Abstract
Objectives: There is increasing evidence that the presence of an inflammation-based prognostic score (modified Glasgow prognostic score, mGPS) could predict survival in patients with advanced cancer. The aim of this study was to investigate the prognostic value of mGPS in patients with cervical cancer. Methods: We included 238 consecutive patients with cervical cancer in our study. The albumin and serum C-reactive protein (CRP) were measured before initiation of treatment. The relationships between the mGPS and other clinical parameters including body mass index (BMI), white blood cell count, lymphocyte, platelet, hemoglobin, total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) were analyzed. Overall survival (OS) and progression-free survival (PFS) were calculated. Significant prognostic factors were identified using univariate and multivariate analyses. Results: The 5-year OS rate for all patients was 52.1% and 5-year PFS rate was 42.3%. Patients with mGPS of 0, 1 and 2 were 138, 71, 29, respectively. Higher mGPS was related to more advanced disease, including higher FIGO stage, lymph node metastases and lower lymphocyte counts, BMI and hemoglobin level. Performance status (PS), FIGO stage, lymph nodal status and mGPS were independent prognostic indicators for OS and PFS in the multivariate analysis. Conclusions: Higher mGPS is associated with advanced cervical cancer. The mGPS is an easily measurable biomarker which can be used in combination with conventional FIGO stage to predict survival in patients with cervical cancer undergoing chemoradiotherapy.
Keywords: Cervical cancer, inflammation, modified Glasgow prognostic score, chemoradiotherapy, prognosis
Introduction
Cervical cancer is the third most common cancer in women worldwide, with 85% of cases occurring in developing countries, and the peak incidence is in the 40-45 year age group [1,2]. For patients with locally advanced cervical cancer, the current standard of care is primary chemoradiotherapy, consisting of external beam radiotherapy, brachytherapy and cisplatin-based chemotherapy [3]. There is marked heterogeneity in the duration of overall survival among patients with cervical cancer, despite the same stage. Therefore, there is much interest in prognostic factors to permit more accurate patient stratification, which will improve clinical decision making, and may contribute to more rational study design and analysis [4].
It is now recognized that not only the intrinsic properties of cancer cells influence the progression of cancer, but also the host-related factors. There is increasing evidence that inflammatory processes in the tumor microenvironment play an important role in the development of several tumors [5,6]. Persistent human papillomavirus (HPV) infection, presenting a state of chronic inflammation, is the most important factor in the development of cervical cancer [7]. The inflammation-based Glasgow Prognostic Score (GPS) is a scoring system which combines albumin and C-reactive protein (CRP) into a risk stratification score for predicting clinical outcome in patients with cancer [8]. Elevated CRP reflects a state of systemic inflammation, which is generally associated with high cancer risk and poor prognosis. Hypoalbuminemia is indicating the hypercatabolic state in cancer patients, caused by activated cytokines [9]. Recent studies have evaluated the value of mGPS for assessing cancer prognosis in several malignancies including breast cancer, colorectal cancer, pancreatic cancer, gastric cancer, renal cancer, and lung cancer [4,8,10,11]. However, few studies have reported the prognostic value of the mGPS in patients with cervical cancer undergoing chemoradiotherapy.
The purpose of this study was to evaluate the association between mGPS and survival in cervical cancer patients receiving chemoradiotherapy. The relationship between mGPS and other relevant variables such as white blood cell count, neutrophilic granulocyte count, platelet count, hemoglobin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH) and bilirubin was also assessed.
Methods
Ethics statement
Written informed consent from all patients and approval from the independent Institute Research Ethics Committee at Affiliated Cancer Hospital of Zhengzhou University were obtained.
Data collection
Between December 1st, 2004 and March 10th, 2011, a total of 238 patients who were diagnosed with pathologically proven cervical cancer and primarily underwent chemoradiotherapy at the Affiliated Cancer Hospital of Zhengzhou University in China were reviewed retrospectively. The pretreatment work-up included the usual radiographs, laboratory analysis, and computed tomography or magnetic resonance imaging to evaluate the primary tumor extent and lymph nodal status. The patients were staged using the International Federation of Gynecology and Obstetrics (FIGO) stage system. All patients underwent regular follow-up at our hospital. The date of last follow-up was October 31st, 2014.
