Abstract
Minimally invasive surgery (MIS) for pancreatic adenocarcinoma has found new avenues for performing pancreaticoduodenectomy (PD) procedures, a historically technically challenging operation. Multiple studies have found laparoscopic PD to be safe, with equivalent oncologic outcomes as compared to open PD. In addition, several series have described potential benefits to minimally invasive PD including fewer postoperative complications, shorter hospital length of stay, and decreased postoperative pain. Yet, despite these promising initial results, laparoscopic PDs have not become widely adopted by the surgical community. In fact, the vast majority of pancreatic resections performed in the United States are still performed in an open fashion, and there are only a handful of surgeons who actually perform purely laparoscopic PDs. On the other hand, robotic assisted surgery offers many technical advantages over laparoscopic surgery including high-definition, 3-D optics, enhanced suturing ability, and more degrees of freedom of movement by means of fully-wristed instruments. Similar to laparoscopic PD, there are now several case series that have demonstrated the feasibility and safety of robotic PD with seemingly equivalent short-term oncologic outcomes as compared to open technique. In addition, having the surgeon seated for the procedure with padded arm-rests, there is an ergonomic advantage of robotics over both open and laparoscopic approaches, where one has to stand up for prolonged periods of time. Future technologic innovations will likely focus on enhanced robotic capabilities to improve ease of use in the operating room. Last but not least, robotic assisted surgery training will continue to be a part of surgical education curriculum ensuring the increased use of this technology by future generations of surgeons.
Keywords: Whipple, minimally invasive surgery (MIS), pancreatic adenocarcinoma, innovation, operative technique
Introduction
Surgery remains a key component of treatment for resectable pancreatic adenocarcinoma. Pancreaticoduodenectomy (PD), or Whipple procedure, for pancreatic head and uncinate process lesions has historically been one of the most difficult abdominal surgical operations and has garnered a well-deserved reputation in by both the medical and lay communities as a risky operation. These challenges include but are not limited to the location of the pancreas in the retroperitoneum, the proximity to major vascular structures, and the unforgiving nature of required anastomoses for functional preservation (1). Mortality rates have dropped dramatically over the past several decades with improvements in preoperative care, intraoperative surgical techniques and instrumentation, as well as post-operative care. One should note that despite improvement in pancreatic fistulae rates, they have not disappeared completely. It is often the improved management of the post-operative complications that has helped drop the mortality rates.
There has been growing academic interest in the relationship between hospital and surgeon volume and their effect on morbidity, mortality, and oncologic outcomes. There is little doubt that with the current healthcare climate and trends in centralization of care into large healthcare systems that this effect will continue for pancreatic and other high risk surgeries (2,3). There is, however, another growing academic focus on improving outcomes following major pancreatic resection through minimally invasive surgical approaches. Indeed, there has already been widespread adoption of both laparoscopic and robotic resections for cancers of the left pancreas to the point that many believe these approaches should become the standard of care (4). Yet, the demanding technical requirements of performing a minimally invasive PD have proven a very steep hill to climb for most. The pancreatic and biliary anastomosis requires meticulous and precise suturing skills that are not easily mastered. Bleeding from structures such as the superior mesenteric vein can be catastrophic if not handled and repaired with delicacy and efficiency. Robotic PD offers the opportunity to overcome several technical challenges associated with laparoscopic PD, while maintaining the benefits of minimally invasive surgery (MIS). Herein, we review the published literature regarding laparoscopic and robotic PD and our institutional series of robotic PD procedures.
Laparoscopic PD
Minimally invasive PD was first reported by laparoscopic approach in 1994 by surgeons Gagner and Pomp (5) who performed a single, purely laparoscopic procedure. Additional reports of laparoscopic PD in porcine animal models concluded more information on the feasibility and safety of this procedure (6,7). In the ensuing two decades, there are only a few fairly small case series of laparoscopic PD demonstrating the safety and feasibility of this surgical technique (8-15). In 2011, a review of 27 published articles regarding laparoscopic PD concluded similar morbidity and mortality rates as compared to open PD (16). Further case series concluded oncologic outcomes comparable to open PD in terms of consistent negative margin resection rates and lymph node retrieval (10,15,16). It should be noted that almost none of these series demonstrated any superiority in terms of morbidity, mortality, or oncologic outcomes. Actually, most of them had significantly higher rates of pancreatic fistulae and longer operative times than open techniques. It is therefore, not a tremendous surprise that most surgeons have been reluctant to adopt the technique of laparoscopic PD for either benign or malignant disease processes.
