Abstract
AIM: This report gives a comprehensive overview of ultrasonography of splenic abnormalities. Certain ultrasonic features are also discussed with pathologic correlation.
METHODS: We review the typical ultrasonic characteristics of a wide range of splenic lesions, illustrating them with images obtained in our institution from 2000 to 2003. One hundred and three patients (47 men, 56 women), with a mean age of 54 years (range 9-92 years), were found to have an abnormal ultrasonic pattern of spleen.
RESULTS: We describe the ultrasonic features of various splenic lesions such as accessory spleen, splenomegaly, cysts, cavernous hemangiomas, lymphomas, abscesses, metastatic tumors, splenic infarctions, hematomas, and rupture, based on traditional gray-scale and color Doppler sonography.
CONCLUSION: Ultrasound is a widely available, noninvasive, and useful means of diagnosing splenic abnormalities. A combination of ultrasonic characteristics and clinical data may provide an accurate diagnosis. If the US appearance alone is not enough, US may also be used to guide biopsy of suspicious lesions.
Keywords: Ultrasonography, Ultrasound, Splenic abnormalities, Spleen
INTRODUCTION
By structure and function, the spleen resembles two separate organs combined in one. On the one hand, it is an organ of the immune system, with the white pulp involved in the maturation of lymphocytes and plasma cells and in the generation of antibodies. On the other had, it has a phagocytic role, with the red pulp removing particulate matter from the blood and participating in the destruction of senescent red blood cells. In early life the red pulp also plays a role in hematopoiesis and the storage and sequestration of blood. The length of the spleen is variable, averaging 11 cm[1]. The weight varies from 50 to 300 g, with an average of 150 g. The normal adult spleen decreases in size with age[2]. The normal appearance of the parenchyma on ultrasound (US) is very homogeneous and uniform, with an echogenicity slightly greater than that of normal hepatic parenchyma. In comparison with other solid abdominal organs, the spleen is relatively rarely the primary site affected by disease. By contrast to the widely studied lesions of the liver, the US appearance of disease in the spleen is rather nonspecific. An understanding of the history and associated symptoms may help narrow the differential diagnosis. The ability of US to detect focal or diffuse splenic lesions depends on numerous factors: spleen size; size, number, and echogenicity of focal lesions; and ancillary adjacent findings.
MATERIALS AND METHODS
Ultrasonographic data of abdominal imaging with a diagnosis of splenic abnormalities from 2000 to 2003 at Mackay Memorial Hospital were reviewed retrospectively. In this period, 103 patients (47 men, 56 women), with a mean age of 54 years (range 9-92 years), were found to have an abnormal US pattern of spleen (exclusion of splenomegaly in patients with cirrhosis and thalassemia) from about 100 000 US evaluations. The indications for patient to undergo abdominal US were abdominal pain referred from emergency room in 13% of evaluations, abdominal relating symptoms referred from ordinary wards or intensive care units in 11% of evaluations, screening of viral hepatitis, abnormal liver function, liver cirrhosis, hepatoma or other malignancy referred from out-patient departments in 76% of evaluations. The spleen was scanned by a real time ultrasound using a 3.5 to 6 mHz transducer and color Doppler imaging was applied in some cases. The final diagnosis of each lesion in our patients was determined by pathologic, bacteriology examination or clinical history and serial imaging study.
RESULTS
These 103 splenic abnormalities were accessory spleen in 5 patients, true cysts in 22 and pseudocysts in 9 patients, splenic calcification and Gamna-Gandy bodies in 10 patients, cavernous hemangiomas in 15 patients, abscesses is 8 patients, lymphomas in 8 patients, metastatic tumors in 5 patients, splenic infarctions in 10 patients, hematomas and rupture in 11 patients.
