Abstract
Pancreatic cancer is a highly lethal malignancy which is increasing in incidence and mortality. The fourth leading cause of cancer death in the U.S., pancreatic cancer is projected to become the second leading cause of cancer death by 2020. Patients with pancreatic cancer have an abysmal 5-year survival of 6%, and 90% of these patients eventually die from the disease. This is in large part due to the commonly advanced stage of disease at the time of diagnosis. Currently, the only potentially curative therapy for pancreatic carcinoma is complete surgical resection. Patients who undergo incomplete resection with residual disease have similar survival rates to those patients with metastatic disease and should be spared this relatively morbid surgery. Thus, the key to impacting prognosis is the detection of smaller and earlier stage lesions, and the key to optimal management is accurately determining which patients have potentially resectable surgery and which patients would not benefit from surgery. Cross-sectional imaging plays an essential role in both the diagnosis and appropriate staging of pancreatic carcinoma. The diagnosis and staging of pancreatic adenocarcinoma is performed with cross-sectional imaging. Multi-detector computed tomography (MDCT) is the most commonly used, best-validated imaging modality for the diagnosis and staging of pancreatic cancer. Modern contrast-enhanced magnetic resonance imaging (MRI) has been demonstrated to be equivalent to MDCT in detection and staging of pancreatic cancer. Endoscopic ultrasound (EUS) is very sensitive for detecting pancreatic masses; however, due to limitations in adequate overall abdominal staging, it is generally used in addition to or after MDCT. Transabdominal ultrasound and positron emission tomography/computed tomography (PET/CT) have limited roles in the diagnosis and staging of pancreatic cancer. Preoperative imaging is used to characterize patients as having resectable disease, borderline resectable disease, locally advanced disease (unresectable) and metastatic disease (unresectable). As the definitions of borderline resectable and unresectable may vary from institution to institution and within institutions, it is essential to accurately assess and describe the factors relevant to staging including: local extent of tumor, vascular involvement, lymph node involvement and distant metastatic disease. To facilitate this, standardized reporting templates for pancreatic ductal adenocarcinoma have been created and published. Structured reporting for pancreatic cancer has been reported to provide superior evaluation of pancreatic cancer, facilitate surgical planning, and increase surgeons’ confidence about tumor resectability.
Keywords: Pancreatic cancer, staging, multi-detector computed tomography (MDCT), magnetic resonance imaging (MRI)
Introduction
Pancreatic cancer is a highly lethal malignancy which is increasing in incidence and mortality (1,2). Pancreatic adenocarcinoma, the most aggressive form, accounts for 85-95% of all pancreatic malignancies (3). It is estimated that there will be 46,420 new cases of pancreatic cancer diagnosed and 39,590 deaths from pancreatic cancer in the U.S. in 2014 (4). Approximately 90% of patients diagnosed with pancreatic cancer eventually die from the disease (5). Currently, pancreatic cancer is the fourth leading cause of cancer death in the U.S.; however, it is projected to become the second leading cause of cancer death in the U.S. by 2020 (2).
Survival with pancreatic cancer is dismal with only a 6% 5-year survival (2). This is in large part due to the commonly advanced stage of disease at the time of diagnosis (Figure 1). The most common presenting symptoms of pancreatic cancer (i.e., abdominal pain, weight loss, anorexia and asthenia) are nonspecific and no effective screening tool to detect early asymptomatic patients is available (6).
Figure 1.
A 58-year-old man with stage IV pancreatic adenocarcinoma at presentation. (A) Portal venous phase 5 mm axial MDCT image through the pancreatic body and tail reveals slight dilation of the main pancreatic duct and numerous liver metastases; (B) at a more caudal level, the hypovascular mass in the right aspect of the uncinate process and additional hepatic metastases are noted, note the high density plastic biliary stent and the moderately dilated main pancreatic duct (both seen in cross section). MDCT, multi-detector computed tomography.
Currently, the only potentially curative therapy for pancreatic carcinoma is complete surgical resection. However, this therapy is limited to patients whose tumors can be resected with negative pathologic margins (R0 resection) and do not have metastatic disease. Unfortunately, 53% of patients have distant metastatic disease at the time of diagnosis and only 15-20% of patients have potentially resectable disease at the time of diagnosis (2,7). Of those patients deemed resectable prior to surgery, 14-30% of these patients are found to be unresectable at the time of surgery (8,9). Patients who undergo incomplete resection with residual microscopic (R1) or macroscopic (R2) disease have similar survival rates to those patients with metastatic disease and should be spared this relatively morbid surgery (10). Thus, the key to optimal management is accurately determining which patients have potentially resectable surgery and which patients would not benefit from surgery. Cross-sectional imaging plays an essential role in both diagnosing and appropriately staging pancreatic carcinoma (11).
Initial diagnosis
The diagnosis of a solid pancreatic mass is made with cross-sectional imaging modalities including, transabdominal ultrasound, endoscopic ultrasound (EUS), multi-detector computed tomography (MDCT), magnetic resonance imaging (MRI) and positron emission tomography/computed tomography (PET/CT).
Ultrasound
Transabdominal ultrasound
The initial workup of typical symptoms of pancreatic cancer, including upper abdominal pain and jaundice, often starts with transabdominal ultrasound. While ultrasound is readily available, inexpensive, and does not use ionizing radiation, it is not an ideal screening tool for detection of pancreatic masses due to its relatively low sensitivity (11,12). This is in part due to high operator dependence as the sensitivity for detection of pancreatic masses has been reported from 67-90% (13). The pancreas in often not well visualized in obese patients and can be significantly obscured by shadowing bowel gas in both obese and non-obese patients. When pancreatic adenocarcinoma is identified via ultrasound, it is typically a hypoechoic hypovascular mass (Figure 2) with irregular margins. In the absence of a discrete visualized mass, secondary signs of pancreatic cancer including pancreatic duct (PD) dilatation (>2-3 mm) and contour abnormalities can be seen, suggestive of an underlying mass, thus warranting further investigation.
Figure 2.

A 50-year-old man who underwent abdominal sonography for abdominal pain. (A) Transabdominal sonographic transverse image through the pancreatic body and tail in the upper abdomen shows a poorly marginated hypoechoic lesion (arrow); same patient, multiphasic MDCT the next week demonstrates that the small mass in the posterior pancreatic body and the upstream main pancreatic duct are much better seen on the pancreatic parenchymal phase 2.5 mm axial image (arrow on B) acquired at 35 s after the initiation of IV contrast medium compared to the portal venous phase image (arrow on C) acquired at 70 s. MDCT, multi-detector computed tomography.
Endoscopic ultrasound (EUS)
EUS is the dominant endoscopic technique used for the diagnosis and evaluation of pancreatic masses (12). High resolution imaging of the pancreas can be achieved by placing a high frequency probe in close proximity to the pancreas (14). EUS is highly sensitive for the detection of pancreatic masses (sensitivities reported as high as 93-100%) and has a negative predictive value approaching 100%, particularly when used in conjunction with fine needle aspiration (FNA) (13). EUS is useful for the detection of small masses (<2-3 cm) which may be occult on other imaging modalities and for patients with indeterminate findings on prior imaging (15-17). The National Comprehensive Cancer Network (NCCN) guidelines for pancreatic adenocarcinoma state that patients who do not have a pancreatic mass visualized on cross-sectional imaging should undergo further evaluation with EUS and/or endoscopic retrograde cholangiopancreatography (ERCP) as clinically indicated (18). Another advantage of EUS is that pancreatic masses can be detected and characterized without the use of intravenous contrast, which is of particular use for patients with renal dysfunction or other contraindications to intravenous contrast. The typical appearance of pancreatic adenocarcinoma with EUS is a heterogeneous hypoechoic solid mass with irregular borders; however, this appearance is not specific for adenocarcinoma.
