Abstract
The present investigation deals with formulation of nicotinamide-based co-crystals of fenofibrate by different methods and solid-state characterization of the prepared co-crystals. Fenofibrate and nicotinamide as a coformer in 1:1 molar ratio were used to formulate molecular complexes by kneading, solution crystallization, antisolvent addition and solvent drop grinding methods. The prepared molecular complexes were characterized by powder X-ray diffractometry, differential scanning calorimetry, Fourier transform infrared spectroscopy, nuclear magnetic resonance spectroscopy and in vitro dissolution study. Considerable improvement in the dissolution rate of fenofibrate from optimized co-crystal formulation was due to an increased solubility that is attributed to the super saturation from the fine co-crystals is faster because of large specific surface area of small particles and prevention of phase transformation to pure fenofibrate. In vitro dissolution study showed that the formation of co-crystals improves the dissolution rate of fenofibrate. Nicotinamide forms the co-crystals with fenofibrate, theoretically and practically.
Keywords: Co-crystal, nicotinamide, dissolution, fenofibrate
Currently only 8% of new drug candidates have both high solubility and high permeability. More than 40-60% of commercially available active pharmaceutical ingredients (APIs) were reported to have poor water solubility, leading to limitation of their efficacy. Therefore, how to enhance the solubility of APIs remains to be a major problem in improving performance of various drug dosage forms[1]. Enhancement of the solubility and dissolution rate of drugs is currently one of the major challenge for the pharmaceutical industry. Many approaches have been adopted for improving the aqueous solubility of drugs. There have been numerous efforts to improve drug dissolution rates. These include (a) Particle size reduction to increase the surface area; (b) use of water-soluble carriers to form inclusion complexes; (c) solubilization in surfactant systems; (d) use of prodrugs and drug derivatization and (e) manipulation of the solid state of drug substances to improve dissolution i.e. by reducing the crystallinity of drug substances through application of different approaches[2].
The crystalline form of API is generally preferred in pharmaceutical industry for drug delivery because of the inherent and thermodynamic stability of crystalline API. Crystal engineering of APIs has become a subject of considerable interest for formulation experts in recent years. Pharmaceutical co-crystals can be defined as crystalline materials comprised of an API and one or more unique co-crystal formers, which are solids at room temperature. Co-crystals usually firm through different types of interactions that include hydrogen bonding, pi stacking and van der Waals forces. Co-crystals often rely on hydrogen-bonded assemblies between neutral molecules of API and other component. For nonionizable compounds co-crystals enhance pharmaceutical properties by modification of chemical stability, moisture uptake, mechanical behavior, solubility, dissolution rate and bioavailability[3].
Fenofibrate (FNO, isopropyl ester of 2-[4-(4-chlorobenzoyl)phenoxy]-2-methylpropanoic acid) is a widely used hypolipidemic drug. Its pharmacological activity consists in reducing triglyceride and cholesterol concentration in plasma. Solubility and permeability are the fundamental parameters controlling the rate and extent of drug absorption. According to the Biopharmaceutics Classification System (BCS), FNO is a Class II drug with low solubility and high permeability. Bioavailability of FNO solely depends on dissolution rate in the gastrointestinal tract. This drug is used mostly in lipid regulation as it decreases low-density lipoprotein (LDL) and very-low density lipoprotein (VLDL) levels, and increases high-density lipoprotein (HDL) level[4]. The present investigation deals with formulation of co-crystals of fenofibrate by different methods and solid-state characterization of prepared co-crystals.
MATERIALS AND METHODS
Fenofibrate was obtained as a gift sample from Alembic Pharma, Vadodara. Nicotinamide was procured from Loba Chemie Pvt. Ltd. Mumbai. All other chemicals used were of analytical grade.
Theoretical prediction of co-crystal formation:
Solubility parameters for dry solutes may be obtained by group contribution methods. Calculations using Hoy's molar attraction constants, Fedor's substituent constants, and van Kreevalen constants are the currently used methods. In the present investigation, these methods were employed to arrive at the solubility parameter values. The basic steps in Fedor's method are to open the rings and treat the resultant structure as an open chain compound. Then the approximate substituent constants are applied. These are summed and the solubility parameter calculated as square root of the sum of energy of mixing substituent constants divided by the sum of molar volume substituent constants. Hoy's procedure expressed the ratio of molar attraction constant to molar volume. The resultant Δ values of drug and conformers are compared and their solid state miscibility is expressed[5].
