Abstract
AIM: To enhance the radiosensitivity of human colon cancer cells by docetaxel.
METHODS: Immunoliposomal docetaxel was prepared by coupling monoclonal antibody against carcinoembryonic antigen to cyanuric chloride at the PEG terminus of liposome. LoVo adenocarcinoma cell line was treated with immunoliposomal docetaxel or/and irradiation. MTT colorimetric assay was used to estimate cytotoxicity of immunoliposomal docetaxel and radiotoxicity. Cell cycle redistribution and apoptosis were determined with flow cytometry. Survivin expression in LoVo cells was verified by immunohistochemistry. D801 morphologic analysis system was used to semi-quantify immunohistochemical staining of survivin.
RESULTS: Cytotoxicity was induced by immunoliposomal docetaxel alone in a dose-dependent manner. Immunoli-posomal docetaxel yielded a cytotoxicity effect at a low dose of 2 nmol/L. With a single dose irradiation, the relative surviving fraction of LoVo cells showed a dose-dependent response, but there were no significant changes as radiation delivered from 4 to 8 Gy. Compared with liposomal docetaxel or single dose irradiation, strongly radiopotentiating effects of immunoliposomal docetaxel on LoVo cells were observed. A low dose of immunoliposomal docetaxel could yield sufficient radiosensitivity. Immunoliposomal docetaxel were achieved both specificity of the conjugated antibody and drug radiosensitization. Combined with radiation, immunoliposomal docetaxel significantly increased the percentage of G2/M cells and induced apoptosis, but significantly decreased the percentage of cells in G2/G1 and S phase by comparison with liposomal docetaxel. Immunohistochemical analysis showed that the brown stained survivin was mainly in cytoplasm of LoVo cells. Semi-quantitative analysis of the survivin immunostaining showed that the expression of survivin in LoVo cells under irradiation with immunoliposomal docetaxel was significantly decreased.
CONCLUSION: Immunoliposomal docetaxel is strongly effective for target radiosensitation in LoVo colon carcinoma cells, and may offer the potential to improve local radiotherapy.
Keywords: Radiosensitivity, Human colon cancer cell, Docetaxel, Immunoliposomes
INTRODUCTION
Colorectal cancer is one of the most common malignant diseases in the world[1-3]. In the treatment of colorectal cancer, local recurrence is a major problem. To improve the overall survival and reduce the local recurrence rate after surgery, additional radiotherapy is given either preoperatively or postoperatively[4-7]. Docetaxel has substantial radiosensitizing properties for malignant tumors. It inhibits microtubule disassembly and interferes with mitotic spindle function to block cells at G2/M, the most radiosensitive phase of cell cycle[8,9].
Conventional radiosensitizers alone are limited by side effects due to their poor selectivity for tumors. Monoclonal antibody specific tumor antigen makes them possible to enhance the selectivity by a targeted delivery approach[10,11]. Radiation induces apoptosis of neoplastic cells. The extent of radiation-induced apoptosis correlates closely with radiosensitivity[12,13]. Apoptosis is regulated by activators and inhibitors in signal transduction pathways. In this study, we attempted to investigate the radiosensitization of colon carcinomas targeting with docetaxel liposome conjugated antibody specific for carcinoembryonic antigen (CEA). The aim of this study was to selectively deliver of docetaxel and to enhance the radiation sensitivity of human colon cancer cells. In order to increase radiotherapy effects on local tumors and to improve the quality of life and overall survival, radiosensitizers should be delivered into the tumor.
This workshop considered a new approach by use of targeted radiosensitizers to improve radiotherapy.
MATERIALS AND METHODS
Chemical and biological materials
Cholesterol, and soy phosphatidylcholine were purchased from Shanghai Chemical Agent company. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) , cyanuric chloride, O-(2-aminoethyl) polyethylene glycol 3000, N,N-diisopropylethylamine, dimethyl sulfoxide (DMSO), 1.2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE) were purchased from Sigma. Docetaxel was kindly supplied by Jiangsu Hengrui Medicine Co. Monoclonal antibody specific for CEA was purchased from Beijing Zhongshan Biotechnology Co. Immunocyt-ochemical detection kit was from Wuhan Boster Biological Technology Co.