Most likely, the low number of published laparoscopic PD procedures is reflective of the inherent complexity of the operation. Many authors describe a difficult learning curve for successfully completing laparoscopic PD (13). Modifications to laparoscopic PD have been performed to attempt to overcome some of the challenges associated with the procedure. These include a combined approach with mini-laparotomy to facilitate skeletonization of the hepato-duodenal ligament and reconstruction (17). Inherently though, the laparoscopic platform has several limitations including non-articulated instruments, lack of depth perception due to two dimensional imaging and constricted intra-abdominal space. These factors make complex pancreatic operations, which are already difficult by their nature, even more complex (1). Even more advanced procedures such as laparoscopic major vascular resection combined with laparoscopic PD have been described, but as the authors note, this technique requires extensive experience with laparoscopy and experience with open major vascular resection in order to be performed safely (18,19). These challenges when combined together have ushered the way for new technological advancements to improve upon the existing minimally invasive surgical technology.
Robotic PD
Robotic surgery may offer many advantages over laparoscopic surgery including articulation of instruments with almost 540° of motion, elimination of surgeon tremor and binocular enhanced three dimensional vision (20). In addition, there are several ergonomic benefits afforded to the surgeon which likely decrease fatigue in the operating room (21), while the enhanced optic and motion capabilities lead to the more accurate movements needed for resection and suturing of delicate tissues. Simply sitting instead of standing for long periods of time, typical of performing a PD, will no doubt benefit the surgeon and possibly lead to better performance. Magnification and depth perception both allow the surgeon to utilize sutures that would be nearly impossible to use with standard laparoscopy. Sutures such as a 6-0 polypropylene on a BV-1 needle are commonly used during robotic Whipple procedures at our institution. These attributes allow the surgeon to overcome many of the insufficiencies associated with classic laparoscopic surgery, making challenging minimally invasive pancreatic surgeries more feasible.
In the past decade, several groups have successfully performed robotic assisted major pancreatic resections, but the literature shows that they have been slow to expand (20,22-24). The first large series of robotic pancreatic procedures was published by Giulianotti et al. in 2010. This study included 60 robotic PD demonstrating the safety and feasibility of the procedure (22). Unfortunately, this series included procedures where the pancreatic remnant was not anastomosed but rather injected with fibrin glue and oversewn (almost 50%). This was followed by a case series of 132 robotic PD procedures by Zeh and Moser, published in 2013, again concluding the safety and feasibility of robotic technology as compared to laparoscopic and open platforms, with low incidence of conversion (25). It did, however, demonstrate a relatively higher rate of pancreatic fistulae than one might expect from the same or similar high-volume institution for open PDs. Furthermore, they did not find any significant difference in the length of stay. In addition, operative times were significantly higher. Table 1 highlights the largest reported case series of robotic PDs published to date. Operative details including procedure time and estimated blood loss are reported in Table 2, along with details regarding margin status and lymph node retrieval for operations performed for malignancy. For centers reporting length of stay, mean hospital length of stay ranged from 9.8-16.4 days.
Table 1. Largest reported case series of robotic PDs published to date.
| Author | Year | Country | Study type | No. of patients | Malignancy [%] | Comparison (No. of patients compared) |
|---|---|---|---|---|---|---|
| Buchs (26) | 2011 | USA | Prospective, case-matched study | 44 | 33 [75] | Open PD [39] |
| Chalikonda (27) | 2012 | USA | Prospective, case-matched study | 30 | 14 [46.7] | Open PD [30] |
| Zhou (28) | 2011 | China | Prospective, case matched study | 8 | 8 [100] | Open PD [8] |
| Giulianotti (22) | 2010 | USA | Retrospective, case series | 20 | 20 [100] | None |
| Zeh (29) | 2012 | USA | Retrospective, case series | 50 | 37 [74] | None |
| Boggi (30) | 2013 | Italy | Retrospective, case series | 34 | 22 [64.7] | None |
| Lai (31) | 2012 | China | Retrospective, case series | 20 | 15 [75] | Open PD [67] |
| Narula (24) | 2010 | USA | Retrospective, case series | 5 | 1 [20] | None |
PD, pancreaticoduodenectomy.
Table 2. Operative details from the largest reported case series of robotic PDs published to date.
| Author | Operative time (min) | EBL (mL) | Margin negative resection rate (%) | No. of lymph nodes collected | Hospital LOS (days) | Complications |
|---|---|---|---|---|---|---|
| Buchs (26) | 444±93.5 | 387±334 | 41 (93.2) | 16.8 | 13 | No difference in complication rates |
| Chalikonda (27) | 476.2 | 485.8 | 30 (100.0) | 13.2 | 9.79 | Decreased postoperative morbidity following RAPD |
| Zhou (28) | 718±186 | 153±43 | 87.5 | – | 16.4±4.1 | Complications were lower with RAPD |
| Giulianotti (22) | 421 | 394 | 91.7 | 14 | 12.5 | No comparison to open |
| Zeh (29) | 568 | 350 | 89 | 18 | 10 | – |
| Boggi (30) | 597 | 220 | 100 | 32 | – | No comparison to open |
| Lai (31) | 491.5 | 247 | 73.3 | 10 | – | No difference in complications |
| Narula (24) | 420 | – | 100 | 16 | 9.6 | – |
–, information not collected or not available. PD, pancreaticoduodenectomy; EBL, estimated blood loss; LOS, length of stay; RAPD, robotic assisted PD.
