Abstract
The effect of lycopene on serum nitrate-nitrite levels was investigated in diabetic rats. In this investigation, 28 Wistar rats were divided into 4 groups, each of seven rats. These groups were control group, diabetes group, diabetes-lycopene group and lycopene group. The concentration of nitrite and nitrate was detected at high levels in diabetes group, diabetes-lycopene group and lycopene group as compared with the control group (P<0.05). Especially, the increase in the levels of nitrate in diabetes group and lycopene group was statistically significant when compared with diabetes-lycopene group and control group (P<0.05). In addition, we also determined the proportion of nitrite/nitrate for nitric oxide radical formation. Therefore, it is important to investigate the recovery and stability of nitrite and nitrate in samples. As a result of this study, it was observed that the amounts of nitrate and nitrite increased due to oxidative stress in diabetes and also application of antioxidant lycopene caused an increase in the amounts of nitrate and nitrite levels.
Keywords: Diabetic Rat, lycopene, nitrite-nitrate proportion
Diabetes mellitus is a genetically inheritable, chronic, metabolic disease, which is of two types, type I (insulin-dependent diabetes mellitus) and type II (noninsulin-dependent diabetes mellitus) diabetes. The effects of diabetes mellitus can be long-term damage, dysfunction and failure of various organs[1]. In living cells, nitrites and nitrates as metabolites can be converted to nitrosamines by way of interaction with amino compounds in stomach due to strongly acidic conditions[2]. Also, nitrosamines are formed due to the interaction of various nitrating agents (e.g. nitrite, nitrogen oxide) and amines in the foods[3]. In the previous studies, nitrosamine species have been found to be toxic to pancreatic beta cells and increase the risk of developing type I and type II diabetes in animal studies as well as epidemiological investigations of type I diabetes[3].
An inorganic free radical, nitric oxide (NO), is firstly responsible for regulation of vascular tone and is considered as an endogenous mediator derived from endothelium. NO is a biological molecule, which is formed catalytically from the essential amino acid L-arginine[4]. NO regulates many physiological and pathophysiological processes such as cardiovascular, inflammatory, immune and neural functions by passing through cell membranes as a gaseous signal-transmitting molecule[5].
Lycopene is a polyunsaturated unsubstituted alkene that has a symmetric plane. Like the other carotenoids, lycopene is also in tetraterpene (C40H64) structure and it is a combination unit of eight isoprenes (C5H8)[6]. The predominant source of lycopene is tomato and tomato-based products in the human diet. A strong correlation has been found between lycopene content and antioxidant activity[7]. Lycopene or its indirect effects can prevent the oxidative reactions that arise during formation of nitrosamines in human cells.
Seven to eight week old Wistar rats weighing in the range of 250-300 g were procured from the Experimental Animals Unit of Yuzuncu Yil University. These rats were randomly divided into four groups of 7 animals each. They were fed with commercial rat pellets ad libitum and allowed access to clean drinking water. They were kept at room temperature (approximately 28°). The groups were identified as control group (CG), diabetes group (DG), lycopene group (LG) and diabetes-lycopene group (DLG).
All four groups received the following treatments. Rats in the CG were injected intraperitoneally with 45 mg/kg of physiological saline. Rats in the DG received intraperitoneally 45 mg/kg dose of streptozotocin (STZ) dissolved in cold citrate buffer at pH 4.5[8]. Rats in the LG were administered 10 mg/kg/day lycopene (DSM natural Products) dissolved in corn oil by oral gavage for 28 days[9]. Animals in the DLG first received intraperitoneally 45 mg/kg dose of streptozotocin (STZ) dissolved in cold citrate buffer at pH 4.5. After establishing that the animals became diabeteic with a blood glucose level greater than 2.5 mg/ml, they were given 10 mg/kg/day of lycopene dissolved in corn oil by oral gavage for 28 days.
Nitrite and nitrate levels of serum were performed according to the method of Sthar[10] in which 100 μl of serum samples were added to 3 ml of double distilled water followed by the addition of 1 ml of coupling reagent and vortexed. After 10 min, the absorbance was measured at 520 nm (Boeco S-22 UV/Vis Spectrophotometer) and nitrite levels were determined from the absorbance values. As for that nitrate levels determination, 100 μl of serum samples were added to 1 ml CuSO4 (50 μg/ml), 1 ml (NH2)2SO4 (1 mg/ml), 1 ml NaOH (8 mg/ml) and 1 ml coupling reagent, respectively. The coupling reagent contained 300 ml double distilled water, 100 ml H3PO4 (85%), 40 g sulfanilamide and 2 g N-(1-Naphthyl)ethylenediamine dihydrochloride. This mixture was vigorously shaken and the absorbance was measured at 520 nm using a UV/Vis spectrophotometer.
