Abstract
Immature nonvital teeth can often be associated with periapical lesions. Presence of external inflammatory resorption can complicate the treatment plan. A 21-year-old female patient presented with a large periapical lesion in relation to teeth 11 and 12. Tooth 11 was an immature tooth undergoing external inflammatory resorption. Aspiration through the root canal was carried out to evacuate the purulent fluid in the periapical lesion. Triple antibiotic paste was then placed as an intracanal medicament for a period of 2 weeks, followed by calcium hydroxide therapy for a period of 2 months. Mineral trioxide aggregate was then placed as an apical barrier to a thickness of about 4 mm. Obturation of the remainder of the canal space was done after 48 h. Complete periapical healing was evident after 1 year and 6 months. Nonsurgical healing of a large periapical lesion associated with an immature tooth displaying external inflammatory resorption can be successfully achieved.
Keywords: Aspiration, immature tooth, nonsurgical, periapical lesion, resorption
INTRODUCTION
Trauma to teeth often leads to pulpal injury. The injured pulp can then undergo necrosis and such infected pulps lead to periapical lesions. The periapical lesions if left untreated may gradually increase in size leading to the destruction of the surrounding osseous tissue.[1] Another sequela that can occur following trauma to immature teeth is nonclosure of the root apices. These divergent apices pose a challenge to the clinician during obturation.[2,3] External inflammatory resorption usually occurs due to infection from a necrotic tooth following a traumatic injury. The inflammatory response triggers the activation of clastic cells, which are responsible for bone and tooth resorption.[4] T-cells found in periapical lesions express receptor activator of nuclear factor kappa B ligand (RANKL).[5] Its receptor activator of nuclear factor kappa B (RANK) is localized on the surface of progenitor clastic cells. A direct interaction between RANKL and RANK is essential for clastic cell activation.[6,7]
Recently, there have been various clinical studies and case reports advocating regenerative procedures for nonvital immature teeth, which along with revascularization of the canal space aim to bring about continued root development.[2,8,9,10] Surviving stem cells located in the apical papilla appear to play a role in regenerative procedures.[11] In the presence of periapical lesions, residual apical papilla cells may still be present. However, the probability may decrease with chronic periapical lesions.[3] Revascularization procedures for necrotic immature teeth in the presence of external inflammatory resorption is questionable, as theoretically, the resorbing apical region is under the influence of clastic cells, decreasing the probability of survival of the stem cells of apical papilla even more.
Traditionally, multiple and long-term sessions of calcium hydroxide therapy have been used to treat large periapical lesions, external inflammatory root resorption, and to bring about root closure in immature teeth.[12,13,14] However, calcium hydroxide therapy alone may not suffice for infected fluid-filled periapical lesions. Aspiration technique along with placement of a triple antibiotic paste has been recommended in infected fluid-filled lesions.[15] Due to multiple drawbacks of calcium hydroxide, mineral trioxide aggregate has replaced it as an apical barrier material for apexification.[16,17,18] Thus, when a complexity of trauma sequelae present, it would seem logical to utilize the benefits of the above materials in a systematic manner to achieve a successful result.
This case reports the management of a large periapical lesion associated with an immature tooth displaying external inflammatory resorption using an aspiration technique followed by placement of triple antibiotic paste, calcium hydroxide paste, and mineral trioxide aggregate barrier.
CASE REPORT
A 21-year-old female patient reported to the department with a chief complaint of swelling in the maxillary right labial sulcus. The patient's medical history was noncontributory. Dental history revealed trauma to the anterior teeth, with avulsion of the maxillary left central incisor when the patient was 9 years old. There was also a history of trauma for a second time to the anterior teeth a year back. Intraoral clinical examination showed a soft and fluctuant swelling in the labial sulcus region of teeth no. 11 and no. 12. Mesial tilting of tooth no. 11 was evident due to missing tooth no. 21. Teeth no. 11 and no. 12 were discolored and were nonresponsive to both electric and thermal pulp testing. Periodontal probing depths were within normal limits.
The preoperative radiograph displayed a large radiolucency, measuring approximately 13 mm × 11 mm apparently involving the apices of teeth no. 11 and no. 12. Tooth no. 11 revealed a large canal space with an open apex with ragged and irregular margins typical of external inflammatory resorption [Figure 1].
Figure 1.
