Abstract
Aim:
The aim of this study was to compare the effect on fracture strength, pH and calcium ion diffusion from mineral trioxide aggregate (MTA) Fillapex, iRoot SP, and Ultracal when used for repair of simulated root resorption defects.
Materials and Methods:
Three sets of 40 teeth each were used, and biomechanical preparation was done. Resorption cavity was made at 5 mm from the apex. Teeth were filled with different experimental materials. In control group, saline was used. Samples of fracture resistance were stored in an incubator for 3 months and then subjected to the universal testing machine. To evaluate the pH and calcium ion release were checked at 1 day and 1, 2, 3, and 4 weeks intervals. Data were recorded and statistical analysis done by one-way analysis of variance followed by post-hoc Tukey test.
Results:
Highest fracture resistance was seen in MTA Fillapex followed by iRoot SP, control, and then Ultracal group. Teeth filled with iRoot SP showed highest pH and calcium ion release followed by MTA Fillapex and Ultracal group.
Conclusion:
Bioceramic sealers showed high pH, calcium ion release, and good root reinforcement potential. Initial dressing of calcium hydroxide followed by obturation with Gutta-percha and bioceramic sealer may be considered as an alternative treatment modality for inflammatory resorption.
Keywords: Calcium ion diffusion, fracture strength, iRoot SP, mineral trioxide aggregate Fillapex, pH, Ultracal, universal testing machine
INTRODUCTION
Inflammatory root resorption is physiologic or pathologic dissolution of mineralized tissues such as dentin, cementum and/or bone resulting in loss of these tissues by osteoclasts, and osteoclasts like cells. The proposed treatment regimen involves the use of calcium hydroxide as an intracanal medicament. Although this treatment modality has wide acceptance, concerns regarding weakening of roots leading to increased susceptibility to fracture have been raised.[1,2] Moreover, this demands patient compliance for repeated dressings.
The drawbacks of calcium hydroxide encouraged the use of mineral trioxide aggregate (MTA) for the treatment of resorption defects. Drawbacks of MTA include its high cost, difficulty in manipulation and prolonged setting time. This has led to the search for newer bioceramic materials like bioaggregate exhibiting properties similar to MTA with improved handling characteristics, shorter setting time, lower cost.
Recently, George et al. reported that Apexcal, which is a paste form of calcium hydroxide, was more effective as compared to powder form of MTA as it ensured homogenous consistency over time and good contact with dentin, which allowed more release of calcium ions.[3] This has prompted us to use various biomimetic materials in their paste consistency in the form of their respective sealers, available in the premixed syringeable form.
Mineral trioxide aggregate based sealer, Fillapex consists of salicylate resin, diluting resin, natural resin, bismuth trioxide, nanoparticulated silica, MTA, and pigments.[4] It shows a high calcium release[5] that enhances formation of mineralized tissue.[6]
As claimed by the manufacturer, bioaggregate based sealer, iRoot SP is premixed, ready to use injectable white hydraulic cement paste based on bioceramic composition-zirconium oxide, calcium silicate, calcium phosphate, calcium hydroxide, filler, thickening agents. It shows excellent physical and chemical properties can be used for filling root canals with or without Gutta-percha (GP) points. Ghoneim et al. demonstrates a high fracture resistance of roots obturated using iRoot SP sealer and active GP cone.[7] Zhang et al. reported upregulation in mineralization related genes after application of iRootSP sealer.[8]
The effect on the fracture strength of root, the extent, duration of elevated pH, the amount of calcium ion released on external root surface from MTA based sealer and bioaggregate based sealer is not known. Therefore, the aim of this study was to compare the effect on fracture strength, pH and calcium ion diffusion from various biomimetic materials when used for repair of simulated root resorption defects. The null hypothesis is that there is no difference.