Experimental results were expressed as mean±SD of triplicate measurements. Analysis of variance was estimated and significant differences between means was determined using Duncan's Multiple Range test. P values of <0.05 were regarded as significant. Statistical operations were performed using SPSS (version 15.0.0; SPSS Inc., Chicago,IL, USA) for windows.
Nitric oxide (NO) is an extremely unstable molecule and is rapidly converted to nitrite (NO2 -) and nitrate (NO3 -) in vivo and in vitro, therefore serum NO2 - and NO3 - levels have been used in vivo and in vitro as an index of NO generation[11]. NO molecule is a short-lived gaseous lipophilic metabolite produced in almost all tissues and organs, which exerts a variety of biological actions under both physiological and pathological conditions. NO is a paracrine mediator formed from its precursor L-arginine by a family of NO synthases (NOSs) with stoichiometric production of L-citruline[12]. As shown in Table 1, the obtained results of nitrite levels range from 6.63±0.41 to 12.58±0.30 ppm in serum samples (P<0.05). Otherwise, the nitrate levels differ from 21.92±0.39 to 24.68±0.51 ppm in serum samples (P<0.05). As seen from the results that all groups (DG, LG and DLG) have higher nitrite and nitrate levels than control (P<0.05).
TABLE 1.
SERUM NITRITE AND NITRATE LEVELS
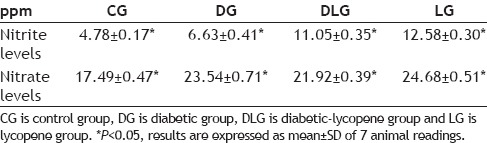
Another important parameter is the proportion of [NO2 -]/[NO3 -] which is an indicator for NO radical formation. NO is rapidly removed by diffusion through tissues into red blood cells, where it is rapidly converted to nitrate by reaction with oxyhemoglobin[13]. This highly reactive cellular radical can cause oxidative damage of biomolecules which could lead to the development of many chronic diseases such as atherosclerosis, cancer, diabetes, rheumatoid arthritis, post-ischemic reperfusion injury, myocardial infarction, chronic inflammation, stroke and septic shock, aging and many more[14]. For this reason, greater importance should be attached to the [NO2 -]/[NO3 -] ratio. The ratio values obtained were, CG (21.46), DG (21.98), DLG (33.52) and LG (33.76) as shown in fig. 1. Both lycopene groups, LG and DLG demonstrated higher [NO2 -]/[NO3 -] values compared to CG and DG. It is interesting to note in this context that in vivo production of inflammatory cytokines in human lung cancer and increase in tumor-associated NO production depended on increased levels of [NO2-]/[NO3-][15,16].
Fig. 1.
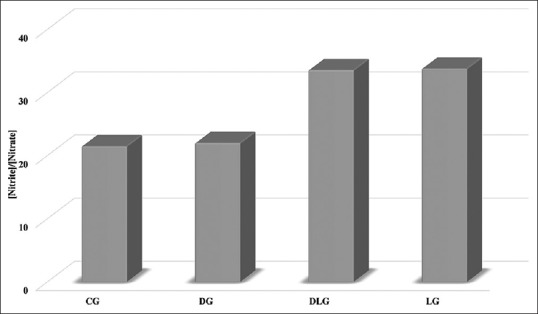
Comparison of serum [nitrite]/[nitrate] proportion.
CG is control group, DG is diabetic group, DLG is diabetic-lycopene group and LG is lycopene group.
The aim of this study is to investigate whether lycopene has protective effect on the distorted antioxidant balance caused by diabetes. Zhao et al.[17], showed that plasma nitrite-nitrate levels gradually declined with time in their study on alloxan-induced diabetic dogs (40-60 mg/kg). It was recorded that plasma nitrite-nitrate levels decreased expressively at the measurements were made at the end of the day on STZ-induced diabetic rats[17]. In contrast, in another study conducted in STZ-induced diabetic rats, it was detected that plasma nitrite-nitrate levels were significantly increased in eight-week diabetic group[18]. Similarly, it was reported that nitrite-nitrate levels in urine specimens of diabetic rats were fairly high as compare to the results of control group and this doesn't change as a result of vitamin-E treatment given as antioxidant. In the same study, it was shown that the amount of nitrotyrosine in urine samples was specifically high and this was partly reduced with antioxidant vitamin-E treatment[19]. The results of this study conform with results obtained in our study.