To evaluate the mGPS, blood test results from the day before treatment (or no more than 1 week before treatment) were used. The mGPS is based on both CRP and albumin. Patients who had both elevated CRP (> 10 mg/L) and hypoalbuminurea (< 35 g/L) were assigned a score of 2. Patients with only elevated CRP (> 10 mg/L) were assigned a score of 1. Patients with neither of these abnormalities were assigned a score of 0 [12].
Radiotherapy protocol and chemotherapy
External beam radiotherapy was administered using 6-10 MV X-rays. The large-field radiation dose was 40-45 Gy to the whole pelvis by either parallel-opposed anteroposterior or four-field box beams, with 1.8-2 Gy/fraction and five fractions weekly. Para-aortic lymph nodes were not routinely included in the treatment field for patients without imaging findings of para-aortic lymphadenopathy. A small-field parametrial boost was given to a dose of up to 57.6-58 Gy using a parallel-opposed anteroposterior field with a 4-cm-wide midline block in patients with Stage IIB or greater disease [13]. The intracavitary brachytherapy boost was given using a 137Cs source. The typical dose to Point A was 5.5-7.5 Gy/fraction for 4-5 fractions, with one fraction weekly. The median cumulative dose and biologically equivalent dose (BED) to Point A was 69 and 87.8 Gy, respectively, with the α/β ratio for tumor effects assumed to be 10 Gy.
Concurrent chemotherapy was administered using regimens that mainly included cisplatin, cisplatin plus 5-fluorouracil or cisplatin plus docetaxel. Chemotherapy was given with intravenous cisplatin 40 mg/m2 once a week during the external beam radiotherapy in 143 patients. Sixty-two patients were treated with two cycles of 60 mg/m2 docetaxel and 80 mg/m2 cisplatin during the external beam radiotherapy. Another 33 patients were treated with two cycles of 80 mg/m2 cisplatin and 500 mg/m2 5-fluorouracil.
Statistical analysis
Continuous data are presented as mean ± standard deviation (SD). The chi-square test, Student’s t-test and Mann-Whitney U test were used to compare data between mGPS and clinical variables. Overall survival (OS) and progression-free survival (PFS) were the study endpoints. Survival analysis was performed using the Kaplan-Meier method, and the differences were analyzed using the log-rank test. The three categories of mGPS (0, 1, 2) were analyzed using pairwise comparisons, with the P-value set at 0.017 (0.05/3) according to Hochberg’s step-up method. Multivariate analyses using the Cox proportional hazards model were used to calculate hazard ratios and to test independent significance by backward elimination of non-significant explanatory variables. P-values were determined using two-sided tests and P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Patients’ characteristics are shown in Table 1. Of the 238 patients, 214 (89.9%) had squamous cell carcinoma, 20 (8.4%) had adenocarcinoma and other 4 (1.7%) patients had adenosquamous carcinoma. The median age of the patients was 52.0 years (range, 34-70 years). Mean CRP and albumin serum levels were 15.7 mg/L and 41.1 g/L, respectively. There were 138 (58.0%) patients with mGPS 0, 71 (29.8%) patients with mGPS 1 and 29 (12.2%) patients with mGPS 2. The median duration of follow-up for patients who were alive at the time of the most recent follow-up was 42.0 months (range, 15-91 months).
Table 1.