Preparation of immunoliposomal docetaxel
Immunoliposomal docetaxel was prepared according to the method described by Bendas[14-17] with minor modifications. Briefly, a 2-fold molar of N, N-diisopropylethylamine and a 3-fold molar cyanuric chloride were added to chloroform solution of DPPE, mixed for 12 h, then DPPE-cyanuric chloride was isolated. DPPE-PEG-OH was prepared by adding a 1-fold molar aminopolyethylene glycol 3000 and a 2-fold molar of N,N-diisopropylethylamine to DPPE-cyanuric chloride. PEG terminus was activated by reaction with 1-fold molar of cyanuric chloride in addition to a 2-fold molar of N,N-diisopropylethylamine. DPPE-cyanuric chloride (cyanur-PEG-PE) was synthesized.
Liposomal docetaxel was prepared with soy phosphati-dylcholine cholesterol 2:1 (molar ratio), cyanur-PEG-PE (0.5 mmoL), docetaxel (1 µmoL). Multilamellar vesicles were extruded through a 200 nm polycarbonate membrane. Antibody conjugated with docetaxel liposomes: anti-CEA-antibody molar ratio of 1 000:1 was prepared in 0.15 mol/L NaCl (pH 8.8). Unbound antibodies were separated by gel permeation chromatography using Sepharose 4B.
Cell culture
LoVo cells, the CEA-expressing human colon adenocarcinoma cell line, were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal calf serum, 100 μg/mL streptomycin, 100 units/mL penicillin. The population doubling time was approximately 30 h. LoVo cells were grown at 37°C in a humidified atmosphere of 50 mL/L CO2.
Treatment with immunoliposomal docetaxel and irradiation
LoVo cells (5 × 103) were plated onto 96-well plates or 6-well plates. Before the administration of appropriate doses of immunoliposomal docetaxel or radiation, cells were plated for 24 h to allow attachment. LoVo cells were incubated with different concentrations of immunoliposomal docetaxel or liposomal docetaxel as equivalent docetaxel doses of 1 nmol/L, 2 nmol/L, 10 nmol/L, 100 nmol/L and 1 000 nmol/L for 2 h at 37°C. After washing thrice with culture medium, the cells with or without radiation were incubated for 24 h or 48 h at 37°C. Cells were X-irradiated at 0, 2, 4, 6 and 8 Gy respectively . Irradiation was performed with 6-MV photons generated by a Philips SL-75 linear accelerator at a dose rate of 1.3 Gy/min. In the combined treatment modality studies, the treated cells were incubated for 24 h before radiation treatment. All doses were delivered without interruption and with the same focus-target distance.
MTT assay
LoVo cells were treated with various concentrations of immunoliposomal docetaxel, and some were exposed to radiation. To measure cell proliferation, the colorimetric method based on MTT assay was used[18,19]. Cells were stained with MTT (50 mg/mL) and incubated for 4 h at 37°C in a humidified atmosphere of 50 mL/L CO2. Then the cells were lysed in DMSO, and the solutions were read in a Bio-Tek spectrophotometer at 570 nm. The production of formazan crystals, and the intensity of color after their dissolution, were proportional to the number of viable cells.
Flow cytometric analysis
At the end of each treatment, LoVo cells were harvested with 0.5 g/L trypsin and 0.2 g/L EDTA. Cells were fixed in 2 mL of 70% cold ethanol at 4°C for 2 h, centrifuged, washed twice with phosphate buffered saline (PBS), and resuspended in 0.5 mL propidium iodide (PI)/RNase A solution. The mixed cells were incubated in the dark at room temperature for 10 min. The fluorescence emission of stained cells was measured with a Becton Dickinson flow cytometer. The number of cells and DNA content were detected by flow cytometry, the fraction of cells in each phase of cell cycle was analyzed. Apoptotic cells were determined by the hypodiploid peak.