MATERIALS AND METHODS
Freshly extracted 120 human permanent single-rooted canines were collected from patients requiring extractions due to caries or periodontal reasons and rinsed in 0.9% unbuffered saline. Radiographs were taken at buccolingual and mesiodistal angulations to confirm the presence of single canal and compare root dimensions and canal morphology. Three sets of 40 teeth each with a similar thickness of radicular dentin at 3 mm, 6 mm, and 9 mm from the apex were selected. This was done using the EasyDent V4 Simple Viewer software. They were debrided to remove organic debris using an ultrasonic scaler, taking care to avoid damage to the cementum and stored in 10% formalin thereafter.
Teeth were decoronated using diamond disk to obtain a final dimension of 10 mm. To working the length of 9 mm was achieved for each tooth by subtracting 1 mm from the final dimension. Biomechanical preparation was done to working length using rotary proTaper system till F5. During biomechanical preparation, canals were irrigated with 3 ml of 5.25% NaOCl for 30 s. Thereafter, standardized simulated resorptive cavities measuring 1 mm in diameter and 0.5 mm deep were made on the distal surface on the middle third of the specimen at 5 mm from the apex using round diamond bur. Smear layer from the root canal was removed using 3 ml of 17% ethylenediaminetetraacetic acid followed by 3 ml of 5.25% NaOCl with a final rinse by 3 ml distilled water.
Three sets of 40 teeth each were taken for evaluation of various methodologies:
Set 1 (n = 40): For evaluation of fracture strength.
Set 2 (n = 40): For evaluation of pH changes.
Set 3 (n = 40): For evaluation of calcium ion diffusion.
Totally, 40 specimens of each set were further subdivided as follows:
Group 1 (n = 10): Filled with iRoot SP sealer (Lot 11002SP, Veriodent, Vancouver, Canada).
Group 2 (n = 10): Filled with MTA Fillapex (Lot 24286, Angelus, Brazil).
Group 3 (n = 10): Filled with Ultracal XS (Lot K064, Ultradent, South Jordan, USA).
Group 4 (n = 10): Filled with saline.
Root canal specimens in all the groups were filled with their respective filling materials. Images were taken with RVG to check for the density of the filling. All the samples were sealed coronally and apically with sticky wax. Varnish coat was applied on the external root surface in specimens of set 2 and set 3 except on the prepared cavity. All samples were stored in 20 ml of distilled water each at 37°C in separate vials.
The pH values of set 2 specimens were measured by dipping the electrode of the pH meter into immersion solution while calcium ion release of set 3 specimens were measured by passing immersion media through atomic absorption spectrometer at intervals of 1 day, 1 week, 2 weeks, 3 weeks, and 4 weeks. The vials containing the specimen were replenished with fresh immersion solution in between different time intervals.
Specimens of set 1 were stored in saline soaked gauze for 3 months in 100% humidity at 37°C. Thereafter, individual specimens were embedded in self-curing acrylic resin block exposing 6 mm. The long axis of each specimen was aligned with the central axis of acrylic resin block and mounted on lower fixed compartment of Instron machine. The upper plate of the machine included a spherical indenter of 5 mm diameter, which was directed toward the long axis of the tooth. The compressive load was applied vertically to the coronal surfaces of roots at a constant loading speed of 1 mm/min. The load at which the failure occurred was recorded.
Results were recorded, and statistical analysis was done using SPSS software (IBM Corp, New York, USA). An one-way analysis of variance with post-hoc analysis (Tukey honestly significant difference) was applied to see the significance among the various groups and at different time intervals. P = 0.05 has been considered as statistically significant.
RESULTS
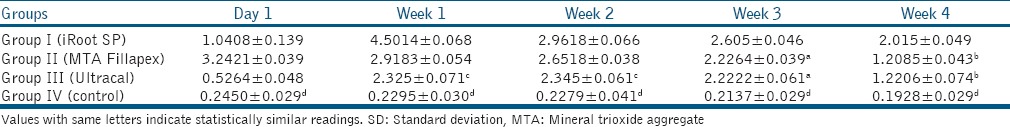
Tables 1-3 show pH, calcium ion release, and fracture resistance values, respectively. At all time periods, Group I (iRoot SP) showed highest pH and calcium ion release followed by Group II (MTA Fillapex), Group III (Ultracal) and Group IV (control) with a statistically significant difference among the groups except between Group II (MTA Fillapex) and Group III (Ultracal) in week 3 for pH values. Group II (MTA Fillapex) showed highest fracture resistance followed by Group I (iRoot SP), Group IV (control) and then Group III (Ultracal) with significant differences among the groups except between Group I (iRoot SP) and Group II (MTA Fillapex).