Diseases of the spleen
Congenital abnormalities Some congenital anomalies of the spleen are common, such as splenic lobulation and accessory spleen, while other conditions are rare, such as wandering spleen[3] and polysplenia[4]. Failure of fusion of splenic tissue results in the formation of an accessory spleen. US appearances usually present as a homogenous, less than 4 cm round contour near the hilum and an echogenicity identical to that of adjacent spleen. Pathologic processes affecting the spleen also affect the accessory spleen, indicating that they have the same developmental origin (Figure 1). An intrapancreatic or intrahepatic accessory spleen is a homogenous mass in the parenchyma of the pancreas or liver that may mimic a neoplastic lesion[5,6]. As it poses no danger, accurate diagnosis is necessary to avoid unnecessary treatment. The diagnosis of ectopic splenic tissue is best made by technetium-99m colloid scintigraphy[7].
Figure 1.
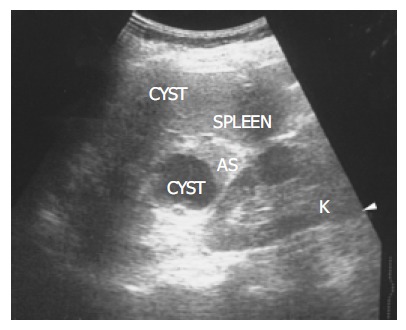
There is a homogenous, round contour near the hilum, identified as an accessory spleen (AS) in a 62-year-old female. One anechoic cyst is noted within the accessory spleen. The pathologic process affecting the spleen (small cyst) also affects the accessory spleen (large cyst).
Splenomegaly and portal hypertension As the spleen is an irregularly shaped organ, no completely satisfactory technique has been developed to measure the volume accurately. Estimating the volume with the formula 0.524 × W × T × (ML + CCL)/2 (where ML = maximum length, W = width, T = thickness, and CCL = craniocaudal length) provides better overall accuracy[8]. The differential diagnosis for splenomegaly includes infection, portal hypertension, storage disorders, blood dyscrasias, and neoplasm. Mild splenomegaly can be seen in infections and portal hypertension. Moderate splenomegaly suggests leukemia, lymphoma, or infectious mononucleosis. Massive splenomegaly is seen in myelofibrosis or chronic myeloid leukemia. The presence of portosystemic collateral vessels, ascites, and cirrhosis of the liver indicates portal hypertension[9]. The presence of space-occupying lesions within an enlarged spleen suggests lymphoma, metastases, or abscesses.
Splenic calcification and Gamna-Gandy bodies Foci of hemosiderin and calcium deposits in the splenic parenchyma secondary to intraparenchymal hemorrhage are called Gamna-Gandy bodies (Figure 2). They are commonly seen in patients with liver cirrhosis and portal hypertension and are also found in patients with splenic vein thrombosis, hemolytic anemia, hemochromatosis, or trauma[10]. Echogenic foci with acoustic shadowing indicating calcification are found in chronic granulomatous infections such as tuberculosis, harmatomas, sickle cell disease[11] and trauma.
Figure 2.
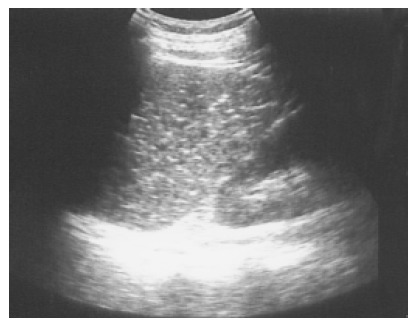
Multiple tiny calcified spots involving almost the entire spleen are found in a 58-year-old female with liver cirrhosis, called Gamna-Gandy bodies.
Splenic cysts Splenic cysts are rarely seen, but they are still probably the commonest splenic lesions. They are much less common than those arising in the kidney, liver, or ovary. They can be true cysts, pseudocysts, or hydatid cysts. The true cyst, also known as an epidermoid cyst, is defined by the presence of an inner endothelial lining. Pseudocysts that lack such a lining are usually secondary to old trauma, a resolved infection, or infarction. Hydatid cysts are formed by the larval stage of the dog tapeworm Echinococcus granulosus. Simple cysts pose less diagnostic problems if they have the classic ultrasonic features of being anechoic and thin-walled with posterior echo enhancement. Splenic cysts usually are located completely within the spleen, thus differing from hepatic or renal cysts which may have an exophytic component. Infection or hemorrhage may cause debris and echogenic contents within thick-walled pseudocysts (Figure 3). Brightly foci with acoustic shadow due to calcification within the wall is also a correlative US feature to pseudocysts. A rare complication of pancreatic pseudocyst may be erosion into the adjacent spleen, where it mimics a huge simple splenic cyst (Figure 4). Rupture of an intrasplenic pancreatic pseudocyst can result in massive hemoperitoneum. Miele stated that internal septa are more frequent in true cysts while parietal calcifications are typical of pseudocysts[12]. However, the final diagnosis is made histologically.