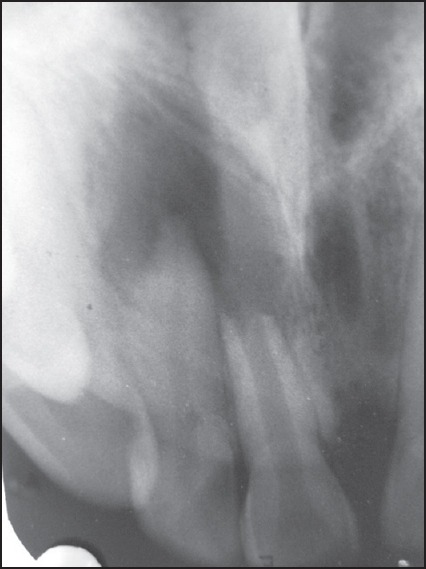
Preoperative radiograph showing periapical radiolucency measuring about 13 mm × 11 mm and immature tooth 11 with external inflammatory resorption
Following the rubber dam application, access cavity preparations were done for both teeth no. 11 and no. 12. There was drainage of purulent exudate from the canal of tooth no. 11. Tooth no. 12 was found to be nonvital, but there was no evidence of exudate from the canal. A 23-gauge needle attached to a 5 ml syringe (Dispovan, Hindustan syringes and medical devices Ltd., India) was inserted into the root canal past the open apex, beyond into the periapical region. The purulent fluid was aspirated while simultaneous digital pressure was applied on the swelling in the labial sulcus. 3% sodium hypochlorite (Vensons India, Bangalore, India) was the irrigant used during instrumentation. Following working length determination, cleaning and shaping of both teeth no. 11 and no. 12 was carried out. Tooth no. 11 was instrumented minimally with a no. 60 H-file (Dentsply Maillefer, Ballaigues, Switzerland), while tooth no. 12 was instrumented with ProTaper NiTi rotary instruments (Dentsply Maillefer, Ballaigues, Switzerland). Digital pressure was applied in the labial vestibule while the canals were dried with paper points (Dentsply Maillefer, Ballaigues, Switzerland) and temporized with Cavit (ESPE, Seefeld, Germany).
At the next appointment after 3 days, a mild swelling was still evident in the labial sulcus. The aspiration through the root canal procedure was repeated. Since, the fluid was purulent in nature, it was decided to place a triple antibiotic paste of ciprofloxacin 500 mg (Ciplox-500, Cipla Ltd., Sikkim, India), metronidazole 400 mg (Aristogyl, Aristo Pharmaceuticals Pvt Ltd., Mumbai, India), and minocycline 100 mg (Minoz 100, Ranbaxy Laboratories Ltd., Solan, India), in tooth no. 11. When the drainage ceased, and there was no more aspirate, a creamy antibiotic paste was prepared and placed in the canal of tooth no. 11 with the help of a no. 55 K-reamer (Dentsply Maillefer, Ballaigues, Switzerland). The paste was restricted only till the canal orifice to minimize tooth discoloration. Calcium hydroxide (Ca-Excel, Ammdent, India) was placed as an intracanal medicament in tooth no. 12. After 1 week, there was no swelling evident in the labial sulcus and the patient was asymptomatic. Tooth no. 12 was obturated, while triple antibiotic paste in tooth no. 11 was kept for a further 1 week. At the next scheduled visit, the triple antibiotic paste was irrigated out of the canal. There was no evidence of any exudate from the canal. Calcium hydroxide was then used as the intracanal medicament, which was refreshed every 15 days. At the end of 2 months, there was a satisfactory decrease in size of the radiolucency and radiographic [Figure 2]. An apical barrier of mineral trioxide aggregate (Angelus, Londrina, Brazil) was then placed to a thickness of about 4 mm. Some amount of hard tissue formation was also evident on the distal apical root end of tooth no. 11, indicating cessation of active external inflammatory resorption [Figure 3]. Absorbable gelatin sponge (Gelfoam, Pfizer, USA) was carefully placed beyond the confines of the canal as an apical matrix. The mineral trioxide aggregate was mixed in a ratio of 3:1 and placed with the help of an amalgam carrier (Pulpdent, USA) and condensed into place with pluggers (Dentsply Maillefer, Ballaigues, Switzerland). A moist cotton pellet was then placed at the canal orifice, and the access cavity was temporized. After 48 h, the mineral trioxide aggregate was checked for complete set and the remainder of the root canal space was obturated with Gutta-percha (Dentsply Maillefer, Ballaigues, Switzerland) and AH plus sealer (Dentsply DeTrey, Konstanz, Germany). The access cavities of both teeth no. 11 and no. 12 were restored with composite resin (Tetri-N-Ceram, Ivoclar Vivadent, Liechtenstein). The recall radiograph after 1 year shows considerable healing of the periapical lesion [Figure 4]. The recall radiograph after 1 year and 6 months [Figure 5] shows complete periapical healing and also the formation of a hard tissue layer over the mineral trioxide aggregate.