Preparation of co-crystals; physical mixture and kneading methods:
Co-crystals of FNO and nicotinamide were prepared by various methods along with physical mixtures. FNO and nicotinamide in 1:1 molar ratio were added in to a mortar and mixed with the help of a spatula and mixed properly. In the kneading method, equimolar amounts of FNO and nicotinamide were taken in a mortar, small amount of ethanol was added to the above mixture and triturated with a pestle to form a knead or damp mass. The mass is then dried in a dessicator and passed through 60 mesh sieve.
Solvent drop grinding and antisolvent addition methods:
In the solvent drop grinding method FNO and nicotinamide were weighed, mixed in 1:1 molar ratio and ground together with addition of 3-4 drops of ethanol. The mixture was ground for 30 min at room temperature[6]. In the antisolvent addition method FNO and nicotinamide in 1:1 molar ratio were dissolved in 20 ml of ethanol using moderate stirring. The solution was then filtered through a Whatman filter paper to remove any undissolved material. Distilled water was then added drop-wise to the above solution with constant stirring to induce co-crystal precipitation. The co-crystals were allowed to dry overnight in a dessicator[3].
Solution co-crystallization method:
FNO and nicotinamide in 1:1 molar ratio were dissolved in 20 ml of ethanol with sonication. The saturated solution was kept overnight to evaporate solvent. The crystals obtained after evaporation of ethanol were allowed to dry in the air[3]
Drug content:
The prepared co-crystals were weighed and process yield was calculated. From the prepared co-crystals powder equivalent to 40 mg FNO was weighed and dissolved in 100 ml 0.5% sodium lauryl sulphate (SLS). The solution was filtered through a Whatmann filter paper and volume was adjusted to 100 ml. After sufficient dilution with 0.5% SLS, samples were analyzed spectrophotometrically at 290 nm and FNO content was calculated[7].
Saturation solubility studies:
Saturation solubility studies were carried out using 0.5% SLS as a solvent. Each excessive quantity (50 mg) of FNO and equivalent prepared co-crystals were taken in screw capped test tubes with fixed volume (10 ml) of 0.5% SLS. The resultant suspension was treated at 37° with 100 rpm in incubator shaker. After 24 h, samples were withdrawn and filtered through 0.2 μ filter. The filtrate was suitably diluted with 0.5% SLS and analyzed at 290 nm in a UV/Vis spectrophotometer[7].
Fourier Transform Infrared spectroscopy (FTIR):
IR spectroscopy was conducted using a Shimadzu FTIR 8300S Spectrophotometer (Shimadzu, Tokyo, Japan) and the spectrum was recorded in the wavelength region of 4000-400 cm−1. The procedure consisted of dispersing a sample (drug alone, or prepared co-crystals) in KBR and compressing into discs by applying a pressure of 5 ton for 5 min in a hydraulic press. The pellet was placed in the light path and the spectrum was recorded.
Differential scanning calorimetry (DSC):
DSC was performed using DSC-60A (Shimadzu, Tokyo, Japan) calorimeter to study the thermal behavior of drug alone and prepared co-crystals. The samples were heated in hermetically sealed aluminum pans under nitrogen flow (30 ml/min) at a scanning rate of 10°/min from 50° to 300°.
Powder X-Ray diffraction studies (PXRD):
The X-ray diffraction patterns of pure drug and the optimized crystals formulation were recorded using Philips analytical X-ray diffractometer (Model: PW 3710, Philips, Almelo, The Netherlands) with a copper target over the interval of 5-70° 2θ-1. The conditions set were, voltage at 40 kV, current at 30 mA, scanning speed at 2°/min while the temperature of acquisition was at room temperature. The detector was scintillation counter detector and the sample holder was non-rotating holder.
Solid state NMR study (SSNMR):
1H NMR spectra for the compounds were recorded on Bruker AX 500 spectrometer (500 MHz) at temperature 25°. 1H NMR Spectra was calibrated with respect to TMS and TMS was used as an internal reference for solvents such as d6-DMSO.