According to literature, after using three different resveratrol applications, it was detected that plasma nitrite-nitrate levels were significantly elevated but this increase didn't change according to resveratrol doses and duration of application[20]. It was detected that endothelial NOS (eNOS) protein expression in myocardial tissue of STZ-induced diabetic rats increased significantly after resveratrol application at the doses of 2.5 mg/kg per day for 15 days[21]. In another study, it was shown that resveratrol application to STZ-induced diabetic rats, at the doses of 5, 10 and 20 mg/kg for 28 days starting from 4th week, reduced the amount of nitrite-nitrate levels in brain tissues, which increased depending on diabetes. A suppressive paradoxical effect of resveratrol on nitric oxide formation could be explained due to its inhibitory effect on inducible NOS (iNOS)[22].
It was observed that eNOS and iNOS mRNA expression and nitrotyrosine immune reactivation on heart tissue of STZ-induced diabetic rats increased significantly after a month process and significant reductions in these parameters were observed after a powerful antioxidant compound curcumin application[23]. In a study in which nitrotyrosine levels were measured in the left ventricle and renal cortex of STZ-induced diabetic rats, it was found that nitrotyrosine levels were lower than control group. But the measured values in aorta were not different from the control group. The amount of nitrotyrosine in these tissues was significantly increased from the control group after insulin treatment[24]. The application of 5000 IU/kg of α-tocopherol in daily diet and 1000 mg/l ascorbic acid in drinking water reduced the nitrotyrosine levels increased after treatment with insulin to control values[25]. It was observed that nitrotyrosine levels did not change after diabetes and applied resveratrol treatment protocols[26].
In conclusion, lycopene is known to be an antioxidant compound which has an important role in eliminating free radicals, released as a result of oxidative stress that develops by depending on various factors. It may be result of antioxidant effect that the level of nitrate was low in diabetes-lycopene group as diabetes group. Besides, it can be said that high levels of nitrite and nitrate in lycopene group like control group may be caused by an inflammatory condition and dose arranging of lycopene should be considered as an important criterion in terms of disrupting the oxidant-antioxidant balance. In summary, lycopene, a natural antioxidant exhibited free radical scavenger properties in living cells and at the same time affected the formation of nitrite/nitrate level.
Financial support and sponsorship:
Nil.
Conflict of interest:
There are no conflicts of interest.
Acknowledgements:
This study is supported by the Project Management Office of Yuzuncu Yil University. The authors are grateful to the Project Management Office of Yuzuncu Yil University (Project no: 2010-SBE-D115). The study protocol was approved by The Local Ethics Committee for Animal Experiments of Yuzuncu Yil University (12 August 2010 and 2010/07 decree no).
Footnotes
Yegin, et al.: Lycopene effect on Serum Nitrite-nitrate in Diabetic Rats
REFERENCES
- 1.Baydaş G, Canatan H, Türkoğlu A. Comparative analysis of the protective effects of melatonin and vitamin E on streptozotocin-induced diabetes mellitus. J Pineal Res. 2002;32:225–30. doi: 10.1034/j.1600-079x.2002.01856.x. [DOI] [PubMed] [Google Scholar]
- 2.Aune D, Ursin G, Veierod MB. Meat consumption and the risk of type 2 diabetes: A systematic review and meta-analysis of cohort studies. Diabetologia. 2009;52:2277–87. doi: 10.1007/s00125-009-1481-x. [DOI] [PubMed] [Google Scholar]
- 3.Abebe EF, Akpabio CJ, Maduagwu EN. Metabolism of precursors of N-nitrosamine in vitro and nitrosamine toxicology in wistar rat. Adv Applied Sci Res. 2013;4:145–51. [Google Scholar]
- 4.Thippeswamy T, McKay JS, Quinn JP, Morris R. Nitric oxide, a biological double-faced janus- Is this good or bad? Histol Histopathol. 2006;21:445–58. doi: 10.14670/HH-21.445. [DOI] [PubMed] [Google Scholar]
- 5.Thomas G. 9th ed. Columbus, OH: McGraw Hill Companies; 2004. Basic and Clinical Pharmacology. [Google Scholar]
- 6.Sevindik H. Ankara: Ankara University Institute of Science Masters Thesis; 2007. Thermal stabilities of lycopene and β-carotene in pink grapefruit juice and tomato pulp. [Google Scholar]