Baseline characteristics of patients (N = 238)
| Characteristic | No. of patients (%) |
|---|---|
| Age (years), median (range) | 52 (34-70) |
| Albumin (g/L) | |
| ≥ 35 | 209 (87.8%) |
| < 35 | 29 (12.2%) |
| C-reactive protein (mg/L) | |
| < 10 | 131 (55.0%) |
| ≥ 10 | 107 (45.0%) |
| ECOG performance status | |
| 0-1 | 211 (88.7%) |
| 2-3 | 27 (11.3%) |
| FIGO stage | |
| IB1 | 3 (1.2%) |
| IB2 | 10 (4.2%) |
| IIA | 19 (8.0%) |
| IIB | 99 (41.6%) |
| IIIA | 32 (13.4%) |
| IIB | 68 (28.6%) |
| IVA | 7 (2.9%) |
| Lymph nodal status | |
| Negative | 164 (68.9%) |
| Positive | 74 (31.1%) |
| Histological type | |
| Squamous cell carcinoma | 214 (89.9%) |
| Adenocarcinoma | 20 (8.4%) |
| Adenosquamous carcinoma | 4 (1.7%) |
| Radiotherapy duration (week) | |
| ≤ 9 | 182 (76.5%) |
| > 9 | 56 (23.5%) |
| Concurrent chemotherapy | |
| PDD | 143 (60.0%) |
| PDD + 5-Fu | 33 (13.9%) |
| PDD + Docetaxel | 62 (26.1%) |
Correlation of mGPS with clinicopathological parameters
As shown in Table 2, a significant association between mGPS and FIGO stage, lymph nodal status and hemoglobin level was ascertained. Patients with higher mGPS presented with more advanced disease, including higher FIGO stage, lymph node metastases and lower hemoglobin level. Eighty-five of the 131 patients (64.9%) with stages III-IV disease were with mGPS 0, whereas only 53 of the 107 patients (49.5%) with stages I-II disease were with mGPS 0 (P < 0.001). The proportion of lymph node metastases in patients with mGPS 2 was significantly higher than patients with mGPS 0 or 58.6% (17 of 29) compared to 27.5% (38 of 138; P = 0.003). No significant relationship was found between the mGPS and age, concurrent chemotherapy, histological type or radiotherapy duration (Table 2).
Table 2.
Relationship between mGPS and clinical characteristics
| Characteristic | mGPS | P | ||
|---|---|---|---|---|
|
|
||||
| mGPS 0 | mGPS 1 | mGPS 2 | ||
| Total | 138 (58.0%) | 71 (29.8%) | 29 (12.2%) | |
| Age (years) | 0.827 | |||
| ≤ 50 | 62 (26.1%) | 34 (14.3%) | 12 (5.0%) | |
| > 50 | 76 (31.9%) | 37 (15.5%) | 17 (7.1%) | |
| FIGO stage | < 0.001 | |||
| I-II | 85 (35.7%) | 41 (17.2%) | 5 (2.1%) | |
| III-IV | 53 (22.3%) | 30 (12.6%) | 24 (10.1%) | |
| Lymph nodal status | 0.003 | |||
| Negative | 100 (42.0%) | 52 (21.8%) | 12 (5.0%) | |
| Positive | 38 (16.0%) | 19 (8.0%) | 17 (7.1%) | |
| Histological type | 0.232 | |||
| SCC | 128 (53.8%) | 61 (25.6%) | 25 (10.5%) | |
| Non-SCC | 10 (4.2%) | 10 (4.2%) | 4 (1.7%) | |
| Hemoglobin level | 0.002 | |||
| < 110 g/L | 6 (2.5%) | 9 (3.8%) | 7(2.9%) | |
| ≥ 110 g/L | 132 (55.5%) | 62 (26.1%) | 22 (9.2%) | |
| Chemotherapy | 0.857 | |||
| PDD | 80 (33.6%) | 45 (18.9%) | 18 (7.6%) | |
| PDD + 5-Fu | 20 (8.4%) | 8 (3.4%) | 5 (2.1%) | |
| PDD + Docetaxel | 38 (16.0%) | 18 (7.6%) | 6 (2.5%) | |
| RT duration (week) | 0.474 | |||
| ≤ 9 | 105 (44.1%) | 57 (23.9%) | 20 (8.4%) | |
| > 9 | 33 (13.9%) | 14 (5.9%) | 9 (3.8%) | |
SCC: squamous cell carcinoma.
The relationship between mGPS and relevant continuous variables is shown in Table 3. Patients with mGPS ≥ 1 had lower lymphocyte counts, body mass index (BMI) and hemoglobin level compared to those with mGPS 0. In contrast, the mean lactate dehydrogenase (LDH) counts in patients with mGPS ≥ 1 were higher than those with mGPS 0. There was no significant difference in the white blood cell counts, platelet, AST, ALT and total bilirubin levels between patients with mGPS 0 and patients with mGPS ≥ 1.
Table 3.