Figure 3.
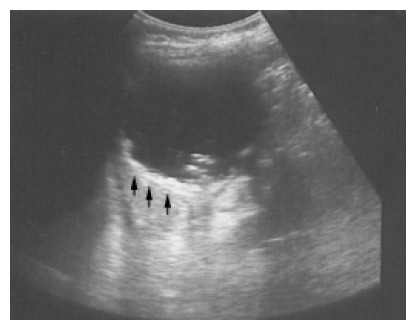
A pseudocyst cyst of the spleen after trauma reveals debris or echogenic contents within the thick wall (arrows) in a 60-year-old female.
Figure 4.
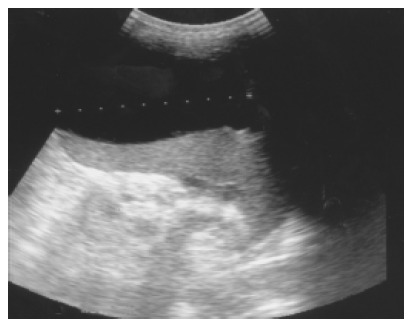
Pancreatic pseudocyst eroding into the adjacent spleen and mimicking a huge splenic simple cyst was noted after an episode of acute alcoholic pancreatitis in a 32-year-old male patient.
Splenic abscess A splenic abscess is a collection of pus, most commonly caused by hematogenous spread of infection from elsewhere. Intravenous drug abusers are predominantly affected. Other causes include penetrating wounds or complications of a hematoma resulting from infarction or trauma. A pyogenic abscess is typically hypoechoic and often has thick, irregular walls. Other findings include gas, progressive enlargement of the lesion, subcapsular extension and collections of extracapsular fluid[13]. If gas has formed within the abscess, hyperechogenicity with distal dirty shadowing can be seen. Through transmission indicates the cystic nature of an abscess even when it contains echogenic material. Color Doppler examination may reveal hypervascularity in the thick wall. The clinical triad suggestive of splenic abscess consists of fever, left upper quadrant pain, and leukocytosis. The typical triad was seen in 44% of patients in one series of 34 cases[14]. Although splenic abscess is rare, there is a high mortality if diagnosis and treatment are delayed. Percutaneous drainage of a single abscess and splenectomy for multiple abscesses are recommended. Fine-needle aspiration is useful when an abscess is suspected, as this may confirm the diagnosis as well allowing culture of the pathogen. In the series noted above, multiple or gas-containing abscesses had a poor prognosis[14].
Hemangioma A hemangioma is characterized by a proliferation of blood-filled spaces lined and separated by endothelium. Cavernous hemangiomas are the most common solid benign lesion seen in the spleen and are found incidentally. The US appearance varies widely from a predominately solid to mixed to a pure cystic lesion. Hemangiomas usually have a periphery and a hypoechoic center with a through transmission character similar to cavernous hemangioma of the liver. Color Doppler may show blood flow within the solid portions. Atypical features are commoner in larger lesions and include heterogeneous echogenicity with hypoechoic areas due to necrosis, hemorrhage, and thrombosis (Figure 5). Rarely, hemangiomas may be large or multiple and can involve the whole spleen (Figure 6). The most frequent complications are rupture and bleeding. Although up to 14% of patients in autopsy series have been reported to have splenic hemangiomas[2], but they are less frequently identified on imaging.
Figure 5.