- 7.Güder A, Korkmaz H, Gökce H, Alpaslan YB, Alpaslan G. Isolation, characterization, spectroscopic properties and quantum chemical computations of an important phytoalexin resveratrol as antioxidant component from Vitis labrusca L. and their chemical compositions. Spectrochim Acta A Mol Biomolecul Spectro. 2014;133:378–95. doi: 10.1016/j.saa.2014.05.056. [DOI] [PubMed] [Google Scholar]
- 8.Karabay G, Zağyapan R, Take G. An Ultrastructural Study of Effects of Streptozotocin Induced Diabetes on Rats Peripheral Nerves. U Med J. 2006;32:77–81. [Google Scholar]
- 9.Rencuzogullari N, Erdogan S. Oral administration of lycopene reverses cadmium-suppressed body weight loss and lipid peroxidation in rats. Biol Trace Elem Res. 2007;118:175–83. doi: 10.1007/s12011-007-0027-7. [DOI] [PubMed] [Google Scholar]
- 10.Sthar HM. Iowa: State Univ. Pres Ames; 1977. Analytical Toxycology Methods Manuel. [Google Scholar]
- 11.Kamal SM, Monseif WA, Nessrin H. Serum nitrite and nitrate levels in cirrhotic patients: Relationship to endotoxemia. Sci Med J ESCME. 1996;8:93–103. [Google Scholar]
- 12.Dellamea BS, Leitão CB, Friedman R, Canani LH. Nitric oxide system and diabetic nephropathy. Diabetol Metab Syndrome. 2014;6:17. doi: 10.1186/1758-5996-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arias-Diaz J, Vara E, Torres-Melero J, Garcia C, Baki W, Ramirez-Armengol JA, et al. Nitrite/Nitrate and cytokine levels in Bronchoalveolar lavage fluid of lung cancer patients. Cancer. 1994;74:1546–51. doi: 10.1002/1097-0142(19940901)74:5<1546::aid-cncr2820740509>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 16.Yegin SC, Yur F, Ceylan E. Effect of lycopene application in rats with experimental diabetes using lipoprotein, paraoxonase and cytokines. J Membrane Biol. 2013;246:621–6. doi: 10.1007/s00232-013-9575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao G, Zhang X, Smith CJ, Xu X, Ochoa M, Greenhouse D. Reduced coronary NO production in conscious dogs after the development of alloxan-induced diabetes. Am J Physiol. 1999;277:268–78. doi: 10.1152/ajpheart.1999.277.1.H268. [DOI] [PubMed] [Google Scholar]
- 18.Korolkiewic R, Rekowski P, Szyk A, Kato S, Yasuhiro T, Kubomi M. Effects of diabetes mellitus on the contractile activity of carbachol and galanin in isolated gastric fundus strips of rats. Pharmacolgy. 1998;57:65–78. doi: 10.1159/000028227. [DOI] [PubMed] [Google Scholar]
- 19.De Young L, Yu D, Bateman RM, Brock GB. Oxidative stress and antioxidant therapy: Their impact in diabetes-associated erectile dysfunction. J Androl. 2004;25:830–6. doi: 10.1002/j.1939-4640.2004.tb02862.x. [DOI] [PubMed] [Google Scholar]
- 20.Tufan C. Ankara: Gazi University Institute of Medicine Science Pharmacology Masters Thesis; 2008. effects of long term resveratrol treatment on serum nitrite/nitrate and nitrotyrosine levels of alloxan diabetic rabbits. [Google Scholar]
- 21.Thirunavukkarasu M, Penumathsa SV, Koneru S, Juhasz B, Zhan L, Otani H, et al. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: Role of nitric oxide, thioredoxin, and heme oxygenase. Free Radical Bio Med. 2007;43:720–9. doi: 10.1016/j.freeradbiomed.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma S, Kulkarni SK, Chopra K. Effect of resveratrol, a polyphenolic phytoalexin, on thermal hyperalgesia in a mouse model of diabetic neuropathic pain. Fund Clin Pharmacol. 2007;21:89–94. doi: 10.1111/j.1472-8206.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 23.Farhangkhoee H, Khan ZA, Chen S, Chakrabarti S. Differential effects of curcumin on vasoactive factors in the diabetic rat heart. Nutr Metab. 2006;3:27–35. doi: 10.1186/1743-7075-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De La Cruz JP, Arrebola MM, González-Correa JA, Martinez-Cerdán E, Moreno A, de la Cuesta FS. Effects of clopidogrel and ticlopidine on experimental diabetic ischemic retinopathy in rats. Arch Pharmacol. 2003;367:204–10. doi: 10.1007/s00210-002-0657-4. [DOI] [PubMed] [Google Scholar]
- 25.Koo JR, Vaziri ND. Effects of diabetes, insulin and antioxidants on NO synthase abundance and NO interaction with reactive oxygen species. Kidney Int. 2003;63:195–200. doi: 10.1046/j.1523-1755.2003.00728.x. [DOI] [PubMed] [Google Scholar]
- 26.Silan C. The effects of chronic resveratrol treatment on vascular responsiveness of streptozotocin-induced diabetic rats. Biol Pharm Bull. 2008;31:897–902. doi: 10.1248/bpb.31.897. [DOI] [PubMed] [Google Scholar]


