Abstract
As a result of technological advances during the past two decades, surgeons now use minimally invasive surgery (MIS) approaches to pancreatic resection more frequently, yet the role of these approaches for pancreatic ductal adenocarcinoma resections remains uncertain, given the aggressive nature of this malignancy. Although there are no controlled trials comparing MIS technique to open surgical technique, laparoscopic distal pancreatectomy for pancreatic adenocarcinoma is performed with increasing frequency. Data from retrospective studies suggest that perioperative complication profiles between open and laparoscopic distal pancreatectomy are similar, with perhaps lower blood loss and fewer wound infections in the MIS group. Concerning oncologic outcomes, there appear to be no differences in the rate of achieving negative margins or in the number of lymph nodes (LNs) resected when compared to open surgery. There are limited recurrence and survival data on laparoscopic compared to open distal pancreatectomy for pancreatic adenocarcinoma, but in the few studies that assess long term outcomes, recurrence rates and survival outcomes appear similar. Recent studies show that though laparoscopic distal pancreatectomy entails a greater operative cost, the associated shorter length of hospital stay leads to decreased overall cost compared to open procedures. Multiple new technologies are emerging to improve resection of pancreatic cancer. Robotic pancreatectomy is feasible, but there are limited data on robotic resection of pancreatic adenocarcinoma, and outcomes appear similar to laparoscopic approaches. Additionally fluorescence-guided surgery represents a new technology on the horizon that could improve oncologic outcomes after resection of pancreatic adenocarcinoma, though published data thus far are limited to animal models. Overall, MIS distal pancreatectomy appears to be a safe and reasonable approach to treating selected patients with pancreatic ductal adenocarcinoma, though additional studies of long-term oncologic outcomes are merited. We review existing data on MIS distal pancreatectomy for pancreatic ductal adenocarcinoma.
Keywords: Laparoscopy, adenocarcinoma, pancreatic neoplasm, distal pancreatectomy, outcomes
Introduction
Pancreatic adenocarcinoma is the fourth leading cause of cancer-related mortality in men and women in the United States. In 2014, it is estimated that there will be 46,420 new cases and 39,590 deaths due to this disease (1). Surgical resection remains the only potentially curative therapy, and several randomized trials support administration of adjuvant chemotherapy or chemoradiation to improve survival outcomes (2-6). Preoperative chemotherapy with or without radiotherapy is recommended for patients with borderline resectable pancreatic adenocarcinoma, albeit no randomized data exist (7).
Distal pancreatic adenocarcinomas of the body or tail of the pancreas comprise only 20-25% of all diagnosed pancreatic adenocarcinomas (8). While more proximal periampullary tumors typically present with jaundice, malabsorption, and pancreatitis, distal tumors are usually associated with vague symptoms including weight loss and abdominal pain (8); consequently, distal cancers present at later stages than proximal cancers and are more likely to be metastatic or locally unresectable at the time of diagnosis (9).
The surgical approach to pancreatic resection for adenocarcinoma is dependent on the location of the tumor along the length of the pancreas. While pancreaticoduodenectomy (Kausch-Whipple procedure) is used to treat select patients with cancers of the pancreatic head, neck, and uncinate process, the operative approach for patients with early stage pancreatic cancer of the body and tail is the distal (or left) pancreatectomy (3). Figure 1 shows cross sectional images of pancreatic ductal adenocarcinoma requiring distal pancreatectomy. Distal pancreatectomy for adenocarcinoma is not commonly performed given the typically advanced stage of presentation of this disease. In an analysis of the Surveillance Epidemiology and End Results (SEER) database from 2003-2009, only 81 distal pancreatectomies for adenocarcinoma were performed on average each year in the United States (10), limiting the ability to study this patient population in a randomized fashion.
Figure 1.