Figure 2.
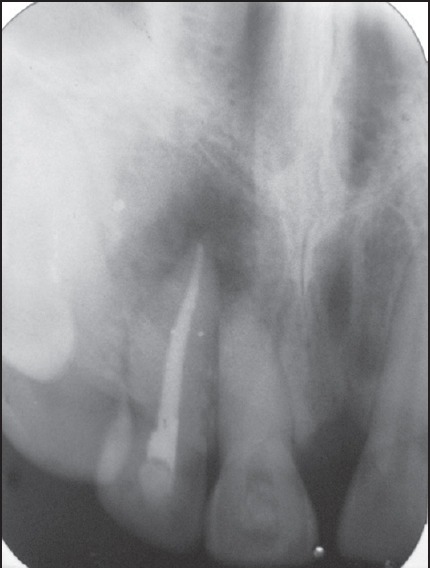
Radiograph after 2 months of calcium hydroxide therapy
Figure 3.
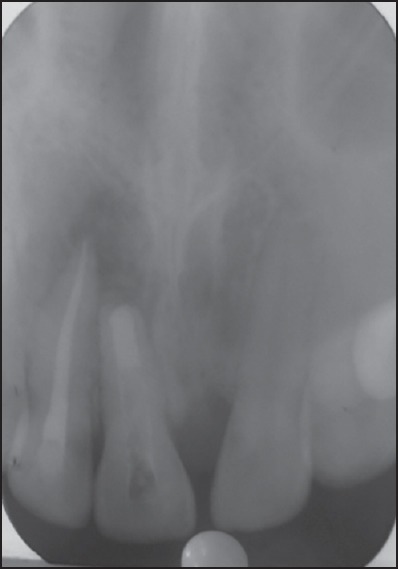
Radiograph after placement of an apical barrier of mineral trioxide aggregate
Figure 4.

Recall radiograph taken after 1 year
Figure 5.

Recall radiograph taken after 1 year and 6 months
DISCUSSION
The above case report presents a triad of trauma sequelae: Open apex, periapical lesion, and external inflammatory resorption. A tooth with extensive external inflammatory resorption may at times mimic an immature tooth.[19] However, in the present case, the wide root canal space of tooth no. 11 indicated an immature tooth, which was undergoing external inflammatory resorption. Tooth no. 12 though nonvital showed complete root development, which could be attributed to the recent trauma that the patient underwent a year back.
A four-step approach was used to manage the case: Aspiration technique + triple antibiotic paste + calcium hydroxide + mineral trioxide aggregate. A periapical lesion increases in size with an increase in the hydrostatic pressure due to fluid accumulation in the bony cavity. This increase in hydrostatic pressure increases the osteoclastic activity.[20,21] The aspiration through the root canal technique aids in decreasing the hydrostatic pressure in the periapical lesion. Application of digital pressure is essential while the aspiration is being done to enhance the complete evacuation of the fluid entrapped in the periapical lesion. Digital pressure application should be continued even while the canal is being dried and temporized to avoid entrapment of air in the bony cavity.[22] However, it has been reported that aspiration techniques alone are successful only in uninfected periapical lesions.[15,23]
Various studies have shown that triple antibiotic paste is beneficial in eliminating bacteria from infected dental tissues.[24,25,26] Metronidazole has a wide bactericidal spectrum against anaerobes while ciprofloxacin and minocycline are effective against bacteria resistant to metronidazole.[25] The purulent nature of the aspirate in the present case necessitated the use of the triple antibiotic paste. Since there was no purulent drainage from tooth no. 12, the triple antibiotic paste was placed only in tooth no. 11. However, there is a lack of evidence that the triple antibiotic paste has antiresorptive properties. Calcium hydroxide has been used for the management of external inflammatory root resorption due to its high alkalinity, which increases the pH of dentin by the diffusion of hydroxyl ions through the dentinal tubules.[13,27] Conventionally, long-term calcium hydroxide has been recommended ranging from 6 to 24 months to allow formation of a hard tissue barrier.[27,28] Calcium hydroxide has also been traditionally used for apexification of nonvital immature teeth.[12] However, this method is time-consuming and the barrier formed is usually incomplete, revealing voids or porosity.[16] Furthermore, long-term exposure of root dentin to calcium hydroxide can lead to a decrease in the fracture resistance of teeth.[17,29] Mineral trioxide aggregate has been successfully used for apexification of nonvital immature teeth.[18,30] It is more advantageous than calcium hydroxide with regard to its sealing ability and reduced treatment time.[31] Reports advocating single session placement of mineral trioxide aggregate as an apical barrier without inter appointment calcium hydroxide placement are scarce.[32] In the present case, the large periapical lesion and external inflammatory resorption negated single session placement of mineral trioxide aggregate. Mineral trioxide aggregate when in direct contact with the tissues forms calcium hydroxide that releases calcium ions for cell attachment and proliferation, modulates cytokine production, encourages differentiation and migration of hard tissue producing cells. Hydroxyapatite is formed on the surface of the mineral trioxide aggregate, thus providing a biologic seal.[33]
CONCLUSIONS
The present case was thus effectively managed using the aspiration technique + triple antibiotic paste + calcium hydroxide + mineral trioxide aggregate. This treatment eliminated the cause of periapical inflammation using the aspiration technique and antibacterial triple antibiotic paste medicament. Calcium hydroxide inhibited the resorptive activity of the clastic cells and mineral trioxide aggregate formed the apical barrier, facilitating a three-dimensional obturation of the remainder of the canal space.
