Abstract
We report on a 26-year-old man who presented with severe jaundice and elevated serum liver enzyme activities after having received a dose of Twinrix® . In his past medical history, jaundice or abnormal liver function tests were never recorded. Following admission, an elevated immunoglobulin G level and antinuclear antibodies at a titer of 320 with a homogenous pattern were found. Histology of a liver biopsy showed marked bridging liver fibrosis and a chronic inflam-mation, compatible with autoimmune hepatitis. Treatment was started with budesonide and ursodeoxycholic acid, and led to complete normalization of the pathological liver function tests. We believe that Twinrix® led to an acute exacerbation of an unrecognized autoimmune hepatitis in our patient. The pathogenesis remains to be clarified. It is tempting to speculate that inactivated hepatitis A virus and/or recombinant surface antigen of the hepatitis B virus -as seen in patients with chronic hepatitis C and unrecognized autoimmune hepatitis who were treated with interferon alpha-might have been responsible for disease exacerbation.
Keywords: Twinrix®, Autoimmune hepatitis, Budesonide
INTRODUCTION
Autoimmune hepatitis (AIH) is a chronic immunity-mediated inflammatory liver disease and is characterized by a marked female preponderance, hypergammaglobulinemia, non-tissue specific autoantibodies and a characteristic good response to immunosuppressive treatment. Without treatment AIH progresses to liver cirrhosis, and in 50% of patients advanced liver fibrosis or complete cirrhosis is already present at the time of diagnosis. Patients usually present with variable unspecific symptoms. In a significant number of patients, the presentation of AIH may be acute or fulminant mimicking acute viral hepatitis[1]. We wish to report on a young male patient, who presented with severe acute exacerbation of a previously unrecognized AIH after the third dose of Twinrix® .
CASE REPORT
A 26-year-old Caucasian man presented in August 2003 with severe jaundice associated with elevated serum liver enzyme activities after having received the third dose of Twinrix® . The first dose was given five months ago, in March 2003, and the second in April 2003. The past medical history of the patient was unremarkable and jaundice or abnormal liver function tests were previously unknown. He denied any alcohol intake or drug (ab)use, and had not been exposed to any blood products. No family history of liver diseases was given. The physical examination showed a moderately enlarged liver without stigmata of chronic liver disease. Blood tests showed elevated alanine aminotransferase (ALT) with 50.4 μmol/s.L (reference interval 0.17-0.60 μmol/s.L), aspartate aminotransferase (AST) with 33.2 μmol/s.L (reference interval <0.17-0.60 µmol/s.L), and serum bilirubin with 17.2 mg/dL (reference interval <1.1 mg/dL), while the γ-globulin fraction was with 23.3% only slightly elevated. Full blood count, creatinine, urea and electrolytes were within the normal limits. Serological tests for viral hepatitis, including hepatitis A, B, and C viruses, and human herpes viruses were all negative. There was no evidence of a metabolic liver disease or biliary obstruction. Because serum liver function tests did not improve on follow-up (Figure 1), a percutaneous liver biopsy was taken in November 2003 (see below). A month later the patient was referred to our University Hospital for further evaluation. On admission, serum ALT was raising again (16.40 μmol/s.L). Additionally, we found elevated immunoglobulin G fraction with 22.70 g/L (reference interval 9-16 g/L) and antinuclear antibodies at a titer of 320 with homogenous immunofluorescence pattern suggestive of AIH. The liver biopsy was re-examined using paraffin sections stained with hematoxylin and eosin (H&E), periodic-acid Schiff (PAS) reagent with and without diastase pretreatment, Masson‘s trichrome stain, reticulin stain, and iron stain. Histologically, marked bridging fibrosis was noted (Figure 2A). A moderate chronic inflammatory infiltrate with few scattered eosinophils was found in the septa, an interface hepatitis was noted and small, partly confluent necroinflammatory foci were present in the parenchyma (Figures 2B and C). A pathological iron deposition or diastase-resistant PAS-positive globuli indicating α 1-antitrypsin deficiency, were not found. The histopathological findings were compatible with advanced AIH. The patients was than treated with budesonide (Budenofalk ), 3 mg tiw and ursodeoxycholic acid (Ursofalk ), 250 mg tiw, which resulted in complete normalization of the liver function tests (Figure 1). The excellent response to immunosuppressive therapy further supports the diagnosis of AIH.