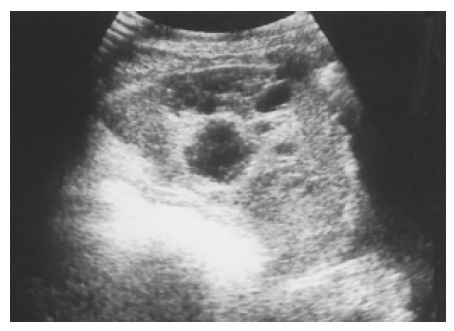
An atypical hemangioma can have mixed echogenicity with a dominant cystic portion. Mild through transmission points to the cystic nature of the hemangioma.
Figure 6.
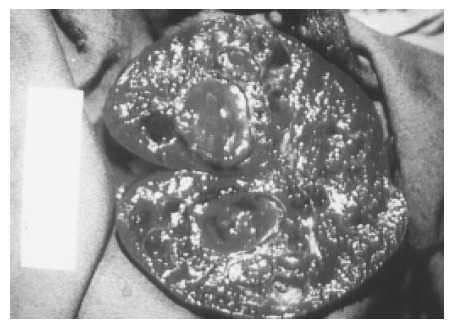
Large and multiple hemangiomas occupy the entire spleen. The hypoechoic areas shown in Figure 5 are filled with blood clots and thrombosis. The 48-year-old male received splenectomy due to LUQ pain caused by venous thrombosis in spleen.
Lymphoma Primary malignant tumors of the spleen are uncommon, with primary lymphoma and angiosarcoma being the most frequently reported. Splenomegaly is a frequent finding in lymphoma, but a normal sized spleen does not exclude the diagnosis[15]. The US patterns correspond to the three macroscopic morphologies: (1) infiltrative and diffuse, (2) miliary and nodular, and (3) focal and cyst-like (Figure 7)[16]. In cyst-like lymphoma, the appearance of a distinct boundary is an important clue in distinguishing lymphoma from cyst[17]. Indistinct boundary echo pattern indicated splenic lymphomas. Hodgkin and non-Hodgkin lymphoma can not be distinguished by the US appearance.
Figure 7.
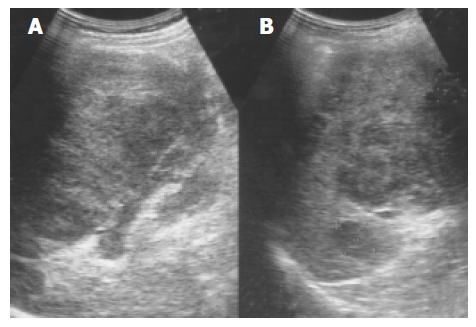
An enlarged spleen (A) demonstrates a diffusely coarse echotexture with several mixed echoic lesions (B) in a 58-year-old male patient.
Metastatic tumor Metastatic involvement of the spleen is very uncommon, usually seen in patients with widespread, terminal malignant disease. The most frequent metastases arise from lymphoma and melanoma, followed by carcinoma of the ovary, breast, lung, and stomach in decreasing order of frequency[2]. Target lesions with a hypoechoic halo suggest metastasis (Figure 8). This appearance is said to be a sign of aggressive behavior, but other authors have postulated that it is due to necrosis or hemorrhage. Miliary tuberculosis, atypical mycobacteria, and pneumocystis carinii, especially in immunocompromised patients, can also result in multiple hypoechoic focal lesions[18]. These should be differentiated from splenic metastases.
Figure 8.
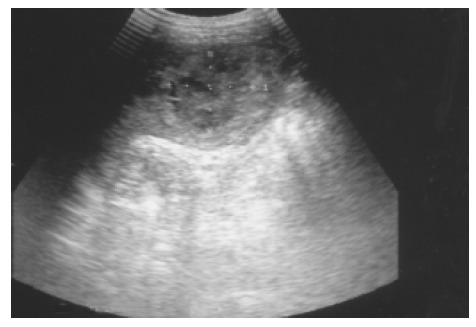
Splenic metastasis from a lung carcinoma in a 68-year-old male is seen as a hypoechoic mass with a target sign.