EUS is an invasive procedure; however, it is generally safe, and has reported procedural complication rates as low as 1.1-3% (19). The most commonly reported complications are bleeding (1-4%), pancreatitis (1-2%), perforation (0.03%) and tumor seeding of the biopsy tract (20). Peritoneal tumor seeding with EUS-FNA is a rare complication and occurs less frequently with EUS-FNA than with percutaneous biopsy (21). The major limitation of EUS that impacts patient care and management decision making is the inability to stage disease beyond the pancreas, thus it is generally used in addition to or after MDCT.
Multi-detector computed tomography (MDCT)
MDCT is widely available and the most commonly used, best-validated imaging modality for the evaluation of a patient with a suspected pancreatic mass (11,18). The reported sensitivity of MDCT for the detection of pancreatic adenocarcinoma is as high as 89-97% (22). The sensitivity for detecting small masses (≤1.5 cm) is lower and has been reported to be 67% (23). The typical appearance of pancreatic adenocarcinoma on MDCT is an ill-defined mass which is hypoenhancing relative to the avidly-enhancing non-tumoral pancreatic parenchyma (Figure 3). Eleven to twenty-seven percent of adenocarcinomas are isoenhancing to the pancreatic parenchyma and are occult on CT, particularly when small (24-26). In these cases, secondary signs of a pancreatic mass such as abrupt cutoff of the PD with upstream dilatation (Figure 4), mass effect, and contour abnormality may be present (27). Approximately 10% of pancreatic adenocarcinomas do not appear as a focal mass but as diffuse gland enlargement/involvement (28).
Figure 3.

A 60-year-old man who presented to the emergency department with nausea and abdominal pain was found to have possible pancreatic head mass. (A) Portal venous phase 5 mm axial image demonstrates fullness in the pancreatic head, but a mass is not clearly discernable. A multiphasic MDCT examination was performed specifically to evaluate potential pancreatic mass; (B) pancreatic parenchymal phase 2.5 mm axial image better demonstrate the margins of the hypovascular mass in the posterior head region compared to either the initial emergency department CT or (C) the 5 mm portal venous phase image obtained as part of the multiphasic pancreatic scan. MDCT, multi-detector computed tomography.
Figure 4.
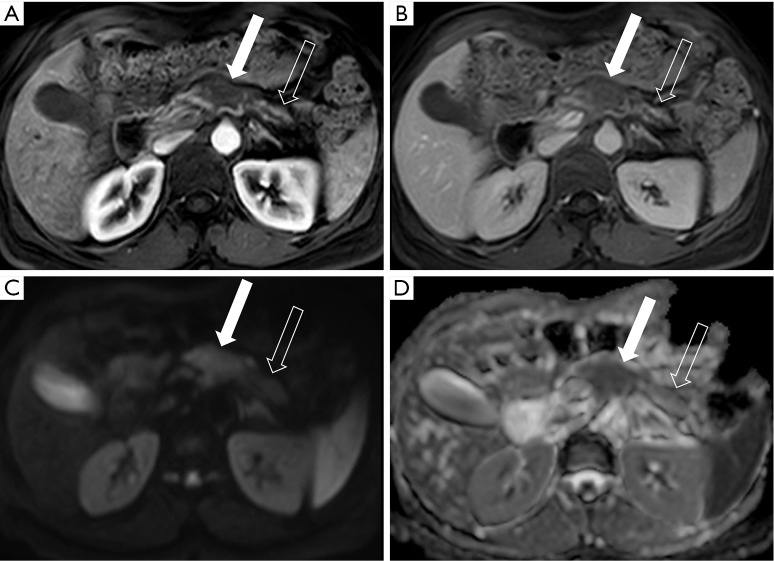
A 63-year-old woman with small pancreatic adenocarcinoma and upstream main pancreatic duct dilation. (A) Coronal reformatted 3 mm MDCT portal venous phase image demonstrates the dilated main pancreatic duct (small arrow) leading in to the 1.0 cm ductal adenocarcinoma (large arrow) in the pancreatic neck region. Note the slightly diminished enhancement of the gland in the body and tail region; the tiny tumor is better depicted on the pancreatic parenchymal phase 2.5 mm axial image (B); compared to the portal venous phase image (C) and appears resectable from a vascular standpoint; however, there is a small metastasis present in the lateral segment of the left lobe of the liver (circle on B). MDCT, multi-detector computed tomography.
Pancreas CT protocols can vary somewhat from institution to institution but typically are multiphasic with thin-section imaging (≤3 mm) and with multi-planar reconstructed (MPR) images (coronal and/or sagittal planes). Post-contrast imaging must include the pancreatic parenchymal phase which is a late arterial phase acquired after a delay of 35-50 s and a portal venous phase which is acquired after a delay of 60-90 s (29,30). The pancreatic parenchymal phase is timed for peak parenchymal enhancement to maximize the difference in enhancement of the hypoenhancing adenocarcinoma and background pancreas in order to increase conspicuity of the mass (31,32) (Figure 2). This phase allows for adequate evaluation for the relationship of the mass with adjacent arterial structures which is essential for staging (31,32). The portal venous phase of imaging provides optimal evaluation for involvement of adjacent veins (mesenteric, portal and splenic) and for the presence of metastatic disease, particularly in the liver (30). However, despite optimal imaging, small metastatic lesions in the liver can be missed on CT resulting in unresectable disease being found at surgery (33).
MPR images are typically included in a pancreas protocol CT as they have been shown to improve evaluation of local extension of tumor and evaluation for vascular involvement (34,35). Curved planar reformatted (CPR) images (Figure 5) are also often included as they have been shown to increase lesion detection and improve evaluation of vascular involvement (36,37).
Figure 5.
A 69-year-old man with a narrowed superior mesenteric vein. (A-D) Successive coronal reformatted images progressing from anterior to posterior demonstrate narrowing of the portal confluence by the hypovascular pancreatic adenocarcinoma in the superior head region, much better depicted, particularly from the standpoint of length of vein involved, on the curved multiplanar reformatted image (E). The axis of this image is aligned with the long axis of the portal vein.
Dual-energy CT (DECT) (Figure 6) is a novel imaging method which utilizes X-ray beams at two different energy levels to increase image contrast on intravenous contrast-enhanced CT images. This is possible because the viewing energies can approach the K-edge of iodine, and the differences in Hounsfield units (HU—CT measure of density or linear attenuation coefficient of tissue) between tumoral and non-tumoral tissue increases. DECT also allows generation of iodine images from the same CT acquisition; these images have high contrast to noise ratios, thus enhancing lesion conspicuity. This advance is important for imaging small pancreatic cancers which tend to be isoattenuating or near isoattenuating to the remainder of the pancreas. Early studies have shown an improvement in lesion detection for patients with pancreatic adenocarcinoma (38-41). Staging can also be improved by review of iodine images and generation of CT angiograms from low energy or iodine datasets (41). It is important to note that dual energy CT techniques are relatively radiation dose neutral examinations, and do not result in significantly increased radiation exposures for patients compared to standard single energy CT (42).