In vitro dissolution studies of co-crystals:
In vitro dissolution studies of solid-state forms of fenofibrate were performed using eight-station USP type II DBK dissolution rate test apparatus. The accurately weighed samples equivalent of 40 mg of drug was used. The dissolution profiles of fenofibrate, physical mixtures and co-crystals were determined in 900 ml of 0.5% SLS solution. Dissolution medium was kept in a thermostatically controlled water bath, maintained at 37±0.5° at rotation speed of 100 rpm. Samples were withdrawn periodically and fresh equal volume of dissolution media was introduced in vessels to maintain the sink condition. Samples were filtered through Whatman filter paper, diluted and analyzed at 290 nm by using Shimadzu UV-1601 Pharma Spec, spectrophotometer (Tokyo, Japan)[8].
Stability studies:
Stability studies for the samples were carried out as per ICH guidelines. The samples (each 100 mg, n=3) were kept for stability studies at 40±2° and 75±5% RH for a period of 6 months in an environmental test chamber. The samples were kept in glass vials sealed with rubber plugs. After 6 months the samples were withdrawn and analyzed for appearance, drug content, dissolution, FTIR and X-ray diffraction study.
RESULTS AND DISCUSSION
Co-crystals are miscible systems at a molecular level. It is therefore hypothesized that an indication of the miscibility of the component molecules in the solid state could predict the likelihood of co-crystal formation, which would be useful in co-crystal screening. The fact that most of the drug/coformer systems with Δδ≤7 mp or ≤5 mp showed eutectic/melting point depression indicates that the miscibility predicted by Greenhalgh correlated well with that determined by DSC. If these two conditions are met with then the proposed system should be a co-crystal. In DSC studies of FNO and nicotinamide, co-crystals showed depression in the melting point, whereas difference in δ values are below 5 so, the prepared complex can be called as co-crystals as shown in Table 1[5]. Drug content analysis was performed on co crystals prepared by all methods in triplicate. The FNO content in the prepared co-crystals was in the range of 90-94% as shown in Table 2.
TABLE 1.
THEOROTICAL PREDICTION OF CO-CRYSTAL FORMATION
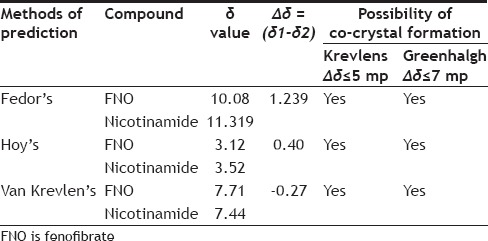
TABLE 2.
DRUG CONTENT AND SOLUBILITY DATA OF FNO AND FNO-NICOTINAMIDE CO-CRYSTALS
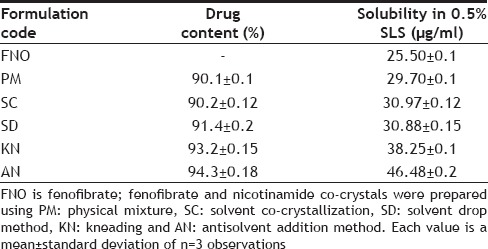
Table 2, summarized experimentally determined solubility of FNO in 0.5% SLS solution. The prepared co-crystals with nicotinamide have shown significantly higher solubility compared to their physical mixture and FNO alone. It is to be expected that FNO would dissolve better in co-crystal form due to a reduction in the crystallinity of FNO and hydrogen bond formation between FNO and the coformer. The co-crystals prepared by antisolvent addition method demonstrated higher solubility than that of FNO and other cocrystals (fig. 1) because addition of an antisolvent which reduces the solute solubility in the resultant system, or changing the solute by chemical reaction producing another substance with much lower solubility[9].
Fig. 1.
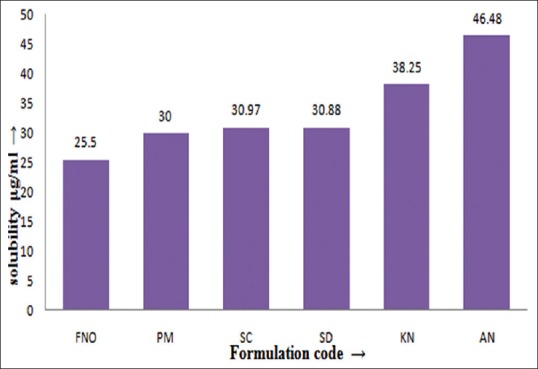
Saturation solubility data of FNO and FNO-nicotinamide cocrystals.