Splenic infarction Infarction can result from either occlusion of the splenic artery or venous thrombosis in the splenic sinusoids. Splenic infarction is typically seen in patients prone to embolic phenomena or, more recently, as a complication of transcatheter arterial embolization of a hepatoma. In splenic vein thrombosis, the entire spleen may be involved, resulting in massive splenomegaly. US typically show a peripheral, wedge-shaped region of hypoechogenicity (Figure 9). Color Doppler imaging may show a lack of perfusion; however, the presence of flow within a lesion does not exclude the diagnosis because the embolus may subsequently lyse. Splenic infarcts may initially be large and then become small and echogenic as fibrosis occurs.
Figure 9.
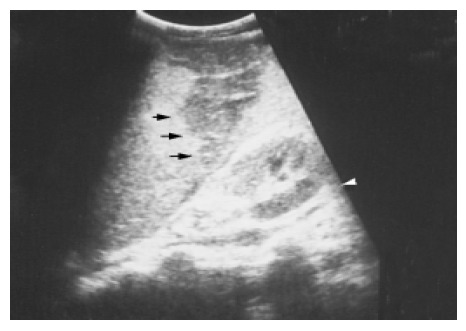
A peripheral wedge-shaped hypoechoic region (arrows) was noted at the upper pole of spleen from an infarction after transcatheter arterial embolization in a 45-year-old male with hepatoma.
Hematoma and rupture The spleen, liver, and kidney are the three intraperitoneal organs most commonly injured by blunt trauma. If the capsule of the injured spleen remains intact, an intraparenchymal or subcapsular hematoma (Figure 10) may result. Echogenicity of a hematoma depends on the stage at which the scan was performed. Fresh blood is liquid and initially echo-free but over the course of several days becomes more echogenic and thus more difficult to identify. Free fluid in the left upper quadrant is strongly suggestive of splenic injury, which must be excluded in such a case[19]. Less frequently, a splenic laceration or rupture is identified as a blood-filled cleft with capsular rupture. (Figures 11 and 12).
Figure 10.
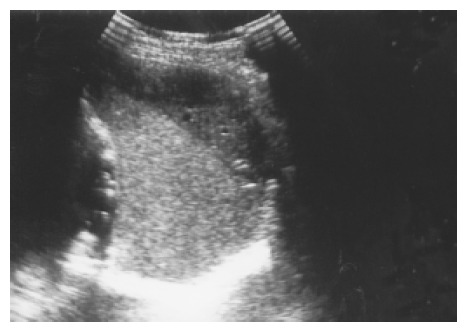
A subcapsular crescent-shaped hypoechoic lesion is noted at the upper pole of spleen.
Figure 11.
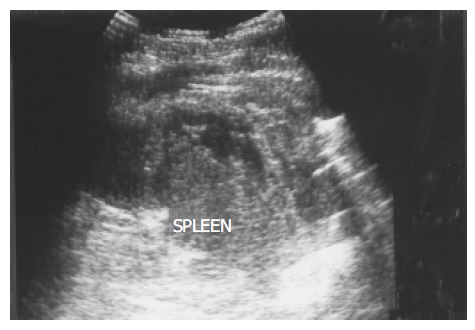
Several linear hypoechoic foci and a subcapsular fluid accumulation are noted in a spleen. Loss of the normal architecture was seen after a traffic accident in a 38-year-old male.
Figure 12.
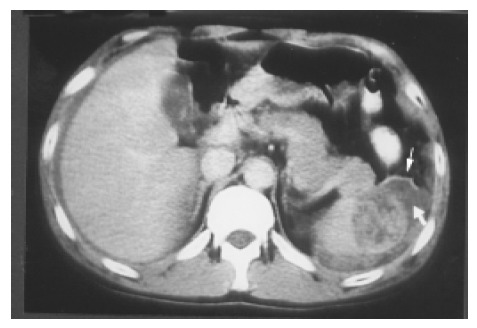
A splenic laceration and rupture (in Figure 11) is identified as a blood-filled cleft (lower arrow) and capsular rupture (upper arrow) on contrast CT.