When compared to open PD, several case series have reported similar postoperative morbidity and complication rates following robotic PD (26,28,31). One comparison study noted a significantly lower postoperative complication rate following robotic PD (25% vs. 75%, P=0.05) (28). As reported by Chalikonda et al., patients who underwent robotic PD had a significantly shorter length of stay when compared to open PD (9.79 vs. 13.26 days, P=0.043) (27). In addition, procedure related oncologic surgical outcomes appear to be equivalent when comparing robotic to open PD, in terms of resection margin negative rates and number of lymph nodes harvested at the time of surgery (27,28,32). In fact, one series notes an improvement in mean lymph node retrieval rate with robotic assisted PD as compared to open (16.8 vs. 11, P=0.02) (26). This is not to claim that removing more lymph nodes necessarily results in better long-term oncologic outcomes, but it does negate any belief that a minimally invasive approach is inferior to open.
Rates of postoperative pancreatic fistula following robotic PD remain mixed in reports from the literature. From the initial Giulianotti et al. series of robotic pancreatic resections, there was an increased rate of postoperative pancreatic fistula (31.6%) (22). They hypothesized that with improvement in technique and more experience with microsurgery reconstructions, rate of postoperative pancreatic fistula would decline. Lai and colleagues also report a high postoperative pancreatic fistula rate of 35%, but they were all managed conservatively and without need for reoperation (31). Other series however, have noted no difference in postoperative pancreatic fistula rates (27). Finally, robotic PD has been found to be safe in older populations (age >70) with similar rates of morbidity, mortality and outcomes as compared to a younger cohort, thereby precluding age as a contraindication for robotic PD (33).
Two major review series of robotic assisted pancreatic surgery have been published to date. Zhang et al. summarize comparisons of open to robotic pancreatectomy in their 2013 article and conclude through meta-analysis that the procedure is safe with lower associated positive margin rate. Their analysis supports no difference in postoperative pancreatic fistula rate or mortality (34). A second review on robotic pancreas surgery concludes that this approach lead to advantages which may include decreased postoperative pain and blood loss, fewer complications and decreased hospital length of stay with faster recovery (21). These promising findings have led many surgeons to take on even more complex robotic assisted pancreatic resections including extended pancreatectomy with vascular resection for locally advanced pancreatic adenocarcinoma (35).
Robotic assisted HPB surgery—institutional experience
Carolinas Medical Center is a 1,000-bed academic affiliated medical center located in Charlotte, NC. The institution serves as a major referral center for the central and western regions of both North and South Carolina. It is a high volume center for both pancreatic and hepatic resections, (greater than 150 each, annually). Robotic surgery is routinely used at our institution for a variety of general, urologic and gynecologic procedures. The senior author, JBM, who had already been performing robotic HPB procedures at another institution since 2006, initiated the program at CMC in 2008. Over the course of 7 years, we have significantly expanded our experience and have moved beyond the learning curve to a robust practice of liver, pancreas, and biliary operations for both benign and malignant conditions. In particular, our experience with robotic PD has grown significantly with an increasing number of procedures performed each year. Last year the senior author performed 96 robotic HPB procedures. Of note, since program initiation back in 2008, the senior author has performed over 200 open PDs and 150 of other (non-HPB) robotic foregut operations, accentuating the importance of being an experienced HPB and robotic surgeon, before embarking on performing robotic PDs.
In our previous work, we described the learning curve to perform robotic liver, biliary and pancreatic procedures (36). This included a time period of utilizing the robot to perform portions of the dissection for PD with planned conversion to an open procedure for the reconstruction phase. During the robotic surgery learning curve, we became increasingly more comfortable with the reconstructive phase of the operation and significantly more efficient. Now, we routinely perform the entirety of the PD procedure using robotic surgery. As highlighted in our previous work, several robotic HPB procedures during the learning phase were converted to laparoscopy or hand-assisted laparoscopy (36). This is reflective of the challenges encountered with robotic surgery. With the accumulating surgeon’s experience in using robot technology, conversion to laparoscopy, hand assist laparoscopy or open surgery is fairly infrequent.