Immunohistochemistry
Streptoavidin-biotin peroxidase complex technique was used for staining cells. The treated LoVo cells were harvested and plated on slides. The cells were fixed in 95% aqueous alcohol. The endogenous peroxidase activity was blocked by a 3% solution of hydrogen peroxide in methanol for 30 min. Cells were immunostained using monoclonal antibody against human survivin for 1 h at 37°C. After three further washes with PBS, a second biotinylated rabbit anti-mouse antibody was applied for 1 h at room temperature. Following extensive washes with PBS, color development was demonstrated with diaminobenzidine tetrahydrochloride chromagen. The slides were lightly counterstained with hematoxylin. Stained cells were examined using light microscopy and photographed. JD801 morphologic analysis system for color-image measurement was used to semi-quantify immunohistochemical staining of survivin.
RESULTS
Determination of the cytotoxicity of immunoliposomal docetaxel to LoVo cells
To determine the cytotoxicity of immunoliposomal docetaxel to human colon cancer cells, LoVo cells were treated with various concentrations of immunoliposomal docetaxel for 24 or 48 h. Relative survival data for LoVo cells are shown in Figure 1. Under the treatment with 2 nmol/(L·d) immunoliposomal docetaxel for 24 h, survival was decreased 12% as compared with 1 nmol/L a immunoliposomal docetaxel. The results for Figure 1 also indicate that LoVo cells were more sensitive to immunoliposomal docetaxel treatment in 24 h than for 48 h. The cytotoxicity was induced by immunoliposomal docetaxel alone in a dose-dependent manner. The immunoliposomal docetaxel yielded a cytotoxicity effect at a low dose of 2 nmol/L.
Figure 1.
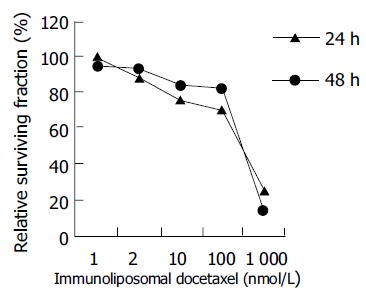
Cytotoxicity of immunoliposomal docetaxel to LoVo cells.
Radiotoxicity to LoVo cells
LoVo cells received a series of test doses of radiation, ranging from 2 to 8 Gy. The radiotoxicity generated is shown in Figure 2. At the doses of 4 to 8 Gy, the reaction to irradiation appeared less pronounced.
Figure 2.

Radiotoxicity to LoVo cells irradiated with single doses.
Target radiopotentiating effect of immunoliposomal docetaxel
To examine whether immunoliposomal docetaxel could target LoVo cells and reveal radiopotentiating effect, these cells were treated with immunoliposomal docetaxel and liposomal docetaxel, respectively, then irradiated at the dose of 2 Gy radiation doses. The control cells were irradiated alone. Using MTT assay, the relative surviving fractions were obtained. The results in Figure 3 showed that immunoliposomal docetaxel was strongly radiopotentiated in LoVo cells, compared with the effects of liposomal docetaxel or single irradiation (P < 0.05). No significant radiopotentiating effects were found after treatment with liposomal docetaxel.
Figure 3.
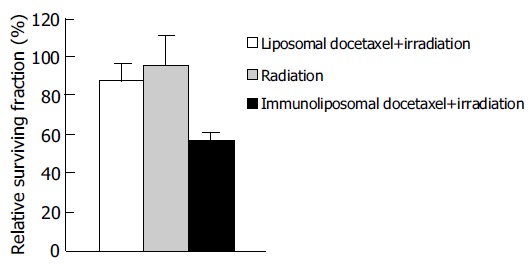
Target radiopotentiating effects of docetaxel immunoliposomes on LoVo cells.