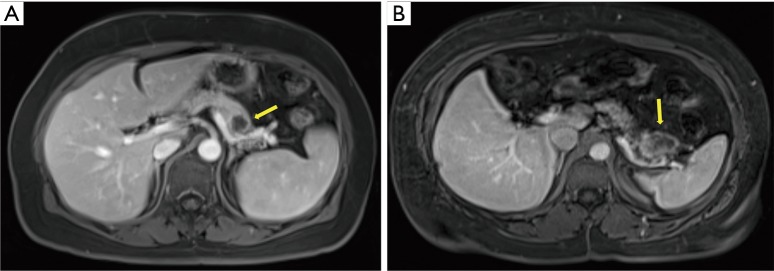
(A,B) Cross-sectional imaging of pancreatic adenocarcinoma of the distal pancreas.
Over the last few decades, laparoscopic surgery has been adopted and is considered the standard approach for resection for many retroperitoneal and abdominal organs (11-15). The adoption of laparoscopic pancreatectomy by the surgical community has been slower to occur secondary to concerns of the technical difficulty and risk of complication; however, since the first series of laparoscopic distal pancreatectomies in 1996, these concerns have been addressed in multiple studies that have supported the safety and benefits of laparoscopic pancreatic surgery (16-20). This review examines patient outcomes after laparoscopic distal pancreatectomy for adenocarcinoma with a focus on the short and long term oncologic outcomes.
Surgical technique
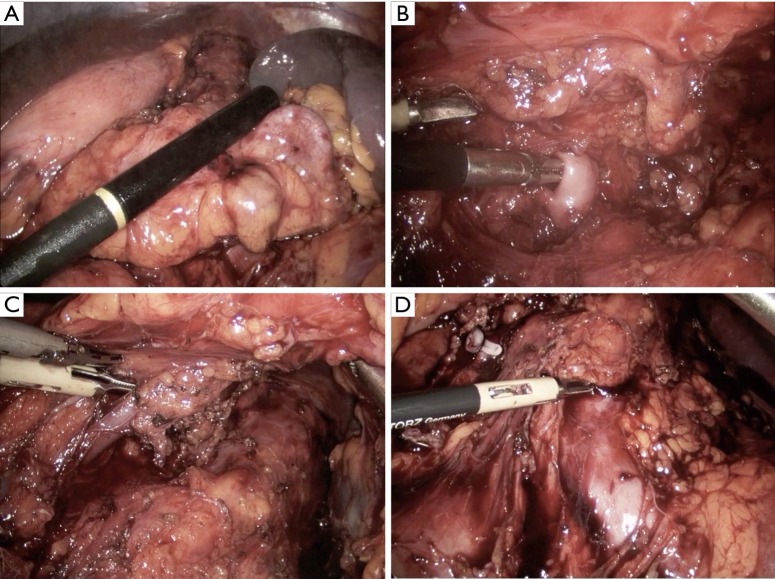
The approaches to laparoscopic distal pancreatectomy are well described elsewhere (21,22), and key operative steps of this technique are shown in Figure 2. Variations of the technique will be discussed, such as: patient positioning, use of hand access ports, the role of splenic preservation, direction and extent of dissection, and role of robotics (which will be covered in a separate section). Figure 3 shows intraoperative images of laparoscopic distal pancreatectomy for adenocarcinoma, and pancreatosplenectomy specimens are demonstrated in Figure 4.
Figure 2.

Key operative steps in laparoscopic distal pancreatectomy and splencetomy for pancreatic adenocarcinoma (23). Available online: http://www.asvide.com/articles/506
Figure 3.
Intraoperative images of laparoscopic distal pancreatectomy for adenocarcinoma illustrating (A) the ultrasound probe over the pancreatic tail tumor; (B) the dissection of the splenic artery; (C) the dissection of the splenic vein; and (D) the splenic artery stump, left renal vein, and left adrenal vein after resection of the specimen.
Figure 4.