Robotic assisted Whipple—operative technique
The DaVinci Si robot (Intuitive Surgical, Sunnyvale, CA) is used to perform all robotic PD’s at Carolinas Medical Center. Our technique has continually evolved over time and is often modified for individual patient characteristics. The patient is placed in the supine position. Pneumoperitoneum is obtained with a Veress needle at the umbilicus and subsequently upsized to a 12 mm port. Three additional robotic 8 mm cannulae, as well as one additional 12 mm camera port (in the right mid-clavicular line) are placed under direct vision. The umbilical trocar site serves as the assistant port during most of the resection portion of the procedure. Upon initial entry, the abdominal cavity is inspected for evidence of metastatic disease, and the round ligament is taken down and preserved for a vascularized pedicle flap as is our institutional experience and routinely performed in open PD. The gallbladder is commonly sutured to the anterior abdominal wall in order to expose the porta hepatis without the need for a Nathanson retractor, which is used in cases where the patient’s gallbladder has previously been removed. The inferior border of the distal gastric antrum and proximal duodenum is mobilized with care to avoid injury to the distal gastric antrum or the pylorus. The right gastric and right gastroepiploic vessels are dissected, sealed, and divided using the robotic bipolar vessel-sealing device. The proximal duodenum is divided distal to the pylorus using a laparoscopic 60 mm stapler device, and the stomach is placed into the left upper quadrant for reconstruction later. The hepatic flexure of the colon is taken down to expose the duodenum. A Kocher maneuver is performed and the ligament of Treitz is mobilized to allow the duodenum to move freely into the right upper quadrant. The common hepatic artery is dissected out and a portal and celiac lymphadenectomy is performed. Intraoperative ultrasound is always performed to confirm the vascular anatomy of the porta hepatis. The gastroduodenal artery is identified, ligated, clipped and divided. The inferior border of the pancreas and the neck are dissected out and mobilized. A tunnel is created underneath the neck the pancreas, on top of the superior mesenteric and portal vein all the way to the superior aspect of the pancreas. An umbilical tape is passed underneath the pancreas. At this point, the neck of the pancreas is transected using the robotic monopolar scissors coupled with saline irrigation to minimize charring of the tissue, a technique which has been previously described (37).
The small bowel is transected about 20 cm distal to the ligament of Treitz. The small bowel mesentery is ligated with a robotic vessel sealing device up towards the base of the uncinate process. Finally, the uncinate process is mobilized away from the superior mesenteric vein and the superior mesenteric artery. The common hepatic duct is then transected just above the cystic duct takeoff. The entire specimen is then placed into a specimen retrieval bag and removed from the abdominal cavity from the slightly enlarged umbilical trocar site. The latter site is partially closed using interrupted sutures around the 12 mm trocar. Then, the camera is moved to this location for the reconstruction phase of the procedure.
For the reconstruction phase of the procedure, the stapled end of the jejunum is brought alongside the transected surface of the pancreas, typically thru a window made in the transverse colonic mesentery. A two layer, end-to-side pancreaticojejunostomy is performed, nearly identical to our open technique. The posterior layer is performed using 5-0 monofilament suture in a running fashion to approximate the capsule of the pancreas with a seromuscular jejunal layer. A small enterotomy (matching the diameter of the pancreatic duct) is created in the jejunum with the electrocautery scissors and a duct-to-mucosal anastomosis is performed using interrupted 6-0 monofilament sutures, typically over a small 8 or 5 French pediatric feeding tube. The anterior layer is completed using an additional 5-0 running monofilament suture. The entire anastomosis is wrapped using the round ligament pedicle flap.
The hepaticojejunostomy is performed approximately 10-15 cm downstream from the pancreaticojejunostomy using a 4-0 or 5-0 monofilament sutures in a running or interrupted fashion, depending on the size of the duct. Finally, an antecolic duodenojejunostomy is performed approximately 50 cm from the biliary anastomosis using absorbable monofilament suture in a running fashion. A single closed suction drain is placed in the right upper quadrant close to the bile duct and the pancreatic anastomosis. All the port sites are closed appropriately.
Evaluation of institutional experience
In order to evaluate our experience with robotic PD, we have recently performed a retrospective cohort analysis of all robotic PD procedures performed at our institution between August 1, 2012 and August 31, 2014, with approval from the Institutional Review Board at Carolinas Medical Center. Study data were collected and managed using REDCap electronic data tools hosted at our institution (38). Variables collected included, but not limited to, patient demographics, operative techniques, oncologic resection quality parameters, morbidity and mortality. A total of 32 patients underwent robotic PD by one, experienced robotic HPB surgeon (JBM), during the reported time period. The intention was to complete the procedure in a completely robotic fashion. Prior to this time period, the senior author had performed segments of a small series of PD’s with planned conversions to open, in order to better study the technical and logistical factors of performing robotic PDs, while minimizing impact on the patient and the operating room in terms of length of procedure. A total of 27 robotic PD performed at our institution were completed without conversion. The remaining five patients (15.6%) required conversion to an open procedure secondary to need for portal or superior mesenteric vein resection. These patients were analyzed as a unique subset. Results from robotic cohort were compared to a contemporaneous series of open PD performed during the same time frame by the four fellowship-trained hepatobiliary surgeons within the CMC HPB Surgery department, and includes the open PDs from the one robotic surgeon (JBM). There were no differences in patient characteristics including age, BMI, sex, or malignant etiology (Table 3). Tumor size, rates of positive margin and number of positive lymph nodes were no different between groups.