Footnotes
Source of Support: Nil
Conflicts of Interest: None declared.
REFERENCES
- 1.Oztan MD. Endodontic treatment of teeth associated with a large periapical lesion. Int Endod J. 2002;35:73–8. doi: 10.1046/j.1365-2591.2002.00455.x. [DOI] [PubMed] [Google Scholar]
- 2.Torabinejad M, Turman M. Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: A case report. J Endod. 2011;37:265–8. doi: 10.1016/j.joen.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Wigler R, Kaufman AY, Lin S, Steinbock N, Hazan-Molina H, Torneck CD. Revascularization: A treatment for permanent teeth with necrotic pulp and incomplete root development. J Endod. 2013;39:319–26. doi: 10.1016/j.joen.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Heithersay GS. Management of tooth resorption. Aust Dent J. 2007;52(1 Suppl):S105–21. doi: 10.1111/j.1834-7819.2007.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 5.Silva MJ, Kajiya M, AlShwaimi E, Sasaki H, Hong J, Ok P, et al. Bacteria-reactive immune response may induce RANKL-expressing T cells in the mouse periapical bone loss lesion. J Endod. 2012;38:346–50. doi: 10.1016/j.joen.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harokopakis-Hajishengallis E. Physiologic root resorption in primary teeth: Molecular and histological events. J Oral Sci. 2007;49:1–12. doi: 10.2334/josnusd.49.1. [DOI] [PubMed] [Google Scholar]
- 7.Tyrovola JB, Spyropoulos MN, Makou M, Perrea D. Root resorption and the OPG/RANKL/RANK system: A mini review. J Oral Sci. 2008;50:367–76. doi: 10.2334/josnusd.50.367. [DOI] [PubMed] [Google Scholar]
- 8.Shah N, Logani A, Bhaskar U, Aggarwal V. Efficacy of revascularization to induce apexification/apexogensis in infected, nonvital, immature teeth: A pilot clinical study. J Endod. 2008;34:919–25. doi: 10.1016/j.joen.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Jadhav G, Shah N, Logani A. Revascularization with and without platelet-rich plasma in nonvital, immature, anterior teeth: A pilot clinical study. J Endod. 2012;38:1581–7. doi: 10.1016/j.joen.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Nagata JY, Gomes BP, Rocha Lima TF, Murakami LS, de Faria DE, Campos GR, et al. Traumatized immature teeth treated with 2 protocols of pulp revascularization. J Endod. 2014;40:606–12. doi: 10.1016/j.joen.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 11.Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. The hidden treasure in apical papilla: The potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34:645–51. doi: 10.1016/j.joen.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank AL. Therapy for the divergent pulpless tooth by continued apical formation. J Am Dent Assoc. 1966;72:87–93. doi: 10.14219/jada.archive.1966.0017. [DOI] [PubMed] [Google Scholar]
- 13.Caliskan MK, Sen BH. Endodontic treatment of teeth with apical periodontitis using calcium hydroxide: A long-term study. Endod Dent Traumatol. 1996;12:215–21. doi: 10.1111/j.1600-9657.1996.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 14.Caliskan MK, Türkün M. Periapical repair and apical closure of a pulpless tooth using calcium hydroxide. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:683–7. doi: 10.1016/s1079-2104(97)90373-5. [DOI] [PubMed] [Google Scholar]
- 15.Fernandes M. Nonsurgical management of a large periapical lesion using aspiration in combination with a triple antibiotic paste and calcium hydroxide. Iran Endod J. 2010;5:179–82. [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg F, Massone EJ, Spielberg C. Evaluation of the dentinal bridge after pulpotomy and calcium hydroxide dressing. J Endod. 1984;10:318–20. doi: 10.1016/S0099-2399(84)80186-7. [DOI] [PubMed] [Google Scholar]