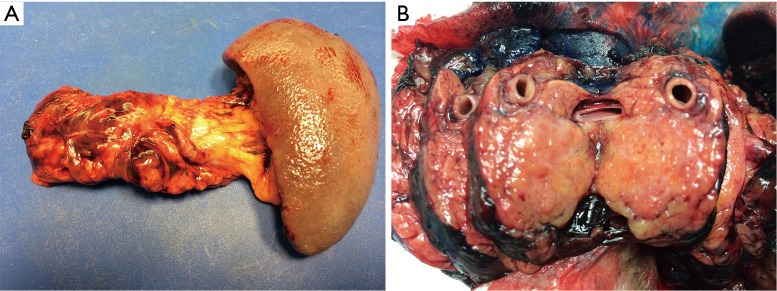
(A) Typical pancreatosplenectomy specimen from distal pancreatectomy for adenocarcinoma; (B) distal pancreatectomy specimen showing a section through the tumor of the pancreatic tail.
Patients are typically positioned either in supine or lazy right lateral decubitus position depending on tumor location and surgeon preference. The advantages of supine position are ease of set up, clearer airway access for anesthesia, and ability to access the pancreatic head and neck if necessary for tumors extending to this location. The benefits of the lateral position include gravity retraction of the stomach and spleen, more direct visualization of the body and tail of the pancreas, and superior surgeon ergonomics and comfort (24).
In the laparoscopic hand-access technique, an abdominal port is placed through which the surgeon’s hand can access the peritoneal cavity during the laparoscopic procedure. Others have described the technical details of laparoscopic hand-assist distal pancreatectomy (18,25,26). Potential advantages to a hand-access approach include preserving the surgeon’s ability of direct palpation of the tumor and anatomy, ease of removal of larger malignant specimens through the hand port, use of manual dissection, and opportunity to apply direct pressure in the case of bleeding. The largest comparative trial of hand access (n=61) compared to total laparoscopic (n=72) distal pancreatectomies is from the authors’ institution (27). Though patients who underwent total laparoscopic procedures had shorter hospital stays (5.3±1.7 vs. 6.8±5.5 days; P=0.032), there was a trend that total laparoscopic procedures had higher rates of conversion to open procedure compared to hand assist (8.5% vs. 3.3%; P=0.21). In the same study, it was found that the hand-access approach was used less frequently in recent cases of laparoscopic distal pancreatectomy compared to earlier cases at a single intuition (25.6% vs. 68.1%; P<0.001) (27). Despite this temporal shift, the hand assist approach still plays an important role in more challenging cases of resection of larger tumors, tumors with increased surrounding inflammation, and in obese patients.
Another option in the laparoscopic approach to distal pancreatectomy is splenic preservation. This can be accomplished through preservation of the splenic vasculature or en bloc resection of the splenic vasculature with preservation of the short gastric vessels to supply the spleen, known as the Warshaw technique (28), although splenic function following this approach is not validated. Multiple studies have addressed the value of splenic preservation with regards to perioperative morbidity and mortality with no clear consensus on recommendations for benign disease (29-31). For patients with malignant disease, vessel-preserving splenic preservation may compromise radial resection margins, as residual pancreatic tissue likely remains following dissection; thus, splenic preservation is not recommended for these patients by the authors.
During a typical open distal pancreatectomy, surgeons mobilize the spleen and dissect under the pancreatic tail and proceed towards the pancreatic neck in a left to right direction, or lateral-to-medial approach, as the operating team is looking down on the target organ. The laparoscopic view is antero-caudal, lending itself to dissection under the gland and a medial-to-lateral approach giving the surgeon access to the splenic vessels first (24). No head to head comparison of these approaches exists.
Radical antegrade modular pancreatosplenectomy (RAMPS) represents an alternate surgical approach to distal pancreatectomy. In this procedure, first described in the context of an open approach in 2003, the surgeon performs the dissection of the pancreas from right to left taking a wider margin where possible, to include the lymphatic tissue surrounding the celiac axis, Gerota’s fascia of the left kidney, and the left adrenal gland when necessary (32). In proceeding with the dissection in this manner, it was hypothesized that one could achieve an improved oncologic resection with a higher likelihood of obtaining negative tangential (mobilization) margins (89%; n=32), increased rates of R0 (microscopically negative) resections (81%; n=32), an improved N1 dissection [mean lymph node (LN) count =18], and a five-year overall survival similar to that of patients undergoing pancreaticoduodenectomy for adenocarcinoma (35.5%) (33,34). Later, the RAMPS technique was adapted for laparoscopic surgery and is an option in the laparoscopic resection of distal pancreatic adenocarcinoma (17).