Table 3. Patients’ demographics, tumor characteristics and oncologic resection quality parameters.
| Variable | Open (N=49) (%) | Robotic (N=27) (%) | P value |
|---|---|---|---|
| Age* (years) | 62.1±12.9 | 63.6±9.8 | 0.59 |
| BMI* (kg/m2) | 26.7±5.5 | 26.8±4.3 | 0.93 |
| Male | 22 (44.9) | 14 (51.9) | 0.56 |
| Malignant etiology | 40 (81.6) | 22 (81.5) | 0.61 |
| Tumor size* (cm) | 3.6±2.5 | 3.0±1.2 | 0.29 |
| Positive margin | 14 (36.8) | 6 (26.1) | 0.39 |
| Positive lymph nodes present | 30 (81.1) | 15 (62.5) | 0.11 |
| No. of positive lymph nodes* (N) | 2.6±2.6 | 2.3±2.9 | 0.69 |
*, mean values.
Primary and secondary endpoints are depicted in Table 4. Overall estimated blood loss was significantly lower with robotic PD (866.8 vs. 466.7 mL, P=0.042), however, operative time was longer (391.1 vs. 527.4 min, P=0.001). Analysis of 30-day postoperative complications (Figure 1) revealed significantly fewer complications in the robotic group (P=0.08). Delayed gastric emptying was the most commonly encountered postoperative complication and it was significantly less in the robotic group (30.6% in open vs. 14.4% in robotic PD, P=0.043). There were fewer surgical site infections in the robotic group (26.5% in open vs. 3.7% in robotic PD, P=0.001). Perhaps the most striking finding was the lower rate of pancreatic fistula compared to open (12% vs. 7.4%, P=0.061) in this series, which is the lowest of any published series to date. Actually, if a few more patients were enrolled to the robotic PD group, statistical significance would have been reached (type II error). Mean intensive care unit length of stay was significantly less following robotic PD (2.9 vs. 1.5 days, P=0.048) and mean hospital length of stay was decreased by 1.5 days (P=0.398) (Figure 2). While hospital length of stay was not significantly different in this analysis, it, again, might represent a type II error (Figure 2). There were no deaths within 90 days following robotic PD and there were two deaths following open PD. Overall, our analysis indicates a trend toward many significant benefits associated with robotic PD, including fewer complications and shorter length of stay.
Table 4. Primary and secondary endpoints of the comparison between open and robotic PD procedures.
| Variable | Open, N=49 (%) | Robotic, N=27 (%) | P value |
|---|---|---|---|
| Estimated blood loss (mL)* | 866.8±931.5 | 466.7±452.3 | 0.042 |
| Operative time (min)* | 391.1±141.8 | 527.4±87.7 | 0.001 |
| Hospital length of stay (days)* | 11.5±7.1 | 10.1±5.8 | 0.398 |
| 30-day complications | 33 (67.4) | 11 (40.7) | 0.008 |
| Delayed gastric emptying | 15 | 4 | 0.043 |
| Surgical Site Infections | 13 | 1 | 0.001 |
| Pancreatic fistula | 6 | 2 | 0.061 |
| Hospital length of stay (days)* | 11.5±7.1 | 10.1±5.8 | 0.398 |
| ICU length of stay (days)* | 2.9±3.2 | 1.5±1.2 | 0.048 |
| 30-day readmissions (%) | 14 (29.8) | 6 (22.2) | 0.480 |
| Death (%) | 2 (4.1) | 0 (0) | 0.410 |
Continuous parameters are described by mean value and standard deviation. Categorical parameters are described by absolute numbers and percentages. *, mean values. PD, pancreaticoduodenectomy.
Figure 1.
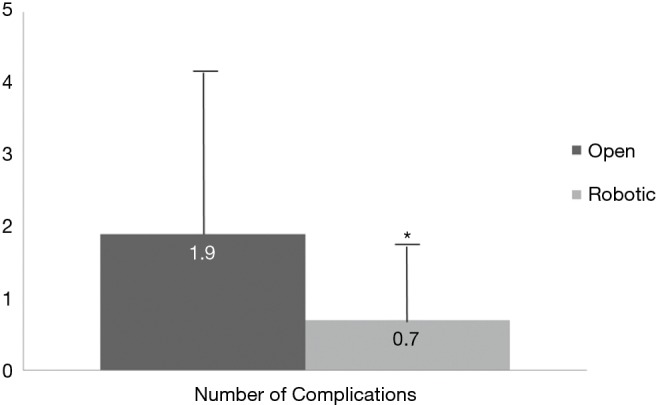
Overall complications. The number of complications, including, but not limited to, delayed gastric emptying, surgical site infection and pancreatic anastomosis leak rate was lower in the robotic group (P=0.008).
Figure 2.