Table 1.
Mean pH and SD at various time intervals in different groups
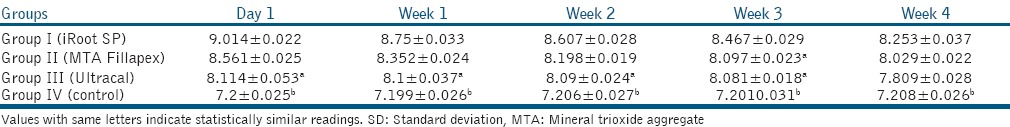
Table 3.
Mean fracture resistance and SD in various groups (in Newtons)

Table 2.
Mean calcium ion release and SD at various time intervals in different groups (mg/L)
DISCUSSION
The results of the present study, showed a difference among various groups in terms of mean pH, calcium ion release, and fracture resistance. Therefore, the null hypothesis was rejected. In our study, Ultracal demonstrates lowest but sustained overall pH and release of calcium ions. This could be attributed to the viscous nature of its vehicle.[9] High pH and calcium ion release by iRoot SP and MTA Fillapex groups can be attributed to the presence of nanoparticles providing a homogenous mixture, high solubility, and good flow characteristics which are important in determining the diffusion of calcium and hydroxyl ions from a material.[5] Furthermore, iRoot SP being hydrophilic, possesses low contact angle, and allows good adaptation with canal walls.[7] At week 1, highest calcium ion release was observed with iRoot SP that decreased gradually. This may be related to the final setting time of this material that occurs between 160 and 240 h in moist medium.[10] At day 1, highest calcium ion release was observed with MTA Fillapex that correlates to its setting time of 5 h as reported by Vitti et al.[11] Candeiro et al. observed higher pH and release of calcium ions by endosequence BC sealer at 168, 240 h as compared to AH plus sealer.[10] Zhang et al. attributed the high antimicrobial activity of iRoot SP to its high pH, active calcium ion diffusion, and its hydrophilicity.[12] As opposed to this study, George et al. showed greater calcium ion release by Apexcal than MTA.[3] Contradictory results obtained with their study may be due to the fact that we have used paste form of MTA based sealer in our study instead of pure MTA in putty consistency. Faria-Júnior et al. compared various epoxy resin-based sealers and MTA based sealers for their pH and antibiofilm activity and attributed the high antimicrobial activity of MTA Fillapex to its high pH.[13]
This study showed higher mean pH and calcium ion release by iRoot SP as compared to MTA Fillapex with statistically significant difference. This may be attributed to higher solubility and flow of iRoot SP than MTA Fillapex. The results of this study are in accordance with that of Borges et al.[5] but contrary to that of Han and Okiji[14] and may be due to the differences in methodologies employed.