As RAMPS is designed in part to improve tangential surgical margin clearance, one must consider the true value of the R0 resection, for which current data are conflicting. In a recent study comparing survival outcomes in patients who underwent RAMPS (n=38) to those who had traditional distal pancreatosplenectomies (n=54), Park et al. found that RAMPS was not independently associated with overall survival (HR: 1.502; 95% CI: 0.796-2.834; P=0.209) (35). Jamieson et al. analyzed outcomes of 148 cases of classic or pylorus-preserving pancreatoduodenectomies for pancreatic adenocarcinoma stratifying by margin status (36). Distinguishing between transection margins and tangential or mobilization margins, the study revealed that patients with R1 mobilization (tangential) margins had the same survival as patients with R0 resections (P=0.52), while R1 transection margins were independently associated with shorter survival (HR: 2.76; 95% CI: 2.12-3.91) (36). This suggested that while R0 transection margins were related to survival, the status of the mobilization margin was not; however, a meta-analysis of randomized controlled trials examining outcomes related to adjuvant therapy after pancreatic resection for pancreatic adenocarcinoma found that margin status, in general, was not an independent predictor of survival (R1: HR 1.10; 95% CI: 0.94-1.29; P=0.24) (37). Though this study challenged the value of negative resection margins, surgical doctrine currently recommends R0 resection, and the RAMPS approach can increase R0 rates.
Patient selection
In surgical planning, multiple factors must be considered in choosing candidates for laparoscopic distal pancreatectomy. These include medical comorbidities, size of the tumor, adjacent organ involvement, and major vascular involvement. Differences between patient populations undergoing laparoscopic and open distal pancreatectomy were considered in a multi-institutional retrospective study from the Central Pancreas Consortium (CPC; representing a collaboration of academic US institutions with high volumes of pancreatic surgery) (38). In this study of patients who underwent distal pancreatectomy for all pathologies between 1999 and 2008, 439 patients underwent open-approach procedures while 254 patients had a laparoscopic procedure. There was no difference in age (>65 years: 30% vs. 31%; P=NS) or ASA class (>2: 54% vs. 49%; P=NS). Additionally, patients had similar BMIs (>27: 45% vs. 51%; P=NS). Open procedures were more frequently done for pancreatic adenocarcinoma (29% vs. 9%; P<0.001) and larger tumors (>3.5 cm: 58% vs. 40%; P<0.001) with longer postoperative specimens (>8.5 cm: 59% vs. 46%; P=0.002) and more frequent splenectomy (90% vs. 66%; P<0.001). For laparoscopic distal pancreatectomy, no assessed preoperative factor increased the risk of major complication or pancreatic fistula (38).
A study from the authors’ institution compared patient populations undergoing laparoscopic distal pancreatectomy in the context of early experience and recent experience (27). One hundred thirty two patients over 11 years were divided into groups of 66 based on timing of resection representing the early and present experience of the institution. Eleven of these patients had pancreatic adenocarcinoma. There was no observed difference between the temporal groups in age, sex, and obesity rate. In more recent cases, patients had a higher rate of comorbidities (Charleston comorbidity score ≥3: 40.9% vs. 16.7%; P=0.003). There were increased tumors in the body and neck in the more recent experience (74.2% vs. 26.3%; P<0.001). Additionally, a trend was appreciated in increased mean size of tumors in the recent experience (4.0±2.8 vs. 3.3±1.5 cm; P=0.09). Despite the increase in more proximal tumors and increased comorbidities in the recent cohort undergoing laparoscopic distal pancreatectomy, there were no differences in perioperative complications rates between early and recent experience, thereby suggesting that this technique has acceptable morbidity in these higher risk patients (27).