Saturation solubility data performed in 0.5 % sodium lauryl sulphate. FNO is fenofibrate; fenofibrate and nicotinamide co-crystals were prepared using PM- physical mixture, SC- solvent co-crystallization, SD- solvent drop method, KN- kneading and AN- antisolvent addition method.
From the results of FTIR it was observed that all the important peaks due to functional groups of drug were present in the co-crystals along with some new peaks. The result revealed considerable changes in the IR peaks of fenofibrate in prepared co-crystals when compared to pure drug thereby indicating the presence of hydrogen bonding had occurred in the co-crystals. Specific FNO peaks are observed. The peak at 2982 cm-1 indicated aromatic C-H stretching, peak at 1587 cm-1 indicated C=O stretching whereas, peaks at 1241 and 1087 cm-1 indicated aralkyl and dialkyl ether C-O stretching, respectively. Also, peak at 757 cm-1 indicates presence of halogen-hydrogen interaction. Specific nicotinamide peaks were also observed at 3529, 1283 and 1644 cm-1, which indicate the presence of aromatic –NH2 group, CN- stretching and –C=O stretching respectively. In case of co-crystals prepared by all the methods induce interaction which is indicated by disappearance of characteristics peak of aromatic -NH2 group at 3529 cm-1 that might be involved in the interaction as shown in fig. 2[4].
Fig. 2.
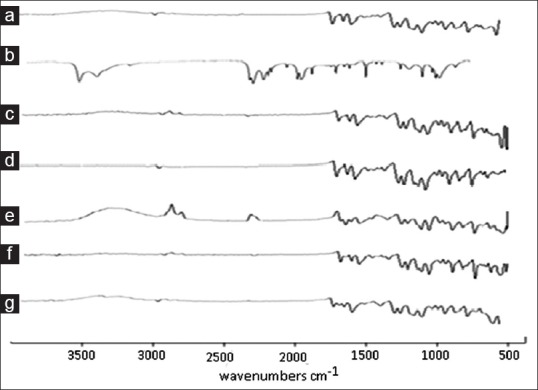
FTIR spectra of FNO, nicotinamide and co-crystals prepared by various methods.
FTIR spectra of (a) fenofibrate (FNO) (b) nicotinamide (c) physical mixture (d) antisolvent addition (e) kneading (f) solvent drop grinding and (g) solution crystallization method.
Fig. 3 represents the thermographs of FNO, physical mixture and FNO co-crystals. FNO showed sharp endothermic peak at 130° at its melting point whereas nicotinamide showed sharp endothermic peak at 128° at its melting point. The prepared co-crystals showed depression in melting point. The physical mixture and co-crystals prepared by solution co-crystallization, solvent drop grinding, kneading and antisolvent addition method showed endothermic peaks at 125, 120, 111 and 104° respectively. The melting point depression with antisolvent method was more due to reduction of particle size and increase in surface area. A depression in melting point of FNO was found in co-crystals, which indicated an interaction of FNO with nicotinamide. The DSC thermograph of FNO co-crystals showed only endothermic peak; the absence of exothermic recrystallization peak may be attributed to interaction between the drug and the conformer, which led to the prevention of conversion of FNO to its more stable form[10].
Fig. 3.
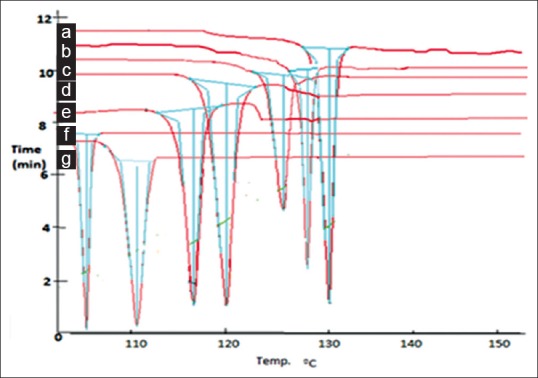
DSC thermograms of FNO, nicotinamide and co-crystals prepared by various methods.
Differential scanning calorimetric thermograms of (a) fenofibrate (FNO) (b) nicotinamide (c) physical mixture (d) solution crystallization, (e) solvent drop grinding (f) kneading and (g) antisolvent addition method.