Cell cycle effects
To determine whether immunoliposomal docetaxel in combination with radiation could increase cellular sensitivity to radiation through cell cycle redistribution, we analyzed the LoVo cells by flow cytometry. After treatment with immunoliposomal docetaxel or liposomal docetaxel, all cells were irradiated at 2 Gy. The response of LoVo cell cycle to radiation is shown in Figure 4. Compared to treatment with liposomal docetaxel, the percentage of G2/M cells treated with immunoliposomal docetaxel was significantly increased (P < 0.01), but the percentages of cells both in G2/G1 phase and in S phase were decreased significantly (P < 0.05). Apoptosis was also monitored by flow cytometry (Figure 4). Apoptosis was significantly increased in LoVo cells due to the combined effects of immunoliposomal docetaxel and radiation.
Figure 4.

Combined of effect immunoliposomal docetaxel and radiation on cell cycle distribution and apoptosis.
Immunohistochemical analysis of survivin
Survivin expression in LoVo cells after irradiation and treatment with immunoliposomal docetaxel was verified by immunocytochemistry. Survivin was positively stained with anti-survivin monoclonal antibody. Representative results are shown in Figure 5.
Figure 5.

Expression of survivin in LoVo cells.
Semiquantitative assessment of survivin staining
Positive staining of survivin was mainly present as diffuse cytoplasmic staining with variable intensity. Integral optical density of survivin was detected semiquantitatively by immunohistochemical staining combined with image analysis. For density measurement, color-images were directly analyzed using D801 morphologic analysis system. The semiquantitative data reported here were directly comparable to image analysis data. Survivin expression in LoVo cells after irradiation and treatment with immunoliposomal docetaxel was significantly decreased in comparision to treatment with liposomal docetaxel (P < 0.001, Figure 6).
Figure 6.
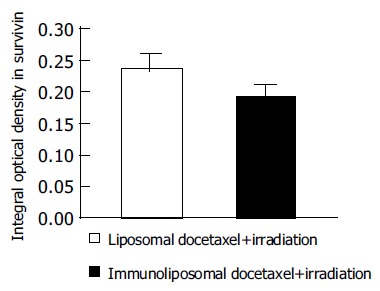
Survivin expression in LoVo cells were determined by quantitative image analysis.
DISCUSSION
Docetaxel plays an important role in the treatment of human malignancies, particularly ovarian and breast cancer[17,18]. It inhibits mitotic progression and induces programmed cell death[19]. For systemic toxicity of docetaxel, the optimal usage is the targeted delivery.
Liposome is used as a potential vector for targeted delivery of radiosensitizers[20]. Liposome is a phospholipid bilayer membrane-bound vesicle capable of encapsulating a wide variety of substances either within their lipid membrane or their central aqueous core. Liposome incorporates polyethylene glycol components and has a prolonged circulation half-life. Liposomal doxorubicin is understanding clinical phase II pilot study in patients with inoperable squamous cell cancer of the head and neck[21]. Maruyama et al[22] introduced a PEG-PE derived lipid with a terminal maleimide group for the reaction with thiolated antibodies. Allen et al[23] synthesized a thiol-reactive PEG anchor for reaction with maleimide-containing antibodies. The terminal coupled antibody shows an increased target binding ability compared with conventional immuno-liposomes[24]. A drawback of these coupling procedures is the need of derivation for the attachment of antibody. Two reagents are needed to activate PEG-derivatives with carboxy groups. In this study, a new liposomal membrane anchor was introduced for the covalent attachment of antibody to liposomes. Anti-CEA-antibody is simply and rapidly coupled to cyanuric chloride at the PEG terminus of liposome without previous derivatization.
Immunoliposomal docetaxel may be an attractive strategy for target radiopotentiation because it can radiosensitize LoVo cells. Docetaxel is targeted by specific anti-CEA-antibody. Our study demonstrate that docetaxel conjugated to a monoclonal antibody specific for CEA tumor-associated antigen could exert efficient and specific cytotoxicity to CEA-expressing LoVo cells. Immuno-liposomal docetaxel alone showed dose-dependent cytotoxicity to LoVo cells. Furthermore, anti-CEA-antibody enhanced the target effects of docetaxel and led to radiosensitization.