Relationship between mGPS and relevant continuous variables
| mGPS = 0 | mGPS ≥ 1 | P | |
|---|---|---|---|
| Body mass index (BMI) | 21.74 ± 4.10 | 21.32 ± 2.84 | 0.036 |
| White blood cell (WBC, count/L) | 7.02 ± 1.84 | 7.48 ± 2.34 | 0.146 |
| Lymphocyte (count/L) | 2.28 ± 0.58 | 1.64 ± 0.39 | < 0.001 |
| Hemoglobin (g/L) | 138.5 ± 13.2 | 121.9 ± 16.2 | 0.018 |
| Platelet (count/L) | 249.2 ± 87.1 | 246.7 ± 72.7 | 0.807 |
| Aspartate aminotransferase (AST, U/L) | 24.2 ± 12.1 | 23.5 ± 9.5 | 0.402 |
| Alanine aminotransferase (ALT, U/L) | 20.8 ± 12.9 | 19.6 ± 10.8 | 0.274 |
| Total bilirubin (mg/dL) | 11.5 ± 3.9 | 11.4 ± 4.9 | 0.498 |
| Lactate dehydrogenase (LDH, U/L) | 169.5 ± 29.3 | 179.7 ± 42.9 | 0.010 |
Continuous data were presented as mean ± standard deviation.
Clinical predictors of OS and PFS
The 5-year OS rate for the entire cohort was 52.1% and the 5-year PFS rate was 42.3%. The median OS time for all patients was 45.0 months and the median PFS time was 41.0 months.
Variables which were predictors of OS using univariate analysis included performance status (P < 0.001), FIGO stage (P = 0.001), lymph nodal status (P < 0.001), concurrent chemotherapy (P = 0.002), albumin level (P < 0.001), CRP level (P < 0.001), mGPS (P < 0.001) and hemoglobin level (P = 0.014) as shown in Table 4. Age, histological type, radiotherapy duration, platelet level, total bilirubin and LDH level were not significant predictors of OS. Performance status (P < 0.001), FIGO stage (P = 0.002), lymph nodal status (P = 0.001), concurrent chemotherapy (P < 0.001), albumin level (P < 0.001), CRP level (P < 0.001), mGPS (P < 0.001) and hemoglobin level (P = 0.006) were significantly associated with PFS. The univariate survival analysis is presented in Table 4.
Table 4.
Univariate analysis of prognostic factors
| Variables | No. | OS P-value | PFS P-value |
|---|---|---|---|
| Age (≤ 50/> 50 years) | 108/130 | 0.425 | 0.579 |
| PS (≤ 1/> 1) | 211/27 | < 0.001 | < 0.001 |
| FIGO stage (I-II/III-IV) | 131/107 | 0.001 | 0.002 |
| LN status (Negative/Positive) | 164/74 | < 0.001 | 0.001 |
| Histological type (SCC/non-SCC) | 214/24 | 0.753 | 0.704 |
| RT duration (≤ 9/> 9 weeks) | 182/56 | 0.641 | 0.902 |
| Chemotherapy (PDD/DP/PF) | 143/62/33 | 0.002 | < 0.001 |
| mGPS (0/1/2) | 138/71/29 | < 0.001 | < 0.001 |
| Albumin (≥ 35/< 35 g/L) | 209/29 | < 0.001 | < 0.001 |
| CRP (< 10/≥ 10 mg/L) | 138/100 | < 0.001 | < 0.001 |
| Hemoglobin (< 11/≥ 11 g/dL) | 22/216 | 0.014 | 0.006 |
| Plt (≥ 200,000/< 200,000/dL) | 181/57 | 0.240 | 0.426 |
| TB (≤ 1.1/> 1.1 mg/dL) | 114/124 | 0.798 | 0.707 |
| LDH (≥ 170/< 170 U/L) | 114/124 | 0.120 | 0.730 |
PS: performance status; LN: lymph node; RT: radiotherapy; CRP: C-reactive protein; Plt: platelet; TB: total bilirubin; LDH: lactate dehydrogenase.
Elevated serum CRP (≥ 10 mg/L) and hypoalbuminemia (< 35 g/L) were associated with shorter OS and PFS in univariate analysis (Figures 1 and 2). When these variables were scored as a combined index (mGPS), the total score provided good stratification of the patients in terms of survival (P < 0.001, Figure 3). The 5-year OS rates for patients with an mGPS of 0, 1 and 2 were 62.3%, 46.2% and 20.1%, respectively. The 5-year PFS rates for patients with an mGPS of 0, 1 and 2 were 50.2%, 34.8% and 17.2%, respectively. An increase in the mGPS correlated significantly with gradual reductions in PFS and OS.