The PXRD pattern of prepared co-crystals exhibited reduction in intensity of peaks compared to pure FNO at the specific angles, however, there are some intense peaks observed at the angles other than drug specific angles in some formulations which are due to the formation of new crystals phases as shown in fig. 4[10].
Fig. 4.
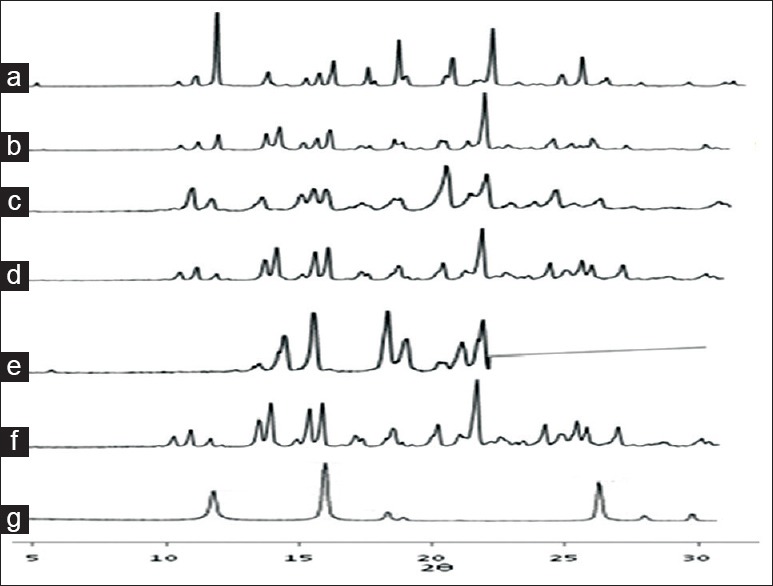
XRD patterns of FNO, nicotinamide and co-crystals prepared by various methods.
X-Ray diffraction patterns of (a) fenofibrate (FNO) (b) physical mixture (c) antisolvent addition (d) kneading (e) solution crystallization (f) solvent drop grinding (g) nicotinamide.
From the 1H NMR spectra of co-crystals of FNO and nicotinamide (fig. 5) it was observed that all the important peaks due to protons in respective functional groups were present in the prepared co-crystals along with some new peaks. The specific peaks of FNO were observed. The peak at 1.2 and 1.6 ppm shows the aromatic proton whereas the peak at 6.9 ppm showed proton attached with -Cl group. The NMR spectra of co-crystals showed disappearance of peak of –NH2 group in nicotinamide, which might indicate the hydrogen bonding between amide group and FNO. This is also supported by the FTIR spectroscopy. Also the appearance of new peaks at 3.2 and 4.9 ppm depicts the hydrogen bonding[11].
Fig. 5.
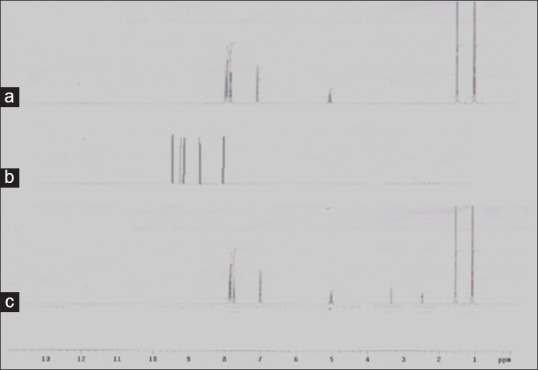
1HNMR Spectra FNO, nicotinamide and co-crystals made by antisolvent addition method.
1HNMR Spectra of (a) fenofibrate (FNO) (b) nicotinamide and (c) FNO-Nicotinamide cocrystals prepared by antisolvent addition method.
The in vitro dissolution profiles (fig. 6) of the co-crystals prepared by various methods were compared with that of pure drug and a physical mixture. The in vitro dissolution rates of all prepared co-crystals were enhanced compared to the corresponding physical mixtures and the drug. Pure drug showed 52.7% drug release at the end of 90 min. whereas, co-crystals prepared by kneading and solvent drop grinding method showed 100.2 and 95.2% after 75 min, respectively. Drug dissolution of above 90% i.e. 103.2% was obtained in 45 min for antisolvent addition method relative to both the physical mixture and the drug alone. The antisolvent addition method produce small, uniform and stable FNO co-crystals with markedly enhanced dissolution rate due to an increased solubility that is attributed to the super saturation from the fine co-crystal is faster because of large specific surface area of small particles and prevention of transformation to pure FNO[12].