Figure 1.
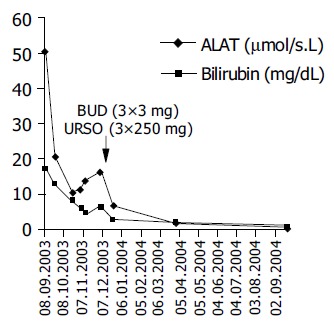
Changes of alanine aminotransferase activity and serum bilirubin level on follow-up and during the therapy with budesonide and ursodeoxycholic acid.
Figure 2.
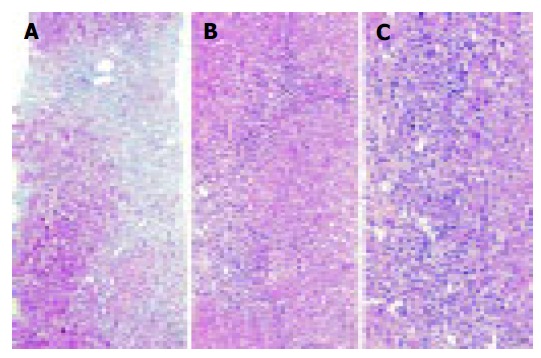
A liver biopsy obtained 3 mo after the first clinical presentation of the patient showed marked bridging fibrosis (A). A moderate chronic inflammatory infiltrate with few scattered eosinophils was found in the septa (B), and an interface hepatitis was also noted (C). The histopathological findings were compatible with AIH. Masson trichrome stain (A) and H&E-stain (B and C). Original magnifications 100x (A and B) and 200x (C).
DISCUSSION
Hepatitis A and B viruses (HAV and HBV) are responsible for considerable morbidity and mortality[2]. Twinrix® is a sterile suspension of inactivated HAV and recombinant surface antigen of the HBV (rHBsAg) and used for active immunization[3]. Vaccination with rHBsAg has been reported to be associated with the development of autoimmune diseases, especially with rheumatoid arthritis[4]. Some patients with vaccination-induced rheumatoid arthritis share the common HLA DR3 and DR4 haplotypes (DRB*0301, *0401)[4]. Evidence exists that AIH has also a specific genetic background including HLA A1, B8 and DR3 or DR4 alleles. The majority of patients with AIH was reported to be either DRB3*0101 or DRB4*0401 positive[1,5].
AIH was reported repeatedly to manifest after viral infections. Some reports suggested that HAV might induce AIH in genetically susceptible persons[6,7], and protracted HAV infection was strongly associated with HLA-DRB*1301, which is a marker for AIH in pediatric patients[8]. The activation of an anti-HBsAg response was, however, not sufficient to induce AIH in an HBV envelope transgenic mouse model[9]. In our patient, who had a previously unrecognized, clinically silent AIH with advanced liver fibrosis, the exposure to Twinrix® led to the development of severe jaundice and an acute, clinically overt exacerbation of chronic liver disease. No other case of AIH after vaccination has been reported yet. Postmarketing studies occassionally described on jaundice and acute hepatitis after the use of Havrix and abnormal liver function tests using Engerix-B[3]. Moreover, vaccination was never reported to lead to chronic liver disease. The pathogenesis of acute exacerbation of this previously clinically silent AIH remains to be clarified. Inactivated HAV and/or rHBsAg are not known to be hepatotoxins, therefore, an intrinsic hepatotoxic effect is highly unlikely. Rather, Twinrix® probably caused an idiosyncratic reaction, i.e. hypers-ensitivity. Several drugs can cause chronic hepatitis, biochemically, serologically, and histologically almost indistinguishable from AIH and have been named drug-induced AIH[10].