Figure 1.
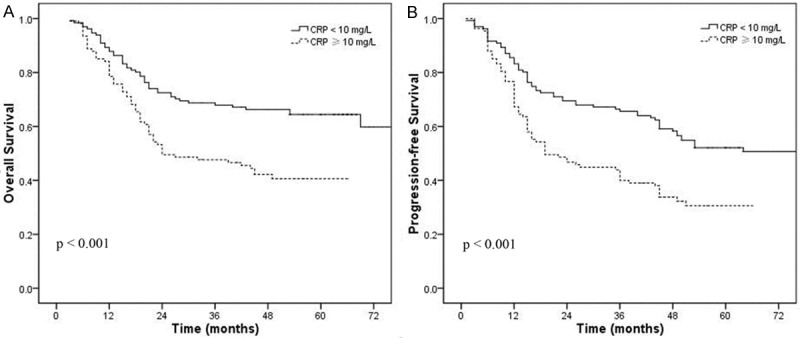
Overall survival (A) and progression-free survival (B) curve in patients with CRP ≥ 10 mg/L vs. CRP < 10 mg/L.
Figure 2.
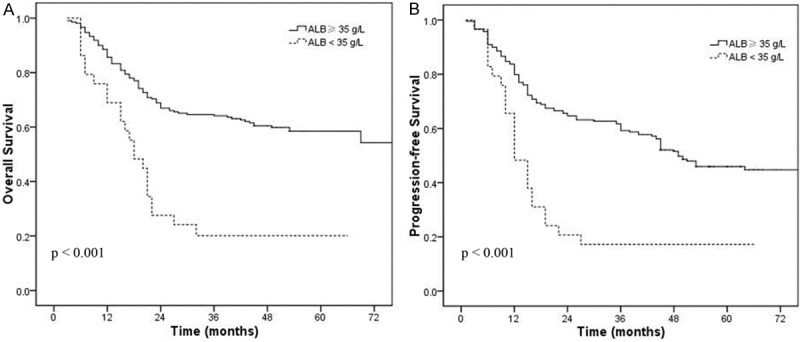
Overall survival (A) and progression-free survival (B) curve in patients with normal vs. lower pretreatment albumin(< 35 g/L) levels.
Figure 3.
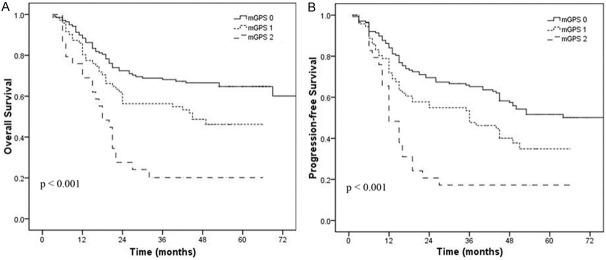
Overall survival (A) and progression-free survival (B) curve in patients with mGPS of 0, 1, 2.
To identify independent prognostic factors, the factors that were found to be significant in univariate analysis were included in multivariate analysis. As there is duplication between albumin, CRP and the mGPS, only the mGPS was entered into the multivariate analysis. Multivariate analysis revealed that performance status (HR 0.362, 95% CI 0.219-0.597; P < 0.001), FIGO stage (HR 1.963, 95% CI 1.329-2.902; P = 0.001), lymph nodal status (HR 2.124, 95% CI 1.428-3.160; P < 0.001) and mGPS (HR 1.820, 95% CI 1.378-2.404; P < 0.001) were independent factors affecting OS in patients with cervical cancer. Performance status (HR 0.413, 95% CI 0.254-0.673; P < 0.001), FIGO stage (HR 1.826, 95% CI 1.287-2.592; P = 0.001), lymph nodal status (HR 1.831, 95% CI 1.272-2.636; P = 0.001), and mGPS (HR 1.642, 95% CI 1.272-2.120; P < 0.001) were independent factors affecting PFS in patients with cervical cancer (Table 5).
Table 5.