Figure 6.

Dual energy MDCT in a 50-year-old man with a small resectable pancreatic ductal adenocarcinoma in the body region (same patient as Figure 2). (A) Low viewing energy (52 keV) axial 2.5 mm image and (B) iodine material density 2.5 mm image demonstrate increased conspicuity of the lesion and its relationship to the adjacent splenic artery (compare to Figure 2B and 2C). MDCT, multi-detector computed tomography.
Magnetic resonance imaging (MRI)
Modern contrast-enhanced MRI has been demonstrated to be equivalent to MDCT in detection and staging pancreatic cancer (43,44). With its superior contrast resolution, MRI provides increased lesion conspicuity and may be better than CT at detecting small cancers (44-46). MRI is particularly useful for the detection and characterization of pancreatic masses that are isoenhancing to the pancreatic parenchyma and not directly seen on CT (25). A limitation of MRI in the detection of pancreatic adenocarcinoma is the susceptibility of MRI to significant degradation by respiratory motion artifact. This is of particular concern when using gadoxetate disodium contrast as it has been associated with increased motion artifact on arterial-phase imaging, which is often critical for detecting these cancers (47,48). The typical appearance of pancreatic adenocarcinoma on MRI is an ill-defined T1 hypointense, T2 hypointense, relatively hypoenhancing mass. Adenocarcinomas usually demonstrate restricted diffusion on diffusion weighted imaging (Figure 7), which may allow for increased detection of tumors even in the unenhanced state (49).
Figure 7.
A 49-year-old woman who underwent upper abdominal MRI to evaluate an incidental hepatic lesion detected on abdominal ultrasound obtained for abdominal pain. (A) Pancreatic parenchymal; and (B) portal venous phase 5 mm axial images well depict the 3.0 cm mass (solid arrows) in the pancreatic body. Note the upstream glandular atrophy and main pancreatic duct dilation (open arrows); the lesion is seen as high (bright) signal on the diffusion weighted image (arrow on C); and is confirmed to have restricted diffusion on the ADC map (arrow on D). MRI, magnetic resonance imaging; ADC, apparent diffusion coefficient.
PET & PET/CT
PET and PET/CT are not routinely used for the initial diagnosis of cancer in patients with clinical suspicion for pancreatic adenocarcinoma. PET/CT is more sensitive for the detection of pancreatic cancer than PET alone (50). The sensitivity and specificity of PET/CT in diagnosing pancreatic carcinoma has been reported to be 89% and 88%, respectively (51). PET/CT may be more sensitive for the diagnosis of pancreatic carcinoma than conventional MDCT and MRI (51). Multiple studies have demonstrated that PET/CT is more sensitive than standard cross-sectional imaging for detecting distant metastatic disease (52,53). Contrast-enhanced PET/CT has also been shown to improve detection of distant metastatic disease when compared with non-contrast PET/CT (54). The typical appearance of pancreatic carcinoma on PET/CT is a focal fluorodeoxyglucose (FDG)-avid mass with CT or MRI characteristics as previously described (Figure 8).
Figure 8.
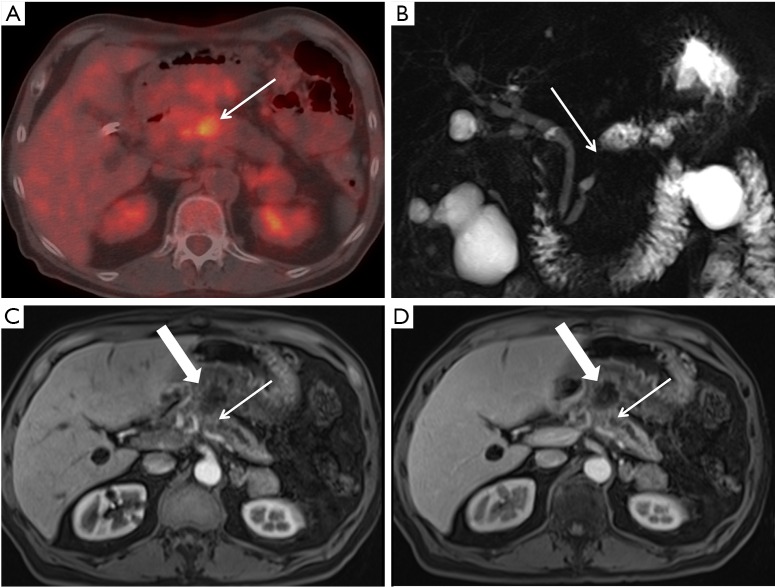
A 75-year-old man with SMV occlusion and locally advanced pancreatic cancer who underwent PET/CT. (A) Axial PET/CT image through the pancreatic body and neck regions reveals an FDG-avid lesion in the midline (arrow). No distant metastatic lesions were detected, but there is abnormal, less FDG avid activity extending toward the gastric antrum; (B) MRCP image demonstrates focal narrowing of the main pancreatic duct (arrow) in the region of the mass, with upstream dilation in the body and tail; (C) pancreatic parenchymal phase 5 mm axial image; and (D) portal venous phase 5 mm axial image demonstrate the abrupt duct cut off by the small pancreatic mass (small arrows), with an inflammatory collection extending towards the stomach. SMV, superior mesenteric vein; PET/CT, positron emission tomography/computed tomography; FDG, fluorodeoxyglucose; MRCP, magnetic resonance cholangiopancreatography.
The role of PET/CT in the initial diagnosis and staging is evolving and not well defined at this time. The NCCN clinical practice guidelines acknowledge the utility of PET/CT in staging pancreatic adenocarcinoma but state that PET/CT is not a substitute for high-quality contrast-enhanced CT but can be used in conjunction with a pancreas-protocol CT as indicated (18).
Staging
Cross-sectional imaging plays an essential role in the staging of pancreatic adenocarcinoma and thus determining the most appropriate therapy for patients. MDCT is the most widely used and validated modality for the staging of pancreatic adenocarcinoma; however, MRI is an equivalent alternative to MDCT for staging. The NCCN practice guidelines recommend that imaging for staging should be done with specialized pancreatic CT or MRI while the consensus statement by the International Study Group of Pancreatic Surgery (ISGPS) recommends evaluation with specialized pancreatic CT (55,56). The decision to use MDCT or MRI should be based on availability, local practice, and local experience/expertise.
Preoperative imaging is used to characterize patients as having resectable disease, borderline resectable disease, locally advanced disease (unresectable without distant metastatic disease) and metastatic disease (unresectable). Borderline resectable disease refers to locally advanced pancreatic adenocarcinoma with involvement of the mesentericoportal veins or local arteries that is in between routinely resectable disease and definitely unresectable disease (56). The exact definitions of borderline resectable and unresectable disease have evolved over recent years and still vary from institution to institution and between different societies. Therefore, it is critical that accurate assessment and reporting of the local extent of disease and the presence and absence of lymph node and distant metastatic disease is performed for optimal management.