- 17.Doyon GE, Dumsha T, von Fraunhofer JA. Fracture resistance of human root dentin exposed to intracanal calcium hydroxide. J Endod. 2005;31:895–7. doi: 10.1097/01.don.0000194542.02521.af. [DOI] [PubMed] [Google Scholar]
- 18.Moore A, Howley MF, O’Connell AC. Treatment of open apex teeth using two types of white mineral trioxide aggregate after initial dressing with calcium hydroxide in children. Dent Traumatol. 2011;27:166–73. doi: 10.1111/j.1600-9657.2011.00984.x. [DOI] [PubMed] [Google Scholar]
- 19.Cotti E, Lusso D, Dettori C. Management of apical inflammatory root resorption: Report of a case. Int Endod J. 1998;31:301–4. doi: 10.1046/j.1365-2591.1998.00162.x. [DOI] [PubMed] [Google Scholar]
- 20.Toller PA. Newer concepts of odontogenic cysts. Int J Oral Surg. 1972;1:3–16. doi: 10.1016/s0300-9785(72)80031-0. [DOI] [PubMed] [Google Scholar]
- 21.Seltzer S. 2nd ed. Philadelphia: Lea and Febiger; 1988. Endodontology; pp. 2391–428. [Google Scholar]
- 22.Fernandes M, De Ataide I. Non-surgical management of a large periapical lesion using a simple aspiration technique: A case report. Int Endod J. 2010;43:536–42. doi: 10.1111/j.1365-2591.2010.01719.x. [DOI] [PubMed] [Google Scholar]
- 23.Hoen MM, LaBounty GL, Strittmatter EJ. Conservative treatment of persistent periradicular lesions using aspiration and irrigation. J Endod. 1990;16:182–6. doi: 10.1016/S0099-2399(06)81968-0. [DOI] [PubMed] [Google Scholar]
- 24.Hoshino E, Kurihara-Ando N, Sato I, Uematsu H, Sato M, Kota K, et al. In-vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int Endod J. 1996;29:125–30. doi: 10.1111/j.1365-2591.1996.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 25.Sato I, Ando-Kurihara N, Kota K, Iwaku M, Hoshino E. Sterilization of infected root-canal dentine by topical application of a mixture of ciprofloxacin, metronidazole and minocycline in situ. Int Endod J. 1996;29:118–24. doi: 10.1111/j.1365-2591.1996.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 26.Takushige T, Cruz EV, Asgor Moral A, Hoshino E. Endodontic treatment of primary teeth using a combination of antibacterial drugs. Int Endod J. 2004;37:132–8. doi: 10.1111/j.0143-2885.2004.00771.x. [DOI] [PubMed] [Google Scholar]
- 27.Fuss Z, Tsesis I, Lin S. Root resorption — diagnosis, classification and treatment choices based on stimulation factors. Dent Traumatol. 2003;19:175–82. doi: 10.1034/j.1600-9657.2003.00192.x. [DOI] [PubMed] [Google Scholar]
- 28.Laux M, Abbott PV, Pajarola G, Nair PN. Apical inflammatory root resorption: A correlative radiographic and histological assessment. Int Endod J. 2000;33:483–93. doi: 10.1046/j.1365-2591.2000.00338.x. [DOI] [PubMed] [Google Scholar]
- 29.Andreasen JO, Munksgaard EC, Bakland LK. Comparison of fracture resistance in root canals of immature sheep teeth after filling with calcium hydroxide or MTA. Dent Traumatol. 2006;22:154–6. doi: 10.1111/j.1600-9657.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 30.Nayar S, Bishop K, Alani A. A report on the clinical and radiographic outcomes of 38 cases of apexification with mineral trioxide aggregate. Eur J Prosthodont Restor Dent. 2009;17:150–6. [PubMed] [Google Scholar]
- 31.Damle SG, Bhattal H, Loomba A. Apexification of anterior teeth: A comparative evaluation of mineral trioxide aggregate and calcium hydroxide paste. J Clin Pediatr Dent. 2012;36:263–8. [PubMed] [Google Scholar]
- 32.Araújo RA, Silveira CF, Cunha RS, De Martin AS, Fontana CE, Bueno CE. Single-session use of mineral trioxide aggregate as an apical barrier in a case of external root resorption. J Oral Sci. 2010;52:325–8. doi: 10.2334/josnusd.52.325. [DOI] [PubMed] [Google Scholar]
- 33.Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review — Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36:400–13. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]