Multivariate analysis of factors influencing OS and PFS in cervical cancer
| Endpoint | Variable | P | HR | 95% CI for HR |
|---|---|---|---|---|
| OS | PS | < 0.001 | 0.362 | 0.219-0.597 |
| FIGO stage | 0.001 | 1.963 | 1.329-2.902 | |
| LN status | < 0.001 | 2.124 | 1.428-3.160 | |
| Chemotherapy | 0.287 | 0.852 | 0.635-1.144 | |
| mGPS | < 0.001 | 1.820 | 1.378-2.404 | |
| Hemoglobin | 0.636 | 0.858 | 0.456-1.617 | |
| PFS | PS | < 0.001 | 0.413 | 0.254-0.673 |
| FIGO stage | 0.001 | 1.826 | 1.287-2.592 | |
| LN status | 0.001 | 1.831 | 1.272-2.636 | |
| Chemotherapy | 0.057 | 0.771 | 0.590-1.008 | |
| mGPS | < 0.001 | 1.642 | 1.272-2.120 | |
| Hemoglobin | 0.877 | 1.047 | 0.585-1.008 |
95% CI, 95% confidence interval; HR, hazards ratio; OS, overall survival; PFS, progression-free survival.
Discussion
To our best knowledge, it is the first study to explore the prognostic value of the mGPS in patients with cervical cancer undergoing chemoradiotherapy. In this study, we revealed that mGPS was an independent prognostic factor in patients with cervical cancer. Besides determining a prognostic value of mGPS, we also correlated mGPS with clinicopathologic parameters and found that a higher mGPS was found more often in patients with advanced FIGO stage, lymph node metastases and lower hemoglobin level.
Higher serum CRP concentration has been shown to associate with tumor stage in various malignancies including cervical cancer [14-16]. The mechanisms by which systemic inflammation reduces survival may be affected by several confounding factors. Firstly, a peritumoral inflammatory infiltrate can promote the tumor progression [5]. The systemic inflammatory response could support the proliferation of malignant cells, promote angiogenesis and metastasis, and subvert adaptive immune responses [5,17,18]. Secondly, the systemic inflammatory response and concomitant nutritional decline reduces patient tolerance to treatment toxicities and diminishes compliance with treatment [19]. In addition, the association of elevated serum CRP levels can impair the T-lymphocytic response to cancer [20], while the intratumoral CD8 (+) T cell infiltrations is a favorable prognostic factor by promoting proliferative activity and IFN-γ secretion [21]. At last, elevated acute-phase response proteins, especially CRP, could lead to cancer-related cachexia [22]. In this study, elevated serum CRP (≥ 10 mg/L) was correlated with poorer OS and PFS in patients with cervical cancer (5 year OS: P < 0.001; 5 year PFS: P < 0.001).
Parallel to the increase in serum CRP level, hypoalbuminemia has been observed in various types of tumors and verified to be a negative prognostic factor [23,24]. The association between hypoalbuminemia and a reduced survival in patients with cervical cancer is influenced by several factors. Firstly, hypoalbuminemia is reflecting a progressive nutritional decline in patients with cancer and is associated to cancer-related cachexia. The diagnostic criteria for cachexia included albumin < 35 g/L [25]. In addition, the development of hypoalbuminemia is often secondary to a systemic inflammatory response and hypoalbuminemia in itself reflected a systemic inflammatory response [23,26]. In this study, hypoalbuminemia (< 35 g/L) was correlated with poorer OS and PFS in patients with cervical cancer (5 year OS: P < 0.001; 5 year PFS: P < 0.001).
The mGPS, based on a combination of elevated CRP (> 10 mg/L) and hypoalbuminemia (< 35 g/L), is indicative of both a systemic inflammatory response and a nutritional decline [27], which could stratify the prognosis of cancer patients better. To date, the mGPS has been shown to be a prognostic factor, independent of TNM stage, including lung, gastrointestinal, and colorectal cancer [12,19,28,29].
In this study, increasing mGPS correlated with more aggressive tumor biology in terms of tumor size, presence of lymph node metastases and lower hemoglobin level. This is consistent with the previous reports [18]. Moreover, in multivariate survival analysis of our study, mGPS remained an independent prognostic factor besides FIGO stage, performance status and lymph node involvement. However, the assessment of FIGO stage is still largely based on physical examination. The limitations of clinical FIGO staging are well appreciated as overdiagnosis and underdiagnosis occur [18]. Therefore, the mGPS could offer a simple, objective and well standardized pre-treatment assessment which can aid in the selection of the optimum treatment strategy and could be used in combination with conventional FIGO staging to predict survival in patients with cervical cancer.