The staging system that is most commonly used by clinicians is the TNM staging system maintained by the American Joint Committee on Cancer (AJCC) (57). This system evaluates local extent of the primary tumor, lymph node involvement, and presence of distant metastatic disease to classify disease and give prognosis (Table 1) (58). The resectability of a tumor is dependent on its location in the pancreas, involvement of local arteries (celiac, superior mesenteric, and hepatic) and veins (superior mesenteric and portal), lymph node involvement, and presence of distant metastatic disease. A step-wise approach to assessment of resectability is utilized in our practice and includes: (I) location of the primary tumor and relation to surrounding organs; (II) evaluation of distant metastatic disease (most commonly in the liver and peritoneum); (III) involvement of the peripancreatic arteries; (IV) involvement of the peripancreatic veins, with description that can allow the surgeon to prepare for potential vein graft; (V) extrapancreatic perineural spread of tumor to the celiac region. If stage IV disease is identified in the liver, a critical analysis of the peripancreatic vessel involvement is not necessary.
Table 1. TNM pancreatic cancer staging (AJCC).
| Stage | Definition |
|---|---|
| Primary tumor (T) | |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor limited to pancreas, ≤2 cm |
| T2 | Tumor limited to pancreas, >2 cm |
| T3 | Extension into peripancreatic tissues (excluding arteries) |
| T4 | Tumor involves celiac axis or superior mesenteric artery |
| Regional lymph nodes (N) | |
| Nx | Regional lymph nodes not assessed |
| N0 | No metastatic regional lymph nodes |
| N1 | Metastatic regional lymph nodes |
| Distant metastasis (M) | |
| M0 | No distant metastatic disease |
| M1 | Distant metastatic disease |
AJCC, American Joint Committee on Cancer.
Tumor location
Approximately 60-70% of pancreatic cancers involve the pancreatic head (3,59). Pancreatic head cancers are defined as those arising to the right of the superior mesenteric–portal vein confluence (58). Approximately 10-20% of pancreatic cancers are in the body and 5-10% are in the tail. Cancers between the mesenteric-portal vein confluence and left lateral margin of the aorta are in the body and those lateral to the aorta are in the tail (58). The location of the tumor determines whether the patient would be treated with a pancreaticoduodenectomy (Whipple procedure) or distal pancreatectomy. The size of the tumor is also important, as it contributes to the T stage and could be important for determining response to the therapy on subsequent studies (60).
Location of the tumor is also important as it determines the route of local spread of disease. With pancreatic adenocarcinoma, there can be direct invasion (Figure 9) of adjacent structures (e.g., duodenum, stomach, adrenal gland, kidney, and colon); however, this does not make disease for a patient unresectable, if this extension can be otherwise adequately and safely resected (61). One route of direct tumor spread that is of particular importance for tumors of the head and uncinate process is perineural invasion (retrograde extension of disease along the neural fascicles of the neurovascular bundles), as it is indicative of a very poor prognosis (62). Perineural invasion (Figure 10) is extremely common with pancreatic carcinomas of the head and uncinate process, being reported in up to 53-100% of cases, and often results in positive resection margins at surgery (63). Adenocarcinomas of the pancreatic head typically spread along the plexus pancreaticus capitalis 1 (PPC1) or gastroduodenal artery (GDA) plexus (if in the dorsal aspect of the head). This can be seen on MDCT as direct contiguous extension of tumor soft tissue extending posterior to the portal vein to along the medial upper margin of the uncinate process or along the GDA to the common hepatic artery (CHA), respectively (63). Adenocarcinomas of the uncinate process typically extend along the PPC2. This can be seen on MDCT as direct contiguous tumor soft tissue extending along the posteroinferior pancreaticoduodenal artery (PIPDA) up to and along the superior mesenteric artery (SMA) (63,64). Note is made that tumor can also extend along this pathway to involve the mesenteric root (63).
Figure 9.
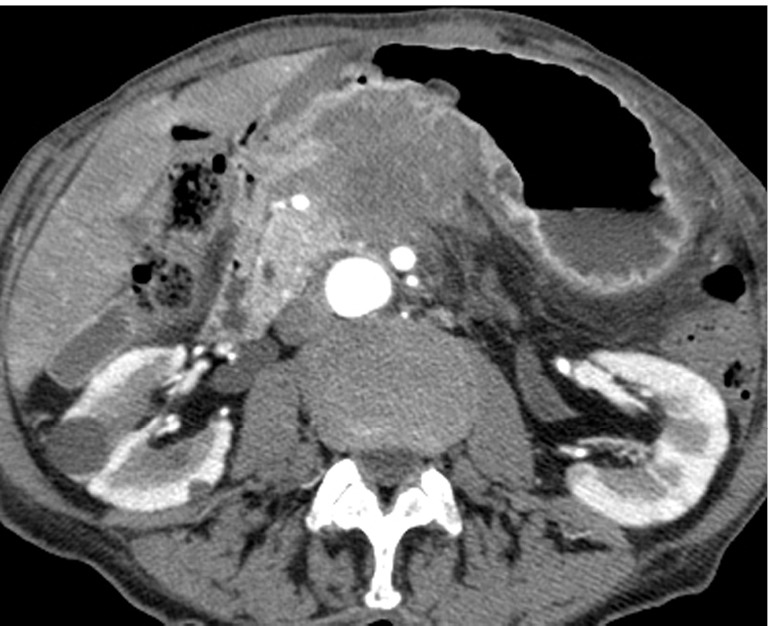
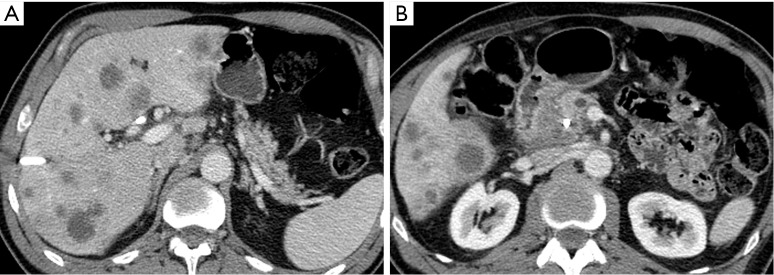
An 85-year-old woman with locally invasive pancreatic adenocarcinoma. Pancreatic parenchymal phase axial image demonstrates the low attenuation hypodense mass in the pancreatic neck/body extending through the posterior antral wall and disrupting the enhancing gastric mucosa.
Figure 10.
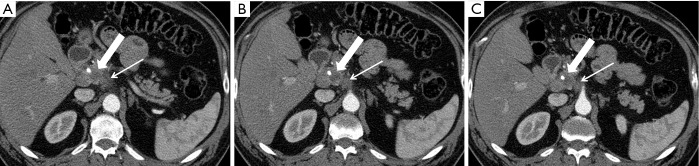
A 61-year-old man with small pancreatic cancer and perineural spread to the celiac ganglion. (A-C) Successively caudal pancreatic parenchymal phase 2.5 mm axial images demonstrate the hypovascular mass in the medial pancreatic head extending posteriorly along the plexus pancreaticus capitalis 1 and abutting the right margin of the celiac trunk. This patient received neoadjuvant therapy.