Fig. 6.
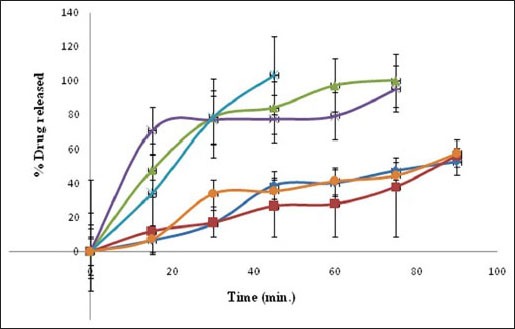
In vitro drug release profile of FNO and FNO nicotinamide co-crystals.
In vitro drug release profile of fenofibrate (FNO) and FNO nicotinamide co-crystals,  fenofibrate;
fenofibrate;  physical mixture;
physical mixture;  kneading;
kneading;  solvent drop grinding;
solvent drop grinding;  antisolvent addition and
antisolvent addition and  solution crystallization method.
solution crystallization method.
The pure FNO and co-crystals prepared by various methods were charged on accelerated stability and monitored for appearance, drug content and in vitro dissolution study at 6 months. The obtained data were mentioned in Tables 3 and 4. Also it was subjected to FTIR and X-ray diffraction study. The stability study reveals no significant variation in appearance, drug content and in vitro dissolution study of pure FNO drug and prepared cocrystals up to six months. Also there were no significant changes in FTIR and XRD spectra of co-crystals after six months as shown in figs. 7 and 8, respectively[13].
TABLE 3.
DRUG CONTENT OF FNO AND FNO-NICOTINAMIDE CO-CRYSTALS AFTER STABILITY STUDIES
TABLE 4.
% DRUG RELEASED FROM FNO AND FNO-NICOTINAMIDE CO-CRYSTALS AFTER STABILITY
Fig. 7.
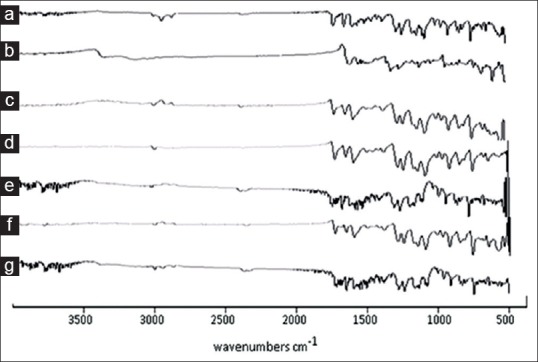
FTIR spectra of FNO, nicotinamide and co-crystals after 6 months of storage.
FTIR spectra taken after 6 months of storage under stability testing conditions of (a) fenofibrate (FNO) (b) nicotinamide (c) physical mixture (d) antisolvent addition (e) kneading (f) solvent drop grinding (g) solution crystallization method.
Fig. 8.
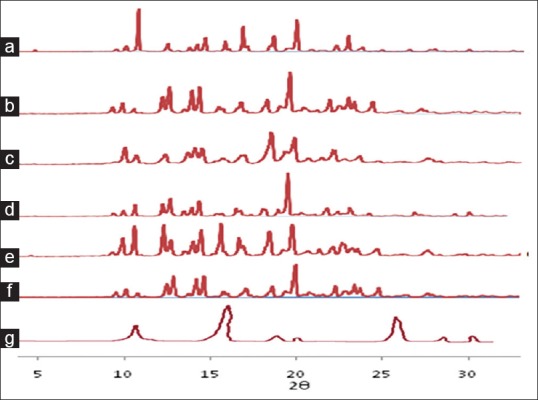
PXRD patterns of FNO, nicotinamide and co-crystals after 6 months of storage.
Powder X-Ray diffraction patterns after 6 months of storage under stability testing conditions from (a) fenofibrate (FNO) (b) physical mixture (c) antisolvent addition (d) kneading (e) solution crystallization method (f) solvent drop grinding and (g) nicotinamide.