Previous studies revealed that decreased Hb levels was associated with poor local control and could reflect an impaired overall survival in patients with cervical cancer [2]. In this study, anemia was found to be related to decreased OS and PFS at univariate but not at multivariate analysis. The pretreatment anemia could be a reflection of the disease process itself, rather than the treatment, and could be caused by bleeding or growth factor inhibitors associated with the tumor.
Although we were able to demonstrate a significant prognostic value of mGPS for long-term outcome in patients with cervical cancer, our study is biased by its retrospective nature. And the study was conducted in a single institution. However, we report on a homogeneous large study population that underwent only chemoradiotherapy for cervical cancer. Larger prospective studies will need to be carried out to confirm these preliminary results.
In conclusion, higher mGPS is associated with advanced cervical cancer. The mGPS is an easily measurable biomarker of prognostic significance in cervical cancer. The mGPS can be used in combination with conventional FIGO stage to predict survival in patients with cervical cancer undergoing chemoradiotherapy.
Disclosure of conflict of interest
None.
References
- 1.Koh WJ, Greer BE, Abu-Rustum NR, Apte SM, Campos SM, Chan J, Cho KR, Cohn D, Crispens MA, DuPont N, Eifel PJ, Gaffney DK, Giuntoli RL 2nd, Han E, Huh WK, Lurain JR 3rd, Martin L, Morgan MA, Mutch D, Remmenga SW, Reynolds RK, Small W Jr, Teng N, Tillmanns T, Valea FA, McMillian NR, Hughes M. Cervical cancer. J Natl Compr Canc Netw. 2013;11:320–343. doi: 10.6004/jnccn.2013.0043. [DOI] [PubMed] [Google Scholar]
- 2.Parker K, Gallop-Evans E, Hanna L, Adams M. Five years’ experience treating locally advanced cervical cancer with concurrent chemoradiotherapy and high-dose-rate brachytherapy: results from a single institution. Int J Radiat Oncol Biol Phys. 2009;74:140–146. doi: 10.1016/j.ijrobp.2008.06.1920. [DOI] [PubMed] [Google Scholar]
- 3.Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802–5812. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crumley AB, McMillan DC, McKernan M, McDonald AC, Stuart RC. Evaluation of an inflammation-based prognostic score in patients with inoperable gastro-oesophageal cancer. Br J Cancer. 2006;94:637–641. doi: 10.1038/sj.bjc.6602998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 6.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kjaer SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst. 2010;102:1478–1488. doi: 10.1093/jnci/djq356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikuta Y, Takamori H, Sakamoto Y, Hashimoto D, Chikamoto A, Kuroki H, Sakata K, Sakamoto K, Hayashi H, Imai K, Nitta H, Hirota M, Kanemitsu K, Beppu T, Baba H. The modified Glasgow Prognostic Score (mGPS) is a good predictor of indication for palliative bypass surgery in patients with unresectable pancreatic and biliary cancers. Int J Clin Oncol. 2014;19:629–633. doi: 10.1007/s10147-013-0613-y. [DOI] [PubMed] [Google Scholar]
- 9.Teunissen SC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de Graeff A. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94–104. doi: 10.1016/j.jpainsymman.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Proctor MJ, Talwar D, Balmar SM, O’Reilly DS, Foulis AK, Horgan PG, Morrison DS, McMillan DC. The relationship between the presence and site of cancer, an inflammation-based prognostic score and biochemical parameters. Initial results of the Glasgow Inflammation Outcome Study. Br J Cancer. 2010;103:870–876. doi: 10.1038/sj.bjc.6605855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou T, Hong S, Hu Z, Hou X, Huang Y, Zhao H, Liang W, Zhao Y, Fang W, Wu X, Qin T, Zhang L. A systemic inflammation-based prognostic scores (mGPS) predicts overall survival of patients with small-cell lung cancer. Tumour Biol. 2015;36:337–343. doi: 10.1007/s13277-014-2623-4. [DOI] [PubMed] [Google Scholar]