Immunoliposomal docetaxel achieved both specificity of the conjugated antibody and drug radiosensitization. Using a low dose of immunoliposomal docetaxel could yield sufficient radiosensitization. Radiation combined with immunoliposomal docetaxel caused a significant increase in the population of G2/M cells. One of the mechanisms may correlate to the effects of docetaxel such as interfering with normal microtubule function, blocking cells in G2/M, and enhancing the sensitivity to apoptosis[25,26]. The presence of arrest in G2/M did not seem to be a sufficient condition to enhance radiation sensitivity. Redistribution of cell cycle due to radiosensitization induced by immunoliposomal docetaxel was observed. Increased apoptosis may have a potential correlation to increased radiosensitivity.
Furthermore, apoptosis may be regulated by a complex balance in signal transduction pathways between apoptosis-activating factors, such as p53 and bax, and antiapoptotic factors, such as the bcl-2 family and inhibitor of apoptosis protein (IAP) family[27]. Survivin, a newly identified member in IAP family, is a strong apoptosis inhibitor. It can be found during fetal development and in colorectal cancer[28]. Expression of survivin may confer a certain degree of radioresistance to rectal cancer cells[29-31]. In the present study, we attempted to find a relationship between the expression of survivin and radiation-induced apoptosis.
Immunohistochemistry examination showed that survivin was mainly in cytoplasm of LoVo cells. Moreover, semiquantitative analysis of the immunohistochemical staining of survivin indicated that a low survivin expression was associated with a significantly higher radiosensitivity after treatment with immunoliposomal docetaxel combined with irradiation. LoVo cells were radiosensitized significantly by decreased expression of survivin. The alteration of survivin expression in LoVo cells may partially explain the enhanced apoptosis and radiation sensitivity.
In conclusion, immunoliposomal docetaxel is strongly effective for target radiosensitizing in LoVo colon carcinoma cells, and may offer the potential to improve local radiotherapy.
Footnotes
Supported by the Department of Science and Technology of Shandong Province
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
References
- 1.Shunyakov L, Ryan CK, Sahasrabudhe DM, Khorana AA. The influence of host response on colorectal cancer prognosis. Clin Colorectal Cancer. 2004;4:38–45. doi: 10.3816/ccc.2004.n.008. [DOI] [PubMed] [Google Scholar]
- 2.Sarid D, Wigler N, Gutkin Z, Merimsky O, Leider-Trejo L, Ron IG. Cutaneous and subcutaneous metastases of rectal cancer. Int J Clin Oncol. 2004;9:202–205. doi: 10.1007/s10147-004-0389-1. [DOI] [PubMed] [Google Scholar]
- 3.Munro AJ, Bentley AH. Deprivation, comorbidity and survival in a cohort of patients with colorectal cancer. Eur J Cancer Care (Engl) 2004;13:254–262. doi: 10.1111/j.1365-2354.2004.00480.x. [DOI] [PubMed] [Google Scholar]
- 4.Hahnloser D, Haddock MG, Nelson H. Intraoperative radiotherapy in the multimodality approach to colorectal cancer. Surg Oncol Clin N Am. 2003;12:993–1013, ix. doi: 10.1016/s1055-3207(03)00091-7. [DOI] [PubMed] [Google Scholar]
- 5.Höcht S, Hammad R, Thiel HJ, Wiegel T, Siegmann A, Willner J, Wust P, Herrmann T, Eble M, Flentje M, et al. Recurrent rectal cancer within the pelvis. A multicenter analysis of 123 patients and recommendations for adjuvant radiotherapy. Strahlenther Onkol. 2004;180:15–20. doi: 10.1007/s00066-004-1130-8. [DOI] [PubMed] [Google Scholar]