The CPC studied patients who underwent laparoscopic distal pancreatectomy to create a risk score to predict development of post-operative complications (39). The preoperative factor that independently correlated with major complications and major pancreatic fistulas (class B or C) was increased BMI (>27: HR 3.27, 95% CI: 1.16-9.60, P<0.05; HR 6.49, 95% CI: 1.79-23.50, P<0.01). Other risk factors included length of pancreas specimen >8 cm and estimated blood loss >150 mL. The increased risk from higher BMI can be helpful in counseling patients pre-operatively (39). Conversely, Boutros et al. found that unselected patients undergoing laparoscopic distal pancreatectomy had similar outcomes to selected patients, implying that selection criteria for laparoscopic approach could be expanded (40).
Outcomes after laparoscopic distal pancreatectomy for adenocarcinoma
Open distal pancreatectomy has long been considered the standard approach to resection of distal pancreatic ductal adenocarcinoma with acceptable morbidity and a perioperative mortality of less than 1% (30). As advanced MIS techniques develop, a laparoscopic approach to managing pancreatic cancer is now an option. There are limited data comparing laparoscopic and open distal pancreatectomy for adenocarcinoma (Table 1). Here, we explore the postoperative outcomes as well as the short-term (nodes and margins) and long-term (recurrence and survival) oncologic outcomes after laparoscopic resection of distal pancreatic ductal adenocarcinoma.
Table 1. Published studies comparing open with laparoscopic distal pancreatectomy for adenocarcinoma.
| Study | Total cases |
Conversions | Mean estimated blood loss (mL) |
Complication rate (%) |
Peri-operative mortality (%) |
Mean tumor size (cm) |
Positive margin (%) |
Mean number of harvested lymph nodes |
Overall survival |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Open | Lap | Open | Lap | Open | Lap | Open | Lap | Open | Lap | Open | Lap | Open | Lap | Open | Lap | ||||||||
| Kooby et al. (41) [2010]a | 189 | 23 | 4 | 790* | 422* | – | – | 0.9 | 0 | 4.5 | 3.5 | 27 | 26 | 12.5 | 13.8 | 16 monthsb,c | 16 monthsb,c | ||||||
| Magge et al. (42) [2013] | 34 | 28 | 5 | 570* | 290* | 50 | 39 | 0 | 0 | 4.5 | 3.7 | 12 | 14 | 12c | 11c | – | HR: 1.11 CI: 0.47-2.62 | ||||||
| Rehman et al. (43) [2013] | 14 | 8 | 0 | 650 | 306 | 42 | 37 | 0 | 0 | 3.2 | 2.2 | 14 | 12 | 14c | 16c | 3 year: 74% | 3 year: 82% | ||||||
| Hu et al. (44) [2014] | 23 | 11 | 0 | 150c | 100c | – | – | 0 | 0 | 3.1 | 2.8 | 0 | 0 | 16.1 | 14.8 | 54 monthsc | 42 monthsc | ||||||
a, multi-institutional; b, case-controlled data; c, median value reported; –, data not available; *, P<0.05; Lap, laparoscopic.
Postoperative surgical outcomes of laparoscopic resection
The first studies to report postoperative outcomes after laparoscopic resections of pancreatic ductal adenocarcinoma had small samples sizes with no comparative element. In a retrospective, multi-centered European trial in 2005, 127 patients who underwent laparoscopic resection for pancreatic neoplasms were studied (19). Twenty-four patients underwent distal pancreatectomy with splenectomy, and only 3 patients had pancreatic adenocarcinoma on pathology. The conversion rate for the entire patient population was 14%, and there were no perioperative deaths. With laparoscopic distal pancreatectomy and splenectomy, the mean OR time was 195 minutes, and 27% of patients had postoperative pancreatic complications. Patients who underwent a laparoscopic procedure had shorter hospital stay compared to those where the procedure was converted to open (7 vs. 11 days; P<0.0021) (19). In 2006, in a single institution study of 16 patients in the US undergoing laparoscopic hand-assisted distal pancreatectomy, only one patient had adenocarcinoma. This patient had an operative time of 224 minutes with 1,250 mL of estimated blood loss. Post-operatively, the patient tolerated a general diet in 3 days and was discharged on post-operative day 4 without complication (18). Though these data suggest that laparoscopy could be performed for pancreatic adenocarcinoma resection in the distal pancreas, they fail to offer comparison between the laparoscopic approach and the standard open approach.