Ultracal group showed least mean fracture resistance values even lower than the control group and the difference was statistically significant. This may be due to the strong alkalinity of calcium hydroxide that leads to neutralization, dissolution or denaturation of the acid proteins, and proteoglycans, which serve as bonding agents between collagen network and hydroxyapatite crystals in dentine.[1,2,15] Similar to our findings, many authors have reported a decrease in the fracture resistance of teeth filled with calcium hydroxide.[1,2,16,17,18]
Mineral trioxide aggregate Fillapex and iRoot SP showed higher mean fracture resistance than the control group with the insignificant difference between the two groups. This may be due to the fact that both are resin-based sealers with the good adhesive property that allows effective reinforcements of the roots filled with these materials.[4] Furthermore, they allow the formation of hydroxyapatite and formation of a chemical bond during the setting reaction of MTA Fillapex[4] and iRoot SP[10] that effectively strengthens the tooth structure. Furthermore, the presence of tissue inhibitor of metalloproteinase-2 in histological sections of dentin filled with MTA prevents the organic matrix from degradation caused by matrix metalloproteinase 2 (MMP 2) and MMP 14 leading to high fracture resistance.[19,20] An iRoot SP has zirconium oxide in its composition, which has been reported to possess high fracture toughness, tensile strength, and lower young's modulus contributing to its reinforcement potential. In addition, the bioceramic sealer is hydrophilic possessing a low contact angle that would allow the sealer to spread easily over the canal wall providing adaptation and good hermetic seal through mechanical interlocking.[7] Similar results have been reported by Sagsen et al.[4] However, in yet another study by these authors, MTA Fillapex showed lower push out bond strength than iRoot SP. They attributed the low push out bond strength to low adhesion capacity of tag-like structures formed by MTA Fillapex. The difference in the result from the present study, might be due to the difference in the methodologies employed, that is, the use of dentin disks in these studies and to the use of these sealers in conjunction with GP points.[21]
The values obtained in this study, cannot be extrapolated to clinical situations as they may be slightly exaggerated than those expected in a clinical scenario. This is due to the absence of GP in root filling in the present study, in contrast, to the use of GP in conjunction with sealer in clinical practice. However, this may be negated by saturation achieved in this study, owing to replacement of immersion media on weekly basis as opposed to clinical condition whereby continuous tissue fluid circulation in areas of resorption might facilitate diffusion rather than equilibration.[22] Further studies should consider replacing the immersion media on a daily basis to prevent saturation of the solution.
The results of the present study showed high pH, prolonged release of calcium ions by bioceramic sealers in conjunction with its root reinforcement potential. Therefore, it is recommended that, for the treatment of inflammatory root resorption, an initial dressing of calcium hydroxide over a period of 1 week followed by obturation with GP and bioceramic sealer may be considered an alternative and beneficial treatment modality as compared to long-term use of calcium hydroxide.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18:134–7. doi: 10.1034/j.1600-9657.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- 2.Sahebi S, Moazami F, Abbott P. The effects of short-term calcium hydroxide application on the strength of dentine. Dent Traumatol. 2010;26:43–6. doi: 10.1111/j.1600-9657.2009.00834.x. [DOI] [PubMed] [Google Scholar]
- 3.George GK, Rajkumar K, Sanjeev K, Mahalaxmi S. Calcium ion diffusion levels from MTA and ApexCal in simulated external root resorption at middle third of the root. Dent Traumatol. 2009;25:480–3. doi: 10.1111/j.1600-9657.2009.00817.x. [DOI] [PubMed] [Google Scholar]
- 4.Sagsen B, Ustün Y, Pala K, Demirbuga S. Resistance to fracture of roots filled with different sealers. Dent Mater J. 2012;31:528–32. [PubMed] [Google Scholar]