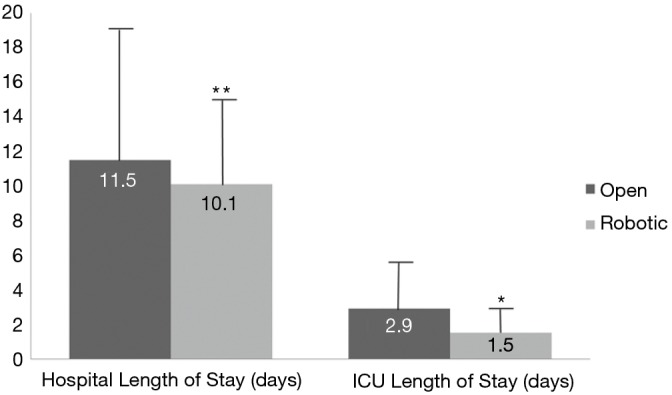
Length of stay. There was significant difference in ICU length of stay between the open and robotic PD group, in favor of the latter. There was a trend for shorter hospital length of stay in robotic group. However, for this observation there is potential for type II error, given the small sample size of the groups. *, P=0.398; **, P=0.048. PD, pancreaticoduodenectomy.
Robotic pancreatectomy: 2015 and beyond
As robotic technology continues to improve and become less expensive and more widely adopted, we will likely see increasing utilization for complex hepatobiliary and pancreatic procedures. Historically, minimally invasive surgical techniques are initially applied to benign disease processes and/or low-grade neoplasms. Subsequently, they are applied to malignant diseases in order to demonstrate similar effectiveness of minimally invasive and open procedures. This appears to be true for pancreatic and peri-ampullary malignancies, including adenocarcinoma, thus far as more surgeons are using a robotic-assisted approach for pancreatic cancer management (28,29). Future reports regarding long-term oncologic effectiveness are still needed to confirm at least equivalency between open and robotic PD.
It is likely that surgeons performing robotic procedures will continue to embrace more challenging pancreatic procedures including vascular resections associated with extended pancreatectomy (35). This has certainly been the senior author’s experience. Simply stated, “the more you do, the more you do.” Early reports are emerging for the use of robotic surgery for total pancreatectomy coupled with autologous islet cell transplantation (39-41), a procedure that historically has been performed by open laparotomy. In addition, robotic instrumentation, both hardware (the actual tools) as well as software, will continue to improve providing access to better equipment, affording better visualization and leading to increased ease of use.
Key to expansion of minimally invasive surgical techniques is access to education and training with new technology. Surgical resident and fellow education for robotic surgery is rapidly expanding in the United States and will no doubt become a requisite component, as it has already done so in both urology and gynecology. The reality is that residents in urology or gynecology who complete their training without robotics are at a significant disadvantage to those who have completed comprehensive robotic training (42). The majority of general surgical residents today will at least have some exposure to robotic surgery during their training (42). More institutions are adopting specialized instruction, educational curriculum, and specific surgical rotations which focus on robotic surgery, indicating the expanding presence of this new technology in formal surgical education (43). The addition of specialized technology, including surgeon instructor consoles, will make it easier to mentor trainees regarding the specifics of robotic assisted surgery and it will hopefully allow them to overcome the learning curve associated with this technology in less time (44).
Finally, disadvantages to robotic surgery include the lack of haptic feedback and cost of equipment purchase and maintenance (45). Increased procedure related costs for robotic pancreatic surgery have been previously described (30,46). This is reflective of both extended time in the operating room, disposables and fixed intraoperative charges. Through retrospective institutional review we have analyzed the associated procedure-related costs comparing robotic PD to open PD. Our findings indicate that while operative charges were significantly higher with robotic PD ($48,857.06 vs. $35,665.34 USD, P=0.009), once inpatient hospital charge and follow-up visit charges were incorporated into total costs associated with robotic PD procedure, there was no significant difference in overall cost ($176,931.50 vs. $182,552.68, P=0.69). We anticipate that future investigations will continue to demonstrate the long-term negligible cost difference between open and robotic procedures due to shorter hospital length of stay and fewer postoperative complications.
Conclusions
Robotic PD for pancreatic adenocarcinoma represents the latest iteration of minimally invasive oncologic surgery. Multiple reported series have found this procedure to be safe and technically feasible. The literature to date supports decreased morbidity associated with robotic PD as compared to open PD, particularly in relevance to wound associated complications and hospital length of stay. Long terms studies are still needed to demonstrate the overall equivalent oncologic outcomes. We anticipate that the future of robotic surgery will find an increasing role for complex abdominal operations, particularly for PD procedures, especially as we incorporate robotic assisted surgery training into current surgical education curriculum.
Acknowledgements
We would like to acknowledge Allyson Cochran for irreplaceable assistance with database management and statistical analysis.
Disclosure: Dr. Martinie serves as a consultant and proctor for Intuitive Surgical. All other authors report no conflicts of interest related to this work.