Biopsy procedures In many cases, pathologic confirmation is necessary to provide a definitive diagnosis. The traditional dry cytologic aspiration is relatively safe but provides only a small sample. Siniluoto et al[20] reported false-negative results for malignancy is about 31% of biopsies using a 22-gauge fine needle aspiration technique. Core-needle biopsies with and 18 or 20-gauge cutting-biopsy needle may increase the diagnostic accuracy by obtaining a sufficiently large specimen. And Katsumi et al[21] reported the safety of this procedure in four cases. All procedures should be carefully planned in advance to avoid hitting the intestine, pancreas, kidneys or pleura as the needle is advanced. Color Doppler imaging helps avoid passing the needle through a large vessel. Endoscopic ultrasound-guided fine needle aspiration may be used in if the lesion is adjacent to the hilum or too small for a CT- or US-guided biopsy. A linear echo endoscope and 22-gauge needles are used to obtain specimens for cytology[22]. To avoid unnecessary morbidity and mortality, laparotomy and splenectomy for pathologic confirmation should be used only when absolutely required.
DISCUSSION
Because splenic abnormalities are relatively rare, the clinician is unlikely to be as familiar with them as with those of the liver. Although some of the experience gained with hepatic US is useful in examining the spleen, there are some differences that must be noted. The classic US finding of posterior echo enhancement suggesting a cyst in the liver or kidney is noted in only 25% of splenic cysts according to Ishida[17] and in our experience (6/22) as well. While the reason for this discrepancy is not known for sure, Ishida suggested it might relate to differences in the acoustic patterns of the parenchyma of the two organs[17]. Splenic cysts are reportedly located completely within the spleen, and we have never found one of 28 cases that extends outside the capsule. Because the spleen is closely surrounded by other organs, it might be difficult for an exophytic cyst to develop. Another difference is that cystic portions occur more commonly and prominently in splenic hemangiomas compared with liver hemangiomas, which are more likely to be solid, in contrast with the variable appearance of hemangiomas in the spleen.
While there have been to differentiate benign from malignant focal lesions in the spleen based on US appearance, the results have been disappointing. There is too much overlap between the echo patterns[23]. Therefore, US should be supplemented with other diagnostic techniques in such cases. However, certain criteria have been proposed that are at least suggestive. For example, malignant lesions are more likely to be multifocal, as with metastatic lesions, or to be diffuse and ill defined because of rapid growth. A soft tissue lesion associated with calcification usually indicates a long term process. Gas in the parenchyma usually originates from bacterial infection and indicates benign disease. Wan et al[24] said that solitary lesions with an anechoic pattern or echogenic foci with gas or calcification are more likely benign, while multifocal or diffuse solid lesions associated with a target sign or extrasplenic abdominal masses are suggestive of malignancy.
Over the past decade, great efforts have been made to improve both tissue harmonic imaging and contrast sonography. The former is a gray-scale imaging technique that uses information from harmonic signals generated by non-linear propagation. This minimizes artifacts from the body, side lobe, and scatter and thus improves the signal-to-noise ratio. Contrast imaging uses gas microbubbles[25], which has dramatically extended the ability of liver US to assess blood flow in a tumor[26]. Preliminary experience with this modality has produced spleen-specific enhancement[27]. Thorelius et al[28] suggested that contrast enhanced sonography might be useful in the detection of lesions in which blood flow is reduced such as infarctions, hematomas, necrotic tissue, and non-vascular cysts in the spleen.
In conclusion, US is a widely available, noninvasive, and useful means of diagnosing splenic abnormalities. A combination of ultrasonic characteristics and clinical data may provide an accurate diagnosis, particularly for such disorders as hemangiomas, splenomegaly secondary to portal hypertension, simple cysts, malignant lesions with a target sign, or lesions associated with other obvious intra-abdominal pathology. In many cases, pathologic confirmation is necessary to make a definitive diagnosis. Fine needle aspiration, core-needle cutting biopsy, and endoscopic ultrasound-guided fine needle aspiration have been developed to assist diagnosis.