One of the first case-controlled comparative trials of laparoscopic versus open distal pancreatectomies was conducted in 2006 (45). In this study, 15 laparoscopic procedures were matched to 15 open procedures. Three of the 15 laparoscopic procedures were converted to open secondary to bleeding and retroperitoneal tumor adherence; these three cases represented the only pancreatic adenocarcinomas included. At that time the authors concluded that their results were unclear as to whether resection of distal pancreatic adenocarcinoma was “consistently feasible with the laparoscopic approach” (45).
In 2008 the CPC published the largest comparative trail to that date (16). This study of 667 patients who underwent distal pancreatectomy between 2002 and 2006 included 159 (24%) attempted laparoscopic resections with mixed pathologies. Twenty (13%) laparoscopic procedures were converted to open. Importantly, 150 patients had pancreatic adenocarcinoma in this study. Resections for pancreatic adenocarcinoma were performed open more frequently than laparoscopically in this population (26% vs. 10%; P<0.001). Cohorts were matched by age, ASA, tumor size, length of resected specimen, and pathology for open (n=200) or laparoscopic (n=142) resection. There was no difference in OR time (216 vs. 230 minutes; P=0.3), development of major pancreatic fistula (18% vs. 11%; P=0.1), major complication (17% vs. 10%; P=0.08), or 30-day mortality (1% vs. 0%; P=0.040). Open procedures had higher estimated blood loss (588 vs. 357 mL, P<0.01), increased wound infections (15% vs. 5%; P=0.004), increased need for drain placement post-operatively (15% vs. 6% P=0.02) and longer hospital stay (9.0 vs. 5.9 days; P<0.01). Laparoscopic resection was independently associated with shorter hospital stays (HR 0.33; CI: 0.19-0.56; P<0.01). From this study, it became clear that laparoscopic distal pancreatectomy is not only feasible, but it could also offer additional benefits as compared to the open approach; yet, the question of oncologic outcomes after laparoscopic resection remained (16).
Short term oncologic outcomes of laparoscopic resection
Resection margins
Though debated, one of the oncologic goals of resection in pancreatic adenocarcinoma is achieving microscopically negative margins (R0). Some small non-comparative studies have shown that laparoscopic resection can frequently achieve R0 resections for pancreatic adenocarcinoma (93-100%) (19,46,47). Multiple comparative studies have found that laparoscopic and open procedures have similar rates of R0 margins on final pathology (74-97% vs. 73-96%; P=NS) (16,41,43,48). The CPC studied 212 patients with resected pancreatic adenocarcinoma and matched open (n=70) and laparoscopic (n=23) resections by age, ASA, and tumor size. They found no difference in positive margin (R1) rates (34% vs. 26%; P=0.61) (41). Few studies have found that laparoscopic margins are more likely to be negative than in open procedures, but DiNorcia et al. report in their series of distal pancreatectomies with mixed pathology that the laparoscopic approach was associated with decreased R1 resections (2.8% vs. 13%; P=0.01); however, the malignancies reported include neuroendocrine tumors and pancreatic adenocarcinoma. Additionally, patients who had procedures that were converted to open were analyzed in the open group, and the groups were not matched such that adverse pathologic factors that could have increased the risk of R1 margins were not considered (31).
In a study by Fernandez-Cruz et al., laparoscopic RAMPS for pancreatic adenocarcinoma was evaluated (17). As discussed previously, the RAMPS approach to distal pancreatectomy potentially offers increased rates of R0 resections with negative tangential margins. Of 13 attempted laparoscopic RAMPS in this study, 3 procedures were converted to open secondary to adhesions to the diaphragm and invasion of the colon. In the 10 RAMPS cases that proceeded laparoscopically, an R0 resection was achieved in 90%, whereas in the converted cases, the R0 rate was only 33%, suggesting that an R1 resection in these patients was associated with more invasive or adherent disease (17). This study does not offer comparison to the open technique. Other small studies of highly selected patients undergoing minimally invasive RAMPS for malignancy in the pancreatic tail reported R0 tangential and transectional margins in 100% of cases (49,50). Yet these patients who had R0 resections were highly selected only to include tumors that were confined to the pancreas, did not invade adjacent organs, and did not approximate the celiac axis (50). Therefore, in highly selected patient populations, MIS RAMPS can offer excellent resection margins.