References
- 1.Winer J, Can MF, Bartlett DL, et al. The current state of robotic-assisted pancreatic surgery. Nat Rev Gastroenterol Hepatol 2012;9:468-76. [DOI] [PubMed] [Google Scholar]
- 2.Enomoto LM, Gusani NJ, Dillon PW, et al. Impact of surgeon and hospital volume on mortality, length of stay, and cost of pancreaticoduodenectomy. J Gastrointest Surg 2014;18:690-700. [DOI] [PubMed] [Google Scholar]
- 3.Swan RZ, Niemeyer DJ, Seshadri RM, et al. The impact of regionalization of pancreaticoduodenectomy for pancreatic Cancer in North Carolina since 2004. Am Surg 2014;80:561-6. [PubMed] [Google Scholar]
- 4.Mesleh MG, Stauffer JA, Asbun HJ. Minimally invasive surgical techniques for pancreatic cancer: ready for prime time? J Hepatobiliary Pancreat Sci 2013;20:578-82. [DOI] [PubMed] [Google Scholar]
- 5.Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408-10. [DOI] [PubMed] [Google Scholar]
- 6.Jones DB, Wu JS, Soper NJ. Laparoscopic pancreaticoduodenectomy in the porcine model. Surg Endosc 1997;11:326-30. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki O, Hirano S, Yano T, et al. Laparoscopic pancreaticoduodenectomy is effective in a porcine model. Surg Endosc 2008;22:2509-13. [DOI] [PubMed] [Google Scholar]
- 8.Cai X, Wang Y, Yu H, et al. Completed laparoscopic pancreaticoduodenectomy. Surg Laparosc Endosc Percutan Tech 2008;18:404-6. [DOI] [PubMed] [Google Scholar]
- 9.Cho A, Yamamoto H, Nagata M, et al. A totally laparoscopic pylorus-preserving pancreaticoduodenectomy and reconstruction. Surg Today 2009;39:359-62. [DOI] [PubMed] [Google Scholar]
- 10.Cho A, Yamamoto H, Nagata M, et al. Comparison of laparoscopy-assisted and open pylorus-preserving pancreaticoduodenectomy for periampullary disease. Am J Surg 2009;198:445-9. [DOI] [PubMed] [Google Scholar]
- 11.Dulucq JL, Wintringer P, Mahajna A. Laparoscopic pancreaticoduodenectomy for benign and malignant diseases. Surg Endosc 2006;20:1045-50. [DOI] [PubMed] [Google Scholar]
- 12.Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg 2010;145:19-23. [DOI] [PubMed] [Google Scholar]
- 13.Kim SC, Song KB, Jung YS, et al. Short-term clinical outcomes for 100 consecutive cases of laparoscopic pylorus-preserving pancreatoduodenectomy: improvement with surgical experience. Surg Endosc 2013;27:95-103. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura Y, Matsumoto S, Yoshioka M, et al. Successful laparoscopic pancreaticoduodenectomy for intraductal papillary mucinous neoplasm: a case report and a reliable technique for pancreaticojejunostomy. J Nippon Med Sch 2012;79:218-22. [DOI] [PubMed] [Google Scholar]
- 15.Pugliese R, Scandroglio I, Sansonna F, et al. Laparoscopic pancreaticoduodenectomy: a retrospective review of 19 cases. Surg Laparosc Endosc Percutan Tech 2008;18:13-8. [DOI] [PubMed] [Google Scholar]
- 16.Gumbs AA, Rodriguez Rivera AM, Milone L, et al. Laparoscopic pancreatoduodenectomy: a review of 285 published cases. Ann Surg Oncol 2011;18:1335-41. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki O, Kondo S, Hirano S, et al. Laparoscopic pancreaticoduodenectomy combined with minilaparotomy. Surg Today 2012;42:509-13. [DOI] [PubMed] [Google Scholar]
- 18.Kendrick ML, Sclabas GM. Major venous resection during total laparoscopic pancreaticoduodenectomy. HPB (Oxford) 2011;13:454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Croome KP, Farnell MB, Que FG, et al. Pancreaticoduodenectomy with major vascular resection: a comparison of laparoscopic versus open approaches. J Gastrointest Surg 2015;19:189-94; discussion 194. [DOI] [PubMed] [Google Scholar]
- 20.Zeh HJ, 3rd, Bartlett DL, Moser AJ. Robotic-assisted major pancreatic resection. Adv Surg 2011;45:323-40. [DOI] [PubMed] [Google Scholar]
- 21.Strijker M, van Santvoort HC, Besselink MG, et al. Robot-assisted pancreatic surgery: a systematic review of the literature. HPB (Oxford) 2013;15:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc 2010;24:1646-57. [DOI] [PubMed] [Google Scholar]
- 23.Zureikat AH, Nguyen KT, Bartlett DL, et al. Robotic-assisted major pancreatic resection and reconstruction. Arch Surg 2011;146:256-61. [DOI] [PubMed] [Google Scholar]
- 24.Narula VK, Mikami DJ, Melvin WS. Robotic and laparoscopic pancreaticoduodenectomy: a hybrid approach. Pancreas 2010;39:160-4. [DOI] [PubMed] [Google Scholar]