Vascular involvement with tumor
Determining vascular involvement is the most important component of determining the resectability of a borderline or locally advanced pancreatic adenocarcinoma. Evaluation of the celiac artery, SMA, CHA, superior mesenteric vein (SMV), and portal vein are essential for accurate staging and determining subsequent therapy. Encasement (>180˚ circumferential contact) of a vessel by tumor (Figure 11) is an imaging sign of vascular invasion with a sensitivity of 84% and specificity of 98% (65). Abutment (≤180˚ circumferential contact) of a vessel with tumor (Figure 12) is not considered a sensitive sign of vessel invasion (65). Addition findings suggestive of vessel invasion are tumor causing vessel deformity (tear-drop configuration) or narrowing (regardless of degree of contact), vessel irregularity, direct invasion into a vessel, and thrombosis (3,66). Note that the degree of vascular contact is best evaluated perpendicular to the long axis of the vessel (Figure 13), so, for example, the SMA and SMV should be assessed on axial images, while a coronal or sagittal reformatted image might better demonstrate involvement of the portal vein and CHA. These imaging signs of vessel invasion were selected to maximize specificity (at the expense of sensitivity) to ensure that patients with clearly unresectable disease did not undergo an unnecessary surgery and to minimize the number of patients with potentially resectable disease being denied surgery.
Figure 11.
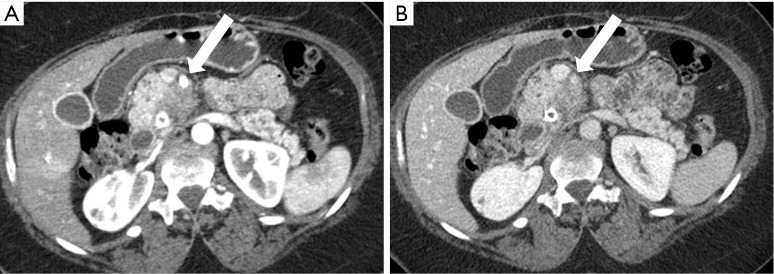
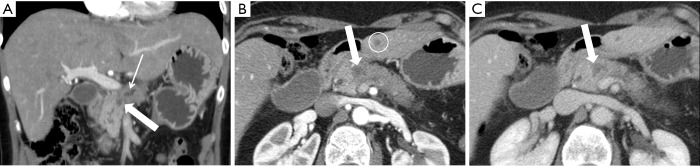
A 55-year-old woman with SMA encasement. (A) Pancreatic parenchymal phase 2.5 mm axial image depicts the relationship of the hypovascular mass in the medial pancreatic head to the SMA (arrow) where there is ≥180° contact indicating encasement; note that this relationship is better seen on this phase of IV contrast administration compared to (B) the portal venous phase 5 mm axial image. SMA, superior mesenteric artery.
Figure 12.
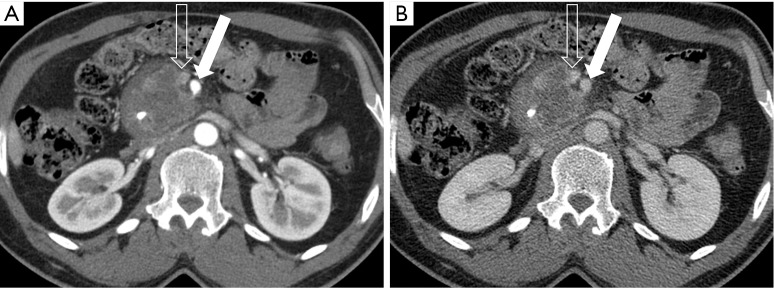
A 52-year-old man with SMA abutment. (A) Pancreatic parenchymal phase 2.5 mm axial image demonstrates contact of the large mass in the pancreatic head with <90° of the SMA (arrow); the SMV (open arrow) is not well evaluated in this phase of contrast, but is better seen on (B) the portal venous phase 5 mm image, where approximately 180° contact is present with slight straightening of the right lateral SMV (open arrow) wall indicating involvement/invasion. SMA, superior mesenteric artery; SMV, superior mesenteric vein.
Figure 13.
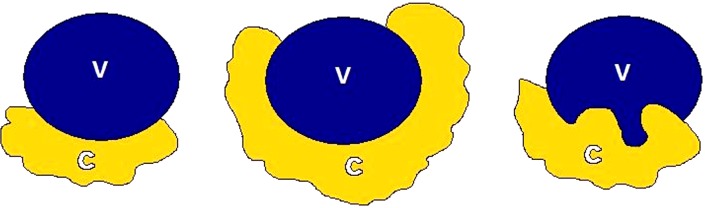
Cartoon depiction of vascular involvement. (A) Abutment of the C with the V; (B) encasement; and (C) involvement/invasion with teardrop deformity. C, cancer; V, vessel.
The exact definition of borderline resectability and unresectability of locally advanced pancreatic cancer is vague, controversial, and varies from institution to institution (67). Differences in imaging practices and interpretation, local surgical skill, and local experience contribute to these varying definitions. Tumors with no evidence of metastatic disease, no definite involvement (abutment or encasement) of the SMV or portal vein, and clear fat planes around the celiac artery, hepatic artery and SMA are considered clearly resectable as per the consensus statements by the NCCN and by the American Hepato-Panceato-Biliary Association (AHPBA)/Society for Surgery of the Alimentary Tract (SSAT)/Society of Surgical Oncology (SSO)/Gastrointestinal Symposium Steering Committee (GSSC)/University of Texas M. D. Anderson Cancer Center (MDACC) (68,69). Note is made that isolated tumor involvement of the pancreaticoduodenal artery does not constitute borderline resectability or unresectability, as this vessel is routinely resected as part of a Whipple procedure.
The MDACC published a classification system for the resectability of pancreatic cancer in 2006 (70). Subsequent consensus guideline statements regarding borderline resectable cancer have been published by the NCCN, the AHPBA/SSAT/SSO/MDACC, and the ISGPS (18,56,68,70,71). The Alliance for Clinical Trials in Oncology (ACTO) has recently published their own definition of borderline resectable disease (67). These are summarized in Table 2. Findings that are not directly related to vascular invasion but otherwise affect surgical planning are extension of the tumor along the CHA to the origins of the right and left hepatic arteries, extension of tumor along the SMA to the first branch, and extension of tumor along the SMV to the most proximal draining vein (72).
Table 2. Different definitions of borderline resectable pancreatic cancer.
| Anatomy | NCCN 2014 | AHPBA/SSAT/SSO | MD Anderson Cancer Center | ISGPS | ACTO |
|---|---|---|---|---|---|
| Superior mesenteric vein/portal vein | Involvement with distortion/narrowing and/or occlusion amenable to reconstruction | Abutment, encasement, or short-segment occlusion amenable to reconstruction | Short-segment occlusion amenable to reconstruction | Involvement with distortion/narrowing and/or occlusion amenable to reconstruction | Tumor-vessel interface ≥180° and/or occlusion amenable to reconstruction |
| Superior mesenteric artery | Abutment (≤180°) | Abutment (≤180°) | Abutment (≤180°) | Abutment (≤180°) | Tumor-vessel interface <180° |
| Common hepatic artery | Abutment or short-segment encasement | Abutment or short-segment encasement | Short segment encasement/abutment | Abutment or short-segment encasement | Short-segment tumor-vessel interface (any degree) amenable to reconstruction |
| Celiac artery | No abutment or encasement | No abutment/encasement | No abutment or encasement | No abutment or encasement | Tumor-vessel interface <180° |
NCCN, National Comprehensive Cancer Network; AHPBA/SSAT/SSO, American Hepato-Pancreato-Biliary Association/Society for Surgery of the Alimentary Tract/Society of Surgical Oncology; ISGPS, International Study Group of Pancreatic Surgery; ACTO, Alliance for Clinical Trials in Oncology.