Footnotes
Science Editor Li WZ Language Editor Elsevier HK
References
- 1.O'Donohue J, Ng C, Catnach S, Farrant P, Williams R. Diagnostic value of Doppler assessment of the hepatic and portal vessels and ultrasound of the spleen in liver disease. Eur J Gastroenterol Hepatol. 2004;16:147–155. doi: 10.1097/00042737-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Andrews MW. Ultrasound of the spleen. World J Surg. 2000;24:183–187. doi: 10.1007/s002689910031. [DOI] [PubMed] [Google Scholar]
- 3.Berkenblit RG, Mohan S, Bhatt GM, Rosenzweig M, Blitz A. Wandering spleen with torsion: appearance on CT and ultrasound. Abdom Imaging. 1994;19:459–460. doi: 10.1007/BF00206940. [DOI] [PubMed] [Google Scholar]
- 4.Harper L, Michel JL, Hameury F, De Napoli-Cocci S, Udomkaewkanjana P, Gruau M, De Clermont H, Bechonnet G. Interest of laparoscopy in polysplenia syndrome. Eur J Pediatr Surg. 2003;13:417–420. doi: 10.1055/s-2003-44735. [DOI] [PubMed] [Google Scholar]
- 5.Ota T, Ono S. Intrapancreatic accessory spleen: diagnosis using contrast enhanced ultrasound. Br J Radiol. 2004;77:148–149. doi: 10.1259/bjr/56352047. [DOI] [PubMed] [Google Scholar]
- 6.Izzo L, Caputo M, Galati G. Intrahepatic accessory spleen: imaging features. Liver Int. 2004;24:216–217. doi: 10.1111/j.1478-3231.2004.00915.x. [DOI] [PubMed] [Google Scholar]
- 7.Ota T, Kusaka S, Mizuno M. A splenic pseudotumor: an accessory spleen. Ann Nucl Med. 2003;17:159–160. doi: 10.1007/BF02988456. [DOI] [PubMed] [Google Scholar]
- 8.Yetter EM, Acosta KB, Olson MC, Blundell K. Estimating splenic volume: sonographic measurements correlated with helical CT determination. AJR Am J Roentgenol. 2003;181:1615–1620. doi: 10.2214/ajr.181.6.1811615. [DOI] [PubMed] [Google Scholar]
- 9.La Seta F, Patti R, Sciarrino E, Valenza F, Costanzo GS, Tesè L, Lagalla R. Ultrasound, spleen and portal hypertension. Radiol Med. 2004;107:332–343. [PubMed] [Google Scholar]
- 10.Dufour JF, Dinkel HP, Zimmermann A. Image of the month. Gamma-Gandy bodies. Gastroenterology. 2003;125:1010, 1294. doi: 10.1016/s0016-5085(03)01250-2. [DOI] [PubMed] [Google Scholar]
- 11.Senol U, Lüleci E, Keser I, Güzeloglu-Kayisli O, Toraman AD, Lüleci G, Canatan D. Sickle-beta-thalassemia and splenic calcification. Abdom Imaging. 2001;26:557. doi: 10.1007/s00261-001-0062-3. [DOI] [PubMed] [Google Scholar]
- 12.Miele V, Galluzzo M, Cortese A, Bellussi A, Valenti M. [Diagnostic imaging of splenic cysts in children] Radiol Med. 1998;95:62–65. [PubMed] [Google Scholar]
- 13.Ng KK, Lee TY, Wan YL, Tan CF, Lui KW, Cheung YC, Cheng YF. Splenic abscess: diagnosis and management. Hepatogastroenterology. 2002;49:567–571. [PubMed] [Google Scholar]
- 14.Changchien CS, Tsai TL, Hu TH, Chiou SS, Kuo CH. Sonographic patterns of splenic abscess: an analysis of 34 proven cases. Abdom Imaging. 2002;27:739–745. doi: 10.1007/s00261-002-0013-7. [DOI] [PubMed] [Google Scholar]
- 15.Goerg C, Schwerk WB, Goerg K, Havemann K. Sonographic patterns of the affected spleen in malignant lymphoma. J Clin Ultrasound. 1990;18:569–574. doi: 10.1002/jcu.1870180708. [DOI] [PubMed] [Google Scholar]