- 5.Borges RP, Sousa-Neto MD, Versiani MA, Rached-Júnior FA, De-Deus G, Miranda CE, et al. Changes in the surface of four calcium silicate-containing endodontic materials and an epoxy resin-based sealer after a solubility test. Int Endod J. 2012;45:419–28. doi: 10.1111/j.1365-2591.2011.01992.x. [DOI] [PubMed] [Google Scholar]
- 6.Salles LP, Gomes-Cornélio AL, Guimarães FC, Herrera BS, Bao SN, Rossa-Junior C, et al. Mineral trioxide aggregate-based endodontic sealer stimulates hydroxyapatite nucleation in human osteoblast-like cell culture. J Endod. 2012;38:971–6. doi: 10.1016/j.joen.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Ghoneim AG, Lutfy RA, Sabet NE, Fayyad DM. Resistance to fracture of roots obturated with novel canal-filling systems. J Endod. 2011;37:1590–2. doi: 10.1016/j.joen.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Li Z, Peng B. Effects of iRoot SP on mineralization-related genes expression in MG63 cells. J Endod. 2010;36:1978–82. doi: 10.1016/j.joen.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 9.Caicedo R, Mercante DE, Alongi DJ. Calcium-ion diffusion of four calcium-hydroxide based materials: Ultracal XS, Vitapex, Roeko calcium-hydroxide plus points and pure calcium hydroxide through radicular dentin. Int J Oral Med Sci. 2004;3:75–82. [Google Scholar]
- 10.Candeiro GT, Correia FC, Duarte MA, Ribeiro-Siqueira DC, Gavini G. Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J Endod. 2012;38:842–5. doi: 10.1016/j.joen.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 11.Vitti RP, Prati C, Silva EJ, Sinhoreti MA, Zanchi CH, de Souza e Silva MG, et al. Physical properties of MTA Fillapex sealer. J Endod. 2013;39:915–8. doi: 10.1016/j.joen.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Shen Y, Ruse ND, Haapasalo M. Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalis. J Endod. 2009;35:1051–5. doi: 10.1016/j.joen.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Faria-Júnior NB, Tanomaru-Filho M, Berbert FL, Guerreiro-Tanomaru JM. Antibiofilm activity, pH and solubility of endodontic sealers. Int Endod J. 2013;46:755–62. doi: 10.1111/iej.12055. [DOI] [PubMed] [Google Scholar]
- 14.Han L, Okiji T. Bioactivity evaluation of three calcium silicate-based endodontic materials. Int Endod J. 2013;46:808–14. doi: 10.1111/iej.12062. [DOI] [PubMed] [Google Scholar]
- 15.Tuna EB, Dinçol ME, Gençay K, Aktören O. Fracture resistance of immature teeth filled with BioAggregate, mineral trioxide aggregate and calcium hydroxide. Dent Traumatol. 2011;27:174–8. doi: 10.1111/j.1600-9657.2011.00995.x. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg B, Murray PE, Namerow K. The effect of calcium hydroxide root filling on dentin fracture strength. Dent Traumatol. 2007;23:26–9. doi: 10.1111/j.1600-9657.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- 17.Doyon GE, Dumsha T, von Fraunhofer JA. Fracture resistance of human root dentin exposed to intracanal calcium hydroxide. J Endod. 2005;31:895–7. doi: 10.1097/01.don.0000194542.02521.af. [DOI] [PubMed] [Google Scholar]
- 18.Zarei M, Afkhami F, Malek Poor Z. Fracture resistance of human root dentin exposed to calcium hydroxide intervisit medication at various time periods: An in vitro study. Dent Traumatol. 2013;29:156–60. doi: 10.1111/j.1600-9657.2012.01158.x. [DOI] [PubMed] [Google Scholar]
- 19.Hatibovic-Kofman S, Raimundo L, Zheng L, Chong L, Friedman M, Andreasen JO. Fracture resistance and histological findings of immature teeth treated with mineral trioxide aggregate. Dent Traumatol. 2008;24:272–6. doi: 10.1111/j.1600-9657.2007.00541.x. [DOI] [PubMed] [Google Scholar]
- 20.Arun A, Subhash TS. Evaluation of fracture resistance of human root dentin when exposed to intra-canal calcium hydroxide, mineral trioxide aggregate and calcium phosphate cement — An in-vitro study. Endodontology. 2012;24:18–27. [Google Scholar]
- 21.Sagsen B, Ustün Y, Demirbuga S, Pala K. Push-out bond strength of two new calcium silicate-based endodontic sealers to root canal dentine. Int Endod J. 2011;44:1088–91. doi: 10.1111/j.1365-2591.2011.01925.x. [DOI] [PubMed] [Google Scholar]
- 22.Heward S, Sedgley CM. Effects of intracanal mineral trioxide aggregate and calcium hydroxide during four weeks on pH changes in simulated root surface resorption defects: An in vitro study using matched pairs of human teeth. J Endod. 2011;37:40–4. doi: 10.1016/j.joen.2010.09.003. [DOI] [PubMed] [Google Scholar]