We have developed FNO co-crystals with nicotinamide. Dissolution rate was determined for the obtained solid-state forms and characterized for FTIR, DSC, PXRD, NMR and compared with the pure FNO. The results revealed that the new solid-state form of fenofibrate with nicotinamide shows higher dissolution rate and are stable. Nicotinamide forms stable co-crystals with FNO, theoretically and practically. Antisolvent addition method is found to be the best because it produced small, uniform and stable FNO co-crystals with markedly enhanced dissolution rate and solubility of FNO.
Financial support and sponsorship:
Nil.
Conflict of interest:
There are no conflicts of interest.
Acknowledgements:
The authors are thankful to the management of Shree Santkrupa College of Pharmacy, Ghogaon for providing excellent research facilities. Also thanks Shivaji University, Kolhapur for providing the sophisticated analytical instrumentation facility (SAIF) of Shivaji University, IIT Mumbai for providing NMR facility.
Footnotes
Shewale, et al.: Formulation and Solid State Characterization of Co-crystals of Fenofibrate
REFERENCES
- 1.Po-Chun H, Hong-Liang L, Shun-Li W, Shan-Yang L. Solid-state thermal behaviour and stability studies of theophylline-citric acid cocrystals prepared by neat cogrinding or thermal treatment. J Solid State Chem. 2012;192:238–45. [Google Scholar]
- 2.Kawabata Y, Wada K, Nakatani M, Yamada S, Onoue S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int J Pharm. 2011;420:1–10. doi: 10.1016/j.ijpharm.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 3.Yadav AV, Shete AS, Dabke AP, Kulkarni PV, Sakhare SS. Co-Crystals: A novel approach to modify physicochemical properties of active pharmaceutical ingredients. Indian J Pharm Sci. 2009;71:359–70. doi: 10.4103/0250-474X.57283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav V, Yadav A. Enhancement of solubility and dissolution rate of fenofibrate by melt granulation technique. Int J PharmTech Res. 2009;2:256–63. [Google Scholar]
- 5.Mohammad MA, Alhalaweh A, Velaga SP. Hansen solubility parameter as a tool to predict cocrystal formation. Int J Pharm. 2011;407:63–71. doi: 10.1016/j.ijpharm.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 6.Patel JR, Carlton RA, Needham TE, Chichester CO, Vogt FG. Preparation, structural analysis, and properties of tenoxicam cocrystals. Int J Pharm. 2012;436:685–706. doi: 10.1016/j.ijpharm.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 7.Shete AS, Yadav AV, Murthy MS. Enhancement of dissolution rate of Irbesartan by Chitosan based Crystal Engineering Technique. Indian J Pharm Educ Res. 2012;46:323–9. [Google Scholar]
- 8.Shevchenko A, Bimbo LM, Miroshnyk I, Haarala J, Jelínková K, Syrjänen K, et al. A new cocrystal and salts of itraconazole: Comparison of solid-state properties, Stability and dissolution behaviour. Int J Pharm. 2012;436:403–9. doi: 10.1016/j.ijpharm.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 9.Giulietti M, Bernardo A. Crystallization by antisolvent addition and cooling. Crystallogr Sci Technol. :379–96. [Google Scholar]
- 10.Tomaszewska I, Karki S, Shur J, Price R, Fotaki N. Pharmaceutical characterisation and evaluation of cocrystals: Importance of in vitro dissolution conditions and type of conformer. Int J Pharm. 2013;453:380–8. doi: 10.1016/j.ijpharm.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 11.Ando S, Kikuchi J, Fujimura Y, Ida Y, Higashi K, Moribe K, et al. Physicochemical Characterization and Structural Evaluation of a Specifci 2:1 Cocrystal of Naproxen-Nicotinamide. J Pharm Sci. 2012;101:3214–21. doi: 10.1002/jps.23158. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Sun C, Hao Y, Jiang T, Zheng L, Wang S. Mechanism of Dissolution Enhancement and Bioavailability of Poorly Water Soluble Celecoxib by Preparing Stable Amorphous Nanoparticles. J Pharm Pharm Sci. 2010;13:589–606. doi: 10.18433/j3530j. [DOI] [PubMed] [Google Scholar]
- 13.Rahman Z, Agarabi C, Zidan AS, Khan SR, Khan MA. Physico-mechanical and Stability Evaluation of Carbamazepine Cocrystal with Nicotinamide. AAPS PharmSciTech. 2011;12:693–704. doi: 10.1208/s12249-011-9603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