- 16.Urrutia M, Mergo PJ, Ros LH, Torres GM, Ros PR. Cystic masses of the spleen: radiologic-pathologic correlation. Radiographics. 1996;16:107–129. doi: 10.1148/radiographics.16.1.107. [DOI] [PubMed] [Google Scholar]
- 17.Ishida H, Konno K, Ishida J, Naganuma H, Komatsuda T, Sato M, Watanabe S. Splenic lymphoma: differentiation from splenic cyst with ultrasonography. Abdom Imaging. 2001;26:529–532. doi: 10.1007/s00261-001-0006-y. [DOI] [PubMed] [Google Scholar]
- 18.Schininà V, Rizzi EB, Mazzuoli G, David V, Bibbolino C. US and CT findings in splenic focal lesions in AIDS. Acta Radiol. 2000;41:616–620. doi: 10.1080/028418500127346018. [DOI] [PubMed] [Google Scholar]
- 19.Richards JR, McGahan PJ, Jewell MG, Fukushima LC, McGahan JP. Sonographic patterns of intraperitoneal hemorrhage associated with blunt splenic injury. J Ultrasound Med. 2004;23:387–394, quiz 395-396. doi: 10.7863/jum.2004.23.3.387. [DOI] [PubMed] [Google Scholar]
- 20.Siniluoto T, Päivänsalo M, Tikkakoski T, Apaja-Sarkkinen M. Ultrasound-guided aspiration cytology of the spleen. Acta Radiol. 1992;33:137–139. [PubMed] [Google Scholar]
- 21.Morita K, Numata K, Tanaka K, Mitsui K, Matsumoto S, Kitamura T, Saito S, Kiba T, Sekihara H. Sonographically guided core-needle biopsy of focal splenic lesions: report of four cases. J Clin Ultrasound. 2000;28:417–424. doi: 10.1002/1097-0096(200010)28:8<417::aid-jcu7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 22.Fritscher-Ravens A, Mylonaki M, Pantes A, Topalidis T, Thonke F, Swain P. Endoscopic ultrasound-guided biopsy for the diagnosis of focal lesions of the spleen. Am J Gastroenterol. 2003;98:1022–1027. doi: 10.1111/j.1572-0241.2003.07399.x. [DOI] [PubMed] [Google Scholar]
- 23.Goerg C, Schwerk WB, Goerg K. Splenic lesions: sonographic patterns, follow-up, differential diagnosis. Eur J Radiol. 1991;13:59–66. doi: 10.1016/0720-048x(91)90058-4. [DOI] [PubMed] [Google Scholar]
- 24.Wan YL, Cheung YC, Lui KW, Tseng JH, Lee TY. Ultrasonographic findings and differentiation of benign and malignant focal splenic lesions. Postgrad Med J. 2000;76:488–493. doi: 10.1136/pmj.76.898.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calliada F, Campani R, Bottinelli O, Bozzini A, Sommaruga MG. Ultrasound contrast agents: basic principles. Eur J Radiol. 1998;27 Suppl 2:S157–S160. doi: 10.1016/s0720-048x(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 26.Wilson SR, Burns PN, Muradali D, Wilson JA, Lai X. Harmonic hepatic US with microbubble contrast agent: initial experience showing improved characterization of hemangioma, hepatocellular carcinoma, and metastasis. Radiology. 2000;215:153–161. doi: 10.1148/radiology.215.1.r00ap08153. [DOI] [PubMed] [Google Scholar]
- 27.Lim AK, Patel N, Eckersley RJ, Taylor-Robinson SD, Cosgrove DO, Blomley MJ. Evidence for spleen-specific uptake of a microbubble contrast agent: a quantitative study in healthy volunteers. Radiology. 2004;231:785–788. doi: 10.1148/radiol.2313030544. [DOI] [PubMed] [Google Scholar]
- 28.Thorelius L. Contrast-enhanced ultrasound for extrahepatic lesions: preliminary experience. Eur J Radiol. 2004;51 Suppl:S31–S38. doi: 10.1016/j.ejrad.2004.03.028. [DOI] [PubMed] [Google Scholar]