In view of the advanced liver fibrosis in our patient, and the relatively recent immunization with Twinrix®, we believe that our patient already suffered from AIH. Drug-induced AIH usually have a long and variable latency period until clinical presentation and was not observed in our patient. Similarly, patients with chronic hepatitis C receiving interferon alpha[11] may also experience an acute exacerbation of AIH. This further supports our assumption that in our patient Twinrix® caused an acute exacerbation rather than an induction of AIH in a previously healthy liver. The therapy with the local active corticosteroid, budesonide, resulted in complete remission of the disease which-based also on our clinical experiences-represents an effective treatment of AIH[12].
Footnotes
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Manns MP, Strassburg CP. Autoimmune hepatitis: clinical challenges. Gastroenterology. 2001;120:1502–1517. doi: 10.1053/gast.2001.24227. [DOI] [PubMed] [Google Scholar]
- 2.Atlanta: Centers for Disease Control and Prevention, 2002 [Google Scholar]
- 3.Prescribing Information GlaxoSmithKline, Rixensart, Belgium, 2003, US License No 1617 [Google Scholar]
- 4.Csepregi A, Nemesanszky E, Rojkovich B, Poor G. Rheumatoid arthritis and hepatitis B virus: evaluating the pathogenic link. J Rheumatol. 2001;28:474–477. [PubMed] [Google Scholar]
- 5.Doherty DG, Donaldson PT, Underhill JA, Farrant JM, Duthie A, Mieli-Vergani G, McFarlane IG, Johnson PJ, Eddleston AL, Mowat AP. Allelic sequence variation in the HLA class II genes and proteins in patients with autoimmune hepatitis. Hepatology. 1994;19:609–615. doi: 10.1002/hep.1840190311. [DOI] [PubMed] [Google Scholar]
- 6.Vento S, Garofano T, Di Perri G, Dolci L, Concia E, Bassetti D. Identification of hepatitis A virus as a trigger for autoimmune chronic hepatitis type 1 in susceptible individuals. Lancet. 1991;337:1183–1187. doi: 10.1016/0140-6736(91)92858-y. [DOI] [PubMed] [Google Scholar]
- 7.Huppertz HI, Treichel U, Gassel AM, Jeschke R, Meyer zum Büschenfelde KH. Autoimmune hepatitis following hepatitis A virus infection. J Hepatol. 1995;23:204–208. doi: 10.1016/0168-8278(95)80336-x. [DOI] [PubMed] [Google Scholar]
- 8.Fainboim L, Cañero Velasco MC, Marcos CY, Ciocca M, Roy A, Theiler G, Capucchio M, Nuncifora S, Sala L, Zelazko M. Protracted, but not acute, hepatitis A virus infection is strongly associated with HLA-DRB*1301, a marker for pediatric autoimmune hepatitis. Hepatology. 2001;33:1512–1517. doi: 10.1053/jhep.2001.24562. [DOI] [PubMed] [Google Scholar]
- 9.Wirth S, Guidotti LG, Ando K, Schlicht HJ, Chisari FV. Breaking tolerance leads to autoantibody production but not autoimmune liver disease in hepatitis B virus envelope transgenic mice. J Immunol. 1995;154:2504–2515. [PubMed] [Google Scholar]
- 10.Liu ZX, Kaplowitz N. Immune-mediated drug-induced liver disease. Clin Liver Dis. 2002;6:755–774. doi: 10.1016/s1089-3261(02)00025-9. [DOI] [PubMed] [Google Scholar]
- 11.García-Buey L, García-Monzón C, Rodriguez S, Borque MJ, García-Sánchez A, Iglesias R, DeCastro M, Mateos FG, Vicario JL, Balas A. Latent autoimmune hepatitis triggered during interferon therapy in patients with chronic hepatitis C. Gastroenterology. 1995;108:1770–1777. doi: 10.1016/0016-5085(95)90139-6. [DOI] [PubMed] [Google Scholar]
- 12.Csepregi A, Treiber G, Klauck S, Malfertheiner P. Budesonid bei Autoimmunhepatitis and Überlappungssyndromen. Z Gastroenterol. 2004;42:908. [Google Scholar]