LN harvest
Current data suggest that a minimum of 12 LNs should be harvested for resections of pancreatic adenocarcinoma based on single institution and SEER data (51,52). If fewer that 12 LNs are resected, the likelihood of underestimating the nodal stage becomes greater. Therefore, patients with fewer than 12 LNs resected who seemingly have N0 disease have shorter median overall survival than N0 patients with greater than 12 LNs resected secondary to occult nodal metastases (16 vs. 23 months; P<0.001) (52).
In the aforementioned non-comparative study of patients undergoing laparoscopic RAMPS, the mean LN harvest was 14.5 (6-20 range) for the ten laparoscopic distal pancreatectomies for pancreatic adenocarcinoma (17). Most studies comparing the number of LNs in laparoscopic and open cases found no significant differences in the number of LNs harvested (31,41,43,48,53). In a matched comparative study of distal pancreatectomies for pancreatic adenocarcinoma, the CPC found similar numbers of LNs for open compared to laparoscopic cases (12.3±8.3 vs. 14.0±8.6; P=0.46) (41). One single institution study of distal pancreatic resection for mixed pathology reported fewer LNs in the laparoscopic group (mean: 4 vs. 10; P=0.04); however, the laparoscopic cohort had fewer patients with pancreatic adenocarcinoma (4.1% vs. 21%; P<0.01), which could have influenced the surgeon’s operative approach to nodal resection (54).
Long-term oncologic outcomes of laparoscopic resection
Few studies offer long-term data on patients after laparoscopic distal pancreatectomy for pancreatic adenocarcinoma. Below, the results from these few studies on recurrence and survival are summarized.
Data are scarce on recurrence of pancreatic adenocarcinoma after laparoscopic resection, and comparative data are limited. Most of our insights into recurrence outcomes originate from non-comparative studies. In 2005, Mabrut et al. conducted a multi-institutional European study of laparoscopic distal pancreatectomies that included 16 patients with a pancreatic malignancy, 4 of which were pancreatic adenocarcinoma (19). During the median 15-month follow up, 23% of patients with malignant tumors had a recurrence. Notably, no patients had evidence of trochar site recurrences (19). The following year, D’Angelica et al. reported a series of laparoscopic hand-assisted distal pancreatectomies, one of which was for adenocarcinoma (18). This patient presented six months post-operatively with liver metastases but no local recurrence (18). Larger comparative trials that report recurrence data are warranted.
In the study by Fernandez-Cruz of laparoscopic RAMPS, 3 of 10 patients died within a year with local recurrence and liver metastases with a median survival of 14 months (17). All patients who underwent laparoscopic RAMPS received adjuvant chemotherapy three weeks post-operatively (17). In a more recent study of patients undergoing laparoscopic distal pancreatectomy for adenocarcinoma, the median survival after resection was 19 months (n=29) (47). In an unmatched single institution study of patients undergoing laparoscopic (n=8) or open (n=14) distal pancreatectomy for pancreatic adenocarcinoma, there was no difference in 3 year overall survival rates (82% vs. 74%; P=0.89) (43). The CPC reported a 16 month median survival after both laparoscopic (n=23) and open (n=70) approaches in matched cohorts (P=0.71) (41). The evidence to date suggests that the recurrence and survival outcomes of laparoscopic distal pancreatectomy for adenocarcinoma are similar to those of open procedures.
Cost outcomes
In evaluating comparative value of surgical techniques, cost must be considered. There are limited financial data on outcomes specific to pancreatic adenocarcinoma pathology after laparoscopic resection; therefore, the data on laparoscopic distal pancreatectomy including resection for all pathology are here reported and are summarized in Table 2.