- 6.Souglakos J, Androulakis N, Mavroudis D, Kourousis C, Kakolyris S, Vardakis N, Kalbakis K, Pallis A, Ardavanis A, Varveris C, et al. Multicenter dose-finding study of concurrent capecitabine and radiotherapy as adjuvant treatment for operable rectal cancer. Int J Radiat Oncol Biol Phys. 2003;56:1284–1287. doi: 10.1016/s0360-3016(03)00275-x. [DOI] [PubMed] [Google Scholar]
- 7.Horgan AF, Finlay IG. Preoperative staging of rectal cancer allows selection of patients for preoperative radiotherapy. Br J Surg. 2000;87:575–579. doi: 10.1046/j.1365-2168.2000.01396.x. [DOI] [PubMed] [Google Scholar]
- 8.Onishi H, Kuriyama K, Yamaguchi M, Komiyama T, Tanaka S, Araki T, Nishikawa K, Ishihara H. Concurrent two-dimensional radiotherapy and weekly docetaxel in the treatment of stage III non-small cell lung cancer: a good local response but no good survival due to radiation pneumonitis. Lung Cancer. 2003;40:79–84. doi: 10.1016/s0169-5002(02)00532-9. [DOI] [PubMed] [Google Scholar]
- 9.Mason KA, Hunter NR, Milas M, Abbruzzese JL, Milas L. Docetaxel enhances tumor radioresponse in vivo. Clin Cancer Res. 1997;3:2431–2438. [PubMed] [Google Scholar]
- 10.Fernando NH, Hurwitz HI. Targeted therapy of colorectal cancer: clinical experience with bevacizumab. Oncologist. 2004;9 Suppl 1:11–18. doi: 10.1634/theoncologist.9-suppl_1-11. [DOI] [PubMed] [Google Scholar]
- 11.Veronese ML, O'Dwyer PJ. Monoclonal antibodies in the treatment of colorectal cancer. Eur J Cancer. 2004;40:1292–1301. doi: 10.1016/j.ejca.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Vávrová J, Rezácová M, Vokurková D, Psutka J. Cell cycle alteration, apoptosis and response of leukemic cell lines to gamma radiation with high- and low-dose rate. Physiol Res. 2004;53:335–342. [PubMed] [Google Scholar]
- 13.Belka C, Jendrossek V, Pruschy M, Vink S, Verheij M, Budach W. Apoptosis-modulating agents in combination with radiotherapy-current status and outlook. Int J Radiat Oncol Biol Phys. 2004;58:542–554. doi: 10.1016/j.ijrobp.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 14.Bendas G, Krause A, Bakowsky U, Vogel J, Rothe U. Targetability of novel immunoliposomes prepared by a new antibody conjugation technique. Int J Pharm. 1999;181:79–93. doi: 10.1016/s0378-5173(99)00002-2. [DOI] [PubMed] [Google Scholar]
- 15.Rossi L, Corvò R. Retinoic acid modulates the radiosensitivity of head-and-neck squamous carcinoma cells grown in collagen gel. Int J Radiat Oncol Biol Phys. 2002;53:1319–1327. doi: 10.1016/s0360-3016(02)02865-1. [DOI] [PubMed] [Google Scholar]
- 16.Raju U, Nakata E, Yang P, Newman RA, Ang KK, Milas L. In vitro enhancement of tumor cell radiosensitivity by a selective inhibitor of cyclooxygenase-2 enzyme: mechanistic considerations. Int J Radiat Oncol Biol Phys. 2002;54:886–894. doi: 10.1016/s0360-3016(02)03023-7. [DOI] [PubMed] [Google Scholar]
- 17.Ray-Coquard I. [Docetaxel and ovarian cancer] Bull Cancer. 2004;91:159–165. [PubMed] [Google Scholar]
- 18.Yardley DA. Gemcitabine and docetaxel in metastatic and neoadjuvant treatment of breast cancer. Semin Oncol. 2004;31:37–44. doi: 10.1053/j.seminoncol.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 19.Mani S, Macapinlac M, Goel S, Verdier-Pinard D, Fojo T, Rothenberg M, Colevas D. The clinical development of new mitotic inhibitors that stabilize the microtubule. Anticancer Drugs. 2004;15:553–558. doi: 10.1097/01.cad.0000131681.21637.b2. [DOI] [PubMed] [Google Scholar]