- 12.Proctor MJ, Morrison DS, Talwar D, Balmer SM, O’Reilly DS, Foulis AK, Horgan PG, McMillan DC. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer. 2011;104:726–734. doi: 10.1038/sj.bjc.6606087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang YT, Wang CC, Tsai CS, Lai CH, Chang TC, Chou HH, Hsueh S, Chen CK, Lee SP, Hong JH. Long-term outcome and prognostic factors for adenocarcinoma/adenosquamous carcinoma of cervix after definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2011;80:429–436. doi: 10.1016/j.ijrobp.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J. Clin. Oncol. 2009;27:2217–2224. doi: 10.1200/JCO.2008.19.8440. [DOI] [PubMed] [Google Scholar]
- 15.Polterauer S, Grimm C, Tempfer C, Sliutz G, Speiser P, Reinthaller A, Hefler LA. C-reactive protein is a prognostic parameter in patients with cervical cancer. Gynecol Oncol. 2007;107:114–117. doi: 10.1016/j.ygyno.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Hefler LA, Concin N, Hofstetter G, Marth C, Mustea A, Sehouli J, Zeillinger R, Leipold H, Lass H, Grimm C, Tempfer CB, Reinthaller A. Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clin Cancer Res. 2008;14:710–714. doi: 10.1158/1078-0432.CCR-07-1044. [DOI] [PubMed] [Google Scholar]
- 17.Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 18.Polterauer S, Grimm C, Seebacher V, Rahhal J, Tempfer C, Reinthaller A, Hefler L. The inflammation-based Glasgow Prognostic Score predicts survival in patients with cervical cancer. Int J Gynecol Cancer. 2010;20:1052–1057. doi: 10.1111/IGC.0b013e3181e64bb1. [DOI] [PubMed] [Google Scholar]
- 19.Scott HR, McMillan DC, Forrest LM, Brown DJ, McArdle CS, Milroy R. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer. 2002;87:264–267. doi: 10.1038/sj.bjc.6600466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang CY, Hsieh MJ, Chiu YC, Li SH, Huang HW, Fang FM, Huang YJ. Higher serum C-reactive protein concentration and hypoalbuminemia are poor prognostic indicators in patients with esophageal cancer undergoing radiotherapy. Radiother Oncol. 2009;92:270–275. doi: 10.1016/j.radonc.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 22.Krzystek-Korpacka M, Matusiewicz M, Diakowska D, Grabowski K, Blachut K, Kustrzeba-Wojcicka I, Terlecki G, Gamian A. Acute-phase response proteins are related to cachexia and accelerated angiogenesis in gastroesophageal cancers. Clin Chem Lab Med. 2008;46:359–364. doi: 10.1515/CCLM.2008.089. [DOI] [PubMed] [Google Scholar]
- 23.McMillan DC. An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc. 2008;67:257–262. doi: 10.1017/S0029665108007131. [DOI] [PubMed] [Google Scholar]
- 24.Deans DA, Tan BH, Wigmore SJ, Ross JA, de Beaux AC, Paterson-Brown S, Fearon KC. The influence of systemic inflammation, dietary intake and stage of disease on rate of weight loss in patients with gastro-oesophageal cancer. Br J Cancer. 2009;100:63–69. doi: 10.1038/sj.bjc.6604828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morley JE, Thomas DR, Wilson MM. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr. 2006;83:735–743. doi: 10.1093/ajcn/83.4.735. [DOI] [PubMed] [Google Scholar]
- 26.McMillan DC, Elahi MM, Sattar N, Angerson WJ, Johnstone J, McArdle CS. Measurement of the systemic inflammatory response predicts cancer-specific and non-cancer survival in patients with cancer. Nutr Cancer. 2001;41:64–69. doi: 10.1080/01635581.2001.9680613. [DOI] [PubMed] [Google Scholar]
- 27.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534–540. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Nozoe T, Iguchi T, Egashira A, Adachi E, Matsukuma A, Ezaki T. Significance of modified Glasgow prognostic score as a useful indicator for prognosis of patients with gastric carcinoma. Am J Surg. 2011;201:186–191. doi: 10.1016/j.amjsurg.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 29.Ishizuka M, Nagata H, Takagi K, Horie T, Kubota K. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann Surg. 2007;246:1047–1051. doi: 10.1097/SLA.0b013e3181454171. [DOI] [PubMed] [Google Scholar]