Accurate restaging of vascular involvement following preoperative neoadjuvant therapy of borderline resectable pancreatic cancers is difficult and somewhat controversial. Neoadjuvant-therapy-induced regional changes decrease the sensitivity of CT for detecting disease resectability (71). Katz et al. demonstrated that while only 0.8% of patients demonstrated downstaging to resectable disease on imaging, 66% of patients were found to be resectable at surgery (73). The ISGPS consensus statement recommends that if neoadjuvant therapy is administered, an exploratory laparotomy with attempted resection should be considered in the absence of disease progression (distant metastasis) on subsequent imaging (56).
In addition to vascular involvement with tumor, relevant variant vascular anatomy is also important to identify and report when determining resectability. For example, multiple jejunal branches inserting high on the SMV near the portal confluence can make vascular resection/reconstruction difficult (74). Arterial variants that can preclude resection include a replaced hepatic artery arising from the SMA (which is involved with tumor) and a low origin of the CHA from the celiac axis with an aberrant course inferior to the portal vein (74).
Nodal disease
Although cross-sectional imaging is not particularly sensitive for the detection of lymph node involvement with pancreatic cancer, MDCT is generally considered the modality of choice. Abnormal appearing region lymph nodes (>1 cm in short axis diameter, rounded morphology, or cystic appearance) that are in the surgical bed are considered nodal metastasis and are generally not a contraindication to surgery; however, if confirmed at surgery, adjuvant chemotherapy is indicated. For cancers in the pancreatic head/neck, this includes lymph nodes along the celiac axis and in the peripancreatic and periportal regions and for cancers in the body/tail this includes lymph nodes along the CHA, celiac axis, splenic artery and splenic hilum. Lymph node involvement outside of the surgical bed is considered distant metastatic disease and is a contraindication for surgery. Therefore, a description of the location of abnormal appearing lymph nodes is the most important aspect of nodal evaluation for staging.
Distant metastatic disease
Distant metastatic disease most commonly occurs in the liver, peritoneum, lungs and bones. As previously stated, lymph node metastases outside of the surgical field are considered distant metastases. The presence of distant metastatic disease makes the primary lesion unresectable. Note that if a patient is scanned initially with a standard abdominal portal venous phase MDCT, and liver metastases along with a primary pancreatic adenocarcinoma are clearly evident, a repeat multiphasic CT is not required to further evaluate, and follow up imaging can also be single portal venous phase. The majority of patients found to have unresectable disease at surgery despite the appearance of resectable disease on state of the art multiphasic MDCT preoperative imaging are due to small metastatic lesions in the liver and peritoneum. Evaluation for hepatic metastatic disease is most often performed with MDCT or MRI; however, MRI is more sensitive for the detection of small metastatic lesions (75). Furthermore, MRI provides better specificity in characterizing indeterminate liver lesions (43), and MRI is often used for further evaluation when MDCT demonstrates indeterminate liver lesions. None of the imaging modalities are sensitive for the detection or early peritoneal disease. Peritoneal thickening/nodularity and/or ascites not otherwise explained should be considered suspicious for metastatic disease. Although PET/CT has been reported to be more sensitive for the detection of distant metastatic disease, the cost-effectiveness has not been proven, and PET/CT is not routinely used in staging (76).
Structured reporting
As imaging plays an essential role in determining the appropriate management of patients with pancreatic adenocarcinoma, an accurate, complete, and concise report is needed to ensure that the pertinent findings are relayed to the referring clinicians. Structured reports have been shown to not only be equally efficient and accurate in conveying information to referring clinicians as free-style reports, they have been shown to be more accepted and preferred by both radiologists and clinicians (77-79). A standardized reporting template for pancreatic ductal adenocarcinoma has been published as a consensus statement of the Society of Abdominal Radiology (SAR) and the American Pancreatic Association (APA) (72). Structured reporting for pancreatic cancer has been reported to provide superior evaluation of pancreatic cancer, facilitate surgical planning, and increase surgeons’ confidence about tumor resectability (80).
Conclusions
Detection and accurate staging of pancreatic carcinoma utilizing abdominal cross sectional state of the art imaging is essential to providing optimal therapy for patients. While specialized pancreatic MDCT is the most commonly used and best-validated modality for diagnosing and staging, MRI is an equally sensitive alternative. A complete and accurate assessment of the primary tumor, its relationship to/involvement of neighboring structures (particularly vascular structures) and distant metastatic disease is required for accurate characterization of disease as resectable, borderline resectable and unresectable. Structured reporting is a good tool for reporting pancreatic adenocarcinoma and has been shown to improve evaluation and surgeons’ confidence in the report.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011;378:607-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matrisian LM, Aizenberg R, Rosenzweig A. The alarming rise of pancreatic cancer deaths in the United States: why we need to stem the tide today. Available online: https://www.pancan.org/wp-content/uploads/2013/01/incidence_report_2012.pdf
- 3.de la Santa LG, Retortillo JA, Miguel AC, et al. Radiology of pancreatic neoplasms: An update. World J Gastrointest Oncol 2014;6:330-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [DOI] [PubMed] [Google Scholar]
- 5.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039-49. [DOI] [PubMed] [Google Scholar]
- 6.Porta M, Fabregat X, Malats N, et al. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol 2005;7:189-97. [DOI] [PubMed] [Google Scholar]
- 7.Al-Hawary MM, Kaza RK, Wasnik AP, et al. Staging of pancreatic cancer: role of imaging. Semin Roentgenol 2013;48:245-52. [DOI] [PubMed] [Google Scholar]
- 8.White R, Winston C, Gonen M, et al. Current utility of staging laparoscopy for pancreatic and peripancreatic neoplasms. J Am Coll Surg 2008;206:445-50. [DOI] [PubMed] [Google Scholar]