- 25.Zureikat AH, Moser AJ, Boone BA, et al. 250 robotic pancreatic resections: safety and feasibility. Ann Surg 2013;258:554-9; discussion 559-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buchs NC, Addeo P, Bianco FM, et al. Robotic versus open pancreaticoduodenectomy: a comparative study at a single institution. World J Surg 2011;35:2739-46. [DOI] [PubMed] [Google Scholar]
- 27.Chalikonda S, Aguilar-Saavedra JR, Walsh RM. Laparoscopic robotic-assisted pancreaticoduodenectomy: a case-matched comparison with open resection. Surg Endosc 2012;26:2397-402. [DOI] [PubMed] [Google Scholar]
- 28.Zhou NX, Chen JZ, Liu Q, et al. Outcomes of pancreatoduodenectomy with robotic surgery versus open surgery. Int J Med Robot 2011;7:131-7. [DOI] [PubMed] [Google Scholar]
- 29.Zeh HJ, Zureikat AH, Secrest A, et al. Outcomes after robot-assisted pancreaticoduodenectomy for periampullary lesions. Ann Surg Oncol 2012;19:864-70. [DOI] [PubMed] [Google Scholar]
- 30.Boggi U, Signori S, De Lio N, et al. Feasibility of robotic pancreaticoduodenectomy. Br J Surg 2013;100:917-25. [DOI] [PubMed] [Google Scholar]
- 31.Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic pancreaticoduodenectomy versus open pancreaticoduodenectomy--a comparative study. Int J Surg 2012;10:475-9. [DOI] [PubMed] [Google Scholar]
- 32.Lai EC, Tang CN. Current status of robot-assisted laparoscopic pancreaticoduodenectomy and distal pancreatectomy: a comprehensive review. Asian J Endosc Surg 2013;6:158-64. [DOI] [PubMed] [Google Scholar]
- 33.Buchs NC, Addeo P, Bianco FM, et al. Outcomes of robot-assisted pancreaticoduodenectomy in patients older than 70 years: a comparative study. World J Surg 2010;34:2109-14. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Wu WM, You L, et al. Robotic versus open pancreatectomy: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:1774-80. [DOI] [PubMed] [Google Scholar]
- 35.Giulianotti PC, Addeo P, Buchs NC, et al. Robotic extended pancreatectomy with vascular resection for locally advanced pancreatic tumors. Pancreas 2011;40:1264-70. [DOI] [PubMed] [Google Scholar]
- 36.Hanna EM, Rozario N, Rupp C, et al. Robotic hepatobiliary and pancreatic surgery: lessons learned and predictors for conversion. Int J Med Robot 2013;9:152-9. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen KT, Zureikat AH, Chalikonda S, et al. Technical aspects of robotic-assisted pancreaticoduodenectomy (RAPD). J Gastrointest Surg 2011;15:870-5. [DOI] [PubMed] [Google Scholar]
- 38.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zureikat AH, Nguyen T, Boone BA, et al. Robotic total pancreatectomy with or without autologous islet cell transplantation: replication of an open technique through a minimal access approach. Surg Endosc 2015;29:176-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giulianotti PC, Kuechle J, Salehi P, et al. Robotic-assisted laparoscopic distal pancreatectomy of a redo case combined with autologous islet transplantation for chronic pancreatitis. Pancreas 2009;38:105-7. [DOI] [PubMed] [Google Scholar]
- 41.Giulianotti P, Gorodner V, Kinzer K, et al. Robot-assisted pancreatoduodenectomy with preservation of the vascular supply for autologous islet cell isolation and transplantation: a case report. J Med Case Rep 2012;6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farivar BS, Flannagan M, Leitman IM. General surgery residents' perception of robot-assisted procedures during surgical training. J Surg Educ 2015;72:235-42. [DOI] [PubMed] [Google Scholar]
- 43.Nelson EC, Gottlieb AH, Müller HG, et al. Robotic cholecystectomy and resident education: the UC Davis experience. Int J Med Robot 2014;10:218-22. [DOI] [PubMed] [Google Scholar]
- 44.Hanly EJ, Miller BE, Kumar R, et al. Mentoring console improves collaboration and teaching in surgical robotics. J Laparoendosc Adv Surg Tech A 2006;16:445-51. [DOI] [PubMed] [Google Scholar]
- 45.Kendrick ML. Laparoscopic and robotic resection for pancreatic cancer. Cancer J 2012;18:571-6. [DOI] [PubMed] [Google Scholar]
- 46.Horiguchi A, Uyama I, Miyakawa S. Robot-assisted laparoscopic pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 2011;18:287-91. [DOI] [PubMed] [Google Scholar]


