and corneal resistance factor after corneal cross-linking for keratoconus. Graefes Arch Clin Exp Ophthalmol. 2012;250:565–573. doi: 10.1007/s00417-011-1897-0. [DOI] [PubMed] [Google Scholar]
- 30.Wittig-Silva C., Chan E., Islam F.M., Wu T., Whiting M., Snibson G.R. Controlled trial of corneal collagen cross-linking in progressive keratoconus: three-year results. Ophthalmology. 2014;121:812–821. doi: 10.1016/j.ophtha.2013.10.028. [DOI] [PubMed] [Google Scholar]