- 9.Friess H, Kleeff J, Silva JC, et al. The role of diagnostic laparoscopy in pancreatic and periampullary malignancies. J Am Coll Surg 1998;186:675-82. [DOI] [PubMed] [Google Scholar]
- 10.Bilimoria KY, Talamonti MS, Sener SF, et al. Effect of hospital volume on margin status after pancreaticoduodenectomy for cancer. J Am Coll Surg 2008;207:510-9. [DOI] [PubMed] [Google Scholar]
- 11.Tamm EP, Balachandran A, Bhosale PR, et al. Imaging of pancreatic adenocarcinoma: update on staging/resectability. Radiol Clin North Am 2012;50:407-28. [DOI] [PubMed] [Google Scholar]
- 12.Tokar JL, Walia R. Diagnostic evaluation of solid pancreatic masses. Curr Gastroenterol Rep 2013;15:347. [DOI] [PubMed] [Google Scholar]
- 13.Săftoiu A, Vilmann P. Role of endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. J Clin Ultrasound 2009;37:1-17. [DOI] [PubMed] [Google Scholar]
- 14.DiMagno EP, Buxton JL, Regan PT, et al. Ultrasonic endoscope. Lancet 1980;1:629-31. [DOI] [PubMed] [Google Scholar]
- 15.Dewitt J, Devereaux BM, Lehman GA, et al. Comparison of endoscopic ultrasound and computed tomography for the preoperative evaluation of pancreatic cancer: a systematic review. Clin Gastroenterol Hepatol 2006;4:717-25; quiz 664. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal B, Krishna NB, Labundy JL, et al. EUS and/or EUS-guided FNA in patients with CT and/or magnetic resonance imaging findings of enlarged pancreatic head or dilated pancreatic duct with or without a dilated common bile duct. Gastrointest Endosc 2008;68:237-42; quiz 334, 335. [DOI] [PubMed]
- 17.Wang W, Shpaner A, Krishna SG, et al. Use of EUS-FNA in diagnosing pancreatic neoplasm without a definitive mass on CT. Gastrointest Endosc 2013;78:73-80. [DOI] [PubMed] [Google Scholar]
- 18.Tempero MA, Arnoletti JP, Behrman S, et al. Pancreatic adenocarcinoma. J Natl Compr Canc Netw 2010;8:972-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eloubeidi MA, Tamhane A. Prospective assessment of diagnostic utility and complications of endoscopic ultrasound-guided fine needle aspiration. Results from a newly developed academic endoscopic ultrasound program. Dig Dis 2008;26:356-63. [DOI] [PubMed] [Google Scholar]
- 20.Adler DG, Jacobson BC, Davila RE, et al. ASGE guideline: complications of EUS. Gastrointest Endosc 2005;61:8-12. [DOI] [PubMed] [Google Scholar]
- 21.Micames C, Jowell PS, White R, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc 2003;58:690-5. [DOI] [PubMed] [Google Scholar]
- 22.Wong JC, Lu DS. Staging of pancreatic adenocarcinoma by imaging studies. Clin Gastroenterol Hepatol 2008;6:1301-8. [DOI] [PubMed] [Google Scholar]
- 23.Legmann P, Vignaux O, Dousset B, et al. Pancreatic tumors: comparison of dual-phase helical CT and endoscopic sonography. AJR Am J Roentgenol 1998;170:1315-22. [DOI] [PubMed] [Google Scholar]
- 24.Prokesch RW, Chow LC, Beaulieu CF, et al. Isoattenuating pancreatic adenocarcinoma at multi-detector row CT: secondary signs. Radiology 2002;224:764-8. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Park SH, Yu ES, et al. Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT: frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology 2010;257:87-96. [DOI] [PubMed] [Google Scholar]
- 26.Yoon SH, Lee JM, Cho JY, et al. Small (≤20 mm) pancreatic adenocarcinomas: analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology 2011;259:442-52. [DOI] [PubMed] [Google Scholar]
- 27.Prokesch RW, Schima W, Chow LC, et al. Multidetector CT of pancreatic adenocarcinoma: diagnostic advances and therapeutic relevance. Eur Radiol 2003;13:2147-54. [DOI] [PubMed] [Google Scholar]
- 28.Brennan DD, Zamboni GA, Raptopoulos VD, et al. Comprehensive preoperative assessment of pancreatic adenocarcinoma with 64-section volumetric CT. Radiographics 2007;27:1653-66. [DOI] [PubMed] [Google Scholar]
- 29.Tamm EP, Silverman PM, Charnsangavej C, et al. Diagnosis, staging, and surveillance of pancreatic cancer. AJR Am J Roentgenol 2003;180:1311-23. [DOI] [PubMed] [Google Scholar]
- 30.Bashir MR, Gupta RT. MDCT evaluation of the pancreas: nuts and bolts. Radiol Clin North Am 2012;50:365-77. [DOI] [PubMed] [Google Scholar]
- 31.Lu DS, Vedantham S, Krasny RM, et al. Two-phase helical CT for pancreatic tumors: pancreatic versus hepatic phase enhancement of tumor, pancreas, and vascular structures. Radiology 1996;199:697-701. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher JG, Wiersema MJ, Farrell MA, et al. Pancreatic malignancy: value of arterial, pancreatic, and hepatic phase imaging with multi-detector row CT. Radiology 2003;229:81-90. [DOI] [PubMed] [Google Scholar]
- 33.Valls C, Andía E, Sanchez A, et al. Dual-phase helical CT of pancreatic adenocarcinoma: assessment of resectability before surgery. AJR Am J Roentgenol 2002;178:821-6. [DOI] [PubMed] [Google Scholar]
- 34.Brügel M, Link TM, Rummeny EJ, et al. Assessment of vascular invasion in pancreatic head cancer with multislice spiral CT: value of multiplanar reconstructions. Eur Radiol 2004;14:1188-95. [DOI] [PubMed] [Google Scholar]
- 35.Ichikawa T, Erturk SM, Sou H, et al. MDCT of pancreatic adenocarcinoma: optimal imaging phases and multiplanar reformatted imaging. AJR Am J Roentgenol 2006;187:1513-20. [DOI] [PubMed] [Google Scholar]
- 36.Vargas R, Nino-Murcia M, Trueblood W, et al. MDCT in Pancreatic adenocarcinoma: prediction of vascular invasion and resectability using a multiphasic technique with curved planar reformations. AJR Am J Roentgenol 2004;182:419-25. [DOI] [PubMed] [Google Scholar]
- 37.Fukushima H, Itoh S, Takada A, et al. Diagnostic value of curved multiplanar reformatted images in multislice CT for the detection of resectable pancreatic ductal adenocarcinoma. Eur Radiol 2006;16:1709-18. [DOI] [PubMed] [Google Scholar]
- 38.Macari M, Spieler B, Kim D, et al. Dual-source dual-energy MDCT of pancreatic adenocarcinoma: initial observations with data generated at 80 kVp and at simulated weighted-average 120 kVp. AJR Am J Roentgenol 2010;194:W27-32. [DOI] [PubMed] [Google Scholar]
- 39.Patel BN, Thomas JV, Lockhart ME, et al. Single-source dual-energy spectral multidetector CT of pancreatic adenocarcinoma: optimization of energy level viewing significantly increases lesion contrast. Clin Radiol 2013;68:148-54. [DOI] [PubMed] [Google Scholar]
- 40.McNamara MM, Little MD, Alexander LF, et al. Multireader evaluation of lesion conspicuity in small pancreatic adenocarcinomas: complimentary value of iodine material density and low keV simulated monoenergetic images using multiphasic rapid kVp-switching dual energy CT. Abdom Imaging 2015;40:1230-40. [DOI] [PubMed] [Google Scholar]
- 41.Chu AJ, Lee JM, Lee YJ, et al. Dual-source, dual-energy multidetector CT for the evaluation of pancreatic tumours. Br J Radiol 2012;85:e891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morgan DE. Dual-energy CT of the abdomen. Abdom Imaging 2014;39:108-34. [DOI] [PubMed] [Google Scholar]