- 20.Harrington KJ, Rowlinson-Busza G, Syrigos KN, Vile RG, Uster PS, Peters AM, Stewart JS. Pegylated liposome-encapsulated doxorubicin and cisplatin enhance the effect of radiotherapy in a tumor xenograft model. Clin Cancer Res. 2000;6:4939–4949. [PubMed] [Google Scholar]
- 21.Koukourakis MI, Koukouraki S, Giatromanolaki A, Archimandritis SC, Skarlatos J, Beroukas K, Bizakis JG, Retalis G, Karkavitsas N, Helidonis ES. Liposomal doxorubicin and conventionally fractionated radiotherapy in the treatment of locally advanced non-small-cell lung cancer and head and neck cancer. J Clin Oncol. 1999;17:3512–3521. doi: 10.1200/JCO.1999.17.11.3512. [DOI] [PubMed] [Google Scholar]
- 22.Maruyama K, Takizawa T, Yuda T, Kennel SJ, Huang L, Iwatsuru M. Targetability of novel immunoliposomes modified with amphipathic poly(ethylene glycol)s conjugated at their distal terminals to monoclonal antibodies. Biochim Biophys Acta. 1995;1234:74–80. doi: 10.1016/0005-2736(94)00263-o. [DOI] [PubMed] [Google Scholar]
- 23.Allen TM, Brandeis E, Hansen CB, Kao GY, Zalipsky S. A new strategy for attachment of antibodies to sterically stabilized liposomes resulting in efficient targeting to cancer cells. Biochim Biophys Acta. 1995;1237:99–108. doi: 10.1016/0005-2736(95)00085-h. [DOI] [PubMed] [Google Scholar]
- 24.Bendas G. Immunoliposomes: a promising approach to targeting cancer therapy. BioDrugs. 2001;15:215–224. doi: 10.2165/00063030-200115040-00002. [DOI] [PubMed] [Google Scholar]
- 25.Berchem GJ, Bosseler M, Mine N, Avalosse B. Nanomolar range docetaxel treatment sensitizes MCF-7 cells to chemotherapy induced apoptosis, induces G2M arrest and phosphorylates bcl-2. Anticancer Res. 1999;19:535–540. [PubMed] [Google Scholar]
- 26.Strunz AM, Peschke P, Waldeck W, Ehemann V, Kissel M, Debus J. Preferential radiosensitization in p53-mutated human tumour cell lines by pentoxifylline-mediated disruption of the G2/M checkpoint control. Int J Radiat Biol. 2002;78:721–732. doi: 10.1080/09553000210141667. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Yu X. Regulation of apoptosis: the ubiquitous way. FASEB J. 2003;17:790–799. doi: 10.1096/fj.02-0654rev. [DOI] [PubMed] [Google Scholar]
- 28.Kim PJ, Plescia J, Clevers H, Fearon ER, Altieri DC. Survivin and molecular pathogenesis of colorectal cancer. Lancet. 2003;362:205–209. doi: 10.1016/S0140-6736(03)13910-4. [DOI] [PubMed] [Google Scholar]
- 29.Asanuma K, Moriai R, Yajima T, Yagihashi A, Yamada M, Kobayashi D, Watanabe N. Survivin as a radioresistance factor in pancreatic cancer. Jpn J Cancer Res. 2000;91:1204–1209. doi: 10.1111/j.1349-7006.2000.tb00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodel C, Haas J, Groth A, Grabenbauer GG, Sauer R, Rodel F. Spontaneous and radiation-induced apoptosis in colorectal carcinoma cells with different intrinsic radiosensitivities: survivin as a radioresistance factor. Int J Radiat Oncol Biol Phys. 2003;55:1341–1347. doi: 10.1016/s0360-3016(02)04618-7. [DOI] [PubMed] [Google Scholar]
- 31.Lu B, Mu Y, Cao C, Zeng F, Schneider S, Tan J, Price J, Chen J, Freeman M, Hallahan DE. Survivin as a therapeutic target for radiation sensitization in lung cancer. Cancer Res. 2004;64:2840–2845. doi: 10.1158/0008-5472.can-03-3547. [DOI] [PubMed] [Google Scholar]