- 43.Koelblinger C, Ba-Ssalamah A, Goetzinger P, et al. Gadobenate dimeglumine-enhanced 3.0-T MR imaging versus multiphasic 64-detector row CT: prospective evaluation in patients suspected of having pancreatic cancer. Radiology 2011;259:757-66. [DOI] [PubMed] [Google Scholar]
- 44.Park HS, Lee JM, Choi HK, et al. Preoperative evaluation of pancreatic cancer: comparison of gadolinium-enhanced dynamic MRI with MR cholangiopancreatography versus MDCT. J Magn Reson Imaging 2009;30:586-95. [DOI] [PubMed] [Google Scholar]
- 45.Schima W, Függer R. Evaluation of focal pancreatic masses: comparison of mangafodipir-enhanced MR imaging and contrast-enhanced helical CT. Eur Radiol 2002;12:2998-3008. [DOI] [PubMed] [Google Scholar]
- 46.Rieber A, Tomczak R, Nüssle K, et al. MRI with mangafodipir trisodium in the detection of pancreatic tumours: comparison with helical CT. Br J Radiol 2000;73:1165-9. [DOI] [PubMed] [Google Scholar]
- 47.Davenport MS, Viglianti BL, Al-Hawary MM, et al. Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology 2013;266:452-61. [DOI] [PubMed] [Google Scholar]
- 48.Pietryga JA, Burke LM, Marin D, et al. Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology 2014;271:426-34. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Miller FH, Chen ZE, et al. Diffusion-weighted MR imaging of solid and cystic lesions of the pancreas. Radiographics 2011;31:E47-64. [DOI] [PubMed] [Google Scholar]
- 50.Tang S, Huang G, Liu J, et al. Usefulness of 18F-FDG PET, combined FDG-PET/CT and EUS in diagnosing primary pancreatic carcinoma: a meta-analysis. Eur J Radiol 2011;78:142-50. [DOI] [PubMed] [Google Scholar]
- 51.Kauhanen SP, Komar G, Seppänen MP, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg 2009;250:957-63. [DOI] [PubMed] [Google Scholar]
- 52.Farma JM, Santillan AA, Melis M, et al. PET/CT fusion scan enhances CT staging in patients with pancreatic neoplasms. Ann Surg Oncol 2008;15:2465-71. [DOI] [PubMed] [Google Scholar]
- 53.Heinrich S, Goerres GW, Schäfer M, et al. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg 2005;242:235-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoneyama T, Tateishi U, Endo I, et al. Staging accuracy of pancreatic cancer: comparison between non-contrast-enhanced and contrast-enhanced PET/CT. Eur J Radiol 2014;83:1734-9. [DOI] [PubMed] [Google Scholar]
- 55.Tempero MA, Arnoletti JP, Behrman SW, et al. Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2012;10:703-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014;155:977-88. [DOI] [PubMed] [Google Scholar]
- 57.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4. [DOI] [PubMed] [Google Scholar]
- 58.Cancer Staging Posters. [accessed 2014 Nov 18]. Available online: https://cancerstaging.org/references-tools/quickreferences/Pages/default.aspx
- 59.Clark LR, Jaffe MH, Choyke PL, et al. Pancreatic imaging. Radiol Clin North Am 1985;23:489-501. [PubMed] [Google Scholar]
- 60.Al-Hawary MM, Francis IR. Pancreatic ductal adenocarcinoma staging. Cancer Imaging 2013;13:360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alexakis N, Halloran C, Raraty M, et al. Current standards of surgery for pancreatic cancer. Br J Surg 2004;91:1410-27. [DOI] [PubMed] [Google Scholar]
- 62.Makino I, Kitagawa H, Ohta T, et al. Nerve plexus invasion in pancreatic cancer: spread patterns on histopathologic and embryological analyses. Pancreas 2008;37:358-65. [DOI] [PubMed] [Google Scholar]
- 63.Deshmukh SD, Willmann JK, Jeffrey RB. Pathways of extrapancreatic perineural invasion by pancreatic adenocarcinoma: evaluation with 3D volume-rendered MDCT imaging. AJR Am J Roentgenol 2010;194:668-74. [DOI] [PubMed] [Google Scholar]
- 64.Patel BN, Giacomini C, Jeffrey RB, et al. Three-dimensional volume-rendered multidetector CT imaging of the posterior inferior pancreaticoduodenal artery: its anatomy and role in diagnosing extrapancreatic perineural invasion. Cancer Imaging 2013;13:580-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu DS, Reber HA, Krasny RM, et al. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol 1997;168:1439-43. [DOI] [PubMed] [Google Scholar]
- 66.Hough TJ, Raptopoulos V, Siewert B, et al. Teardrop superior mesenteric vein: CT sign for unresectable carcinoma of the pancreas. AJR Am J Roentgenol 1999;173:1509-12. [DOI] [PubMed] [Google Scholar]
- 67.He J, Page AJ, Weiss M, et al. Management of borderline and locally advanced pancreatic cancer: where do we stand? World J Gastroenterol 2014;20:2255-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16:1727-33. [DOI] [PubMed] [Google Scholar]
- 69.Tempero MA, Malafa MP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2014;12:1083-93. [DOI] [PubMed] [Google Scholar]
- 70.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006;13:1035-46. [DOI] [PubMed] [Google Scholar]
- 71.Morgan DE, Waggoner CN, Canon CL, et al. Resectability of pancreatic adenocarcinoma in patients with locally advanced disease downstaged by preoperative therapy: a challenge for MDCT. AJR Am J Roentgenol 2010;194:615-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Al-Hawary MM, Francis IR, Chari ST, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology 2014;270:248-60. [DOI] [PubMed] [Google Scholar]
- 73.Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012;118:5749-56. [DOI] [PubMed] [Google Scholar]
- 74.Lall CG, Howard TJ, Skandarajah A, et al. New concepts in staging and treatment of locally advanced pancreatic head cancer. AJR Am J Roentgenol 2007;189:1044-50. [DOI] [PubMed] [Google Scholar]
- 75.Motosugi U, Ichikawa T, Morisaka H, et al. Detection of pancreatic carcinoma and liver metastases with gadoxetic acid-enhanced MR imaging: comparison with contrast-enhanced multi-detector row CT. Radiology 2011;260:446-53. [DOI] [PubMed] [Google Scholar]
- 76.Goh BK. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg 2006;243:709-10; author reply 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sistrom CL, Honeyman-Buck J. Free text versus structured format: information transfer efficiency of radiology reports. AJR Am J Roentgenol 2005;185:804-12. [DOI] [PubMed] [Google Scholar]
- 78.Naik SS, Hanbidge A, Wilson SR. Radiology reports: examining radiologist and clinician preferences regarding style and content. AJR Am J Roentgenol 2001;176:591-8. [DOI] [PubMed] [Google Scholar]
- 79.Schwartz LH, Panicek DM, Berk AR, et al. Improving communication of diagnostic radiology findings through structured reporting. Radiology 2011;260:174-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brook OR, Brook A, Vollmer CM, et al. Structured reporting of multiphasic CT for pancreatic cancer: potential effect on staging and surgical planning. Radiology 2015;274:464-72. [DOI] [PubMed] [Google Scholar]