Table 2. Cost-comparisons of open and laparoscopic distal pancreatectomies.
| Study | Total cases |
Mean operative cost |
Mean length of stay (days) |
Mean total cost of care |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Open | Lap | Open | Lap | Open | Lap | Open | Lap | ||||
| Eom et al. (55) [2008] | 167 | 31 | – | – | 13.5* | 11.5* | $3,401* | $4,884* | |||
| Abu et al. (56) [2012] | 16 | 35 | £5,231* | £6,039* | 11a,* | 7a,* | £15,324 | £10,587 | |||
| Fox et al. (57) [2012] | 76 | 42 | $4,510a | $4,655a | 7a,* | 5a,* | $13,656a,* | $10,842a,* | |||
| Limongelli et al. (58) [2012] | 29 | 16 | €1,989* | €2,889* | 8.8* | 6.4* | € 10,944 | € 9,603 | |||
| Rutz et al. (59) [2014] | 45 | 70 | $4,900* | $5,756* | 6 | 5 | $13,900 | $10,480 | |||
a, median value reported instead of mean. –, data not available; *, P<0.05; Lap, laparoscopic.
A single institution Korean study in 2008 found that the total cost (operating room charges and hospitalization cost) for laparoscopic (n=31) distal pancreatectomies was more expensive than that of the open [167] approach ($4,884.2±1,845.1 vs. $3,401.4±1,247.5; P<0.001) (55). Subsequent studies in Britain and Italy in 2012 showed that though the operating room cost of laparoscopic distal pancreatectomy is higher than open (£6,039/€2,889 vs. £5,231/€1,989; P<0.05), decreased length of hospital stay after laparoscopic procedures (6.3-7 vs. 8.8-11 days; P<0.01) led to equivalent total hospital costs (£10,587/€9,603 vs. £15,324/€10,944; P=0.2) (56,58). Two recent North American studies reported that laparoscopic distal pancreatectomy was less expensive than open distal pancreatectomy in overall hospital cost (57,59). In a study from the author’s institution, 115 patients who underwent uncomplicated distal pancreatectomies from 2009-2013 were assessed (laparoscopic: n=70; open: n=45) (59). Nineteen of these patients had pancreatic adenocarcinoma (laparoscopic: 16%; open: 18%). Again, the operating room cost was higher for patients undergoing laparoscopic procedures ($5,756 vs. $4,900; P=0.02), but the shorter length of stay after laparoscopy (5.2 vs. 7.7 days; P=0.01) led to decreased total variable costs ($10,480 vs. $13,900; P=0.06) (59). These studies show that laparoscopic distal pancreatectomy is a financially reasonable approach to resection. Future goals are aimed towards reducing intraoperative costs further.
Robotic approach to resection of distal pancreatic adenocarcinoma
Rates of robotic surgery have been increasing since its advent over a decade ago (60). Much like laparoscopic surgery initially, there are barriers to the universal adoption of this new approach including overall expense, a steep learning curve, and lack of tactile feedback to the operator. Yet, robotic surgery offers three-dimensional optics, increased freedom of motion, precision, and improved ergonomics for the surgeon (60-62). Consequently, robotic surgery is becoming widespread and versatile.
The surgical approach to robotic conventional distal pancreatectomy with splenectomy and the RAMPS procedure has been well described elsewhere (63-65). One of the first reports of robotics used in pancreatic surgery came from Italy in 2003 (66). In this study, 5 patients underwent robotic distal pancreatectomy, 3 of whom had pancreatic adenocarcinoma. The operating room time was 270 minutes. The mean length of stay was 11 days. One patient had a complication of a pancreatic leak (20%), and there were no post-operative mortalities (66). A similar study from 2010 of 43 patients who underwent distal pancreatectomy by the same author had similar postoperative outcomes: pancreatic leak 20.9% and postoperative mortality of 1.5% (64). Choi et al. report on a case series of 4 patients who underwent robotic RAMPS for pancreatic adenocarcinoma in which 100% had R0 margins with a median LN count of 8.5 (range, 2-23) (65). Multiple other cases of robotic distal pancreatectomy and splenectomy have been reported (63,67-72). The results of these studies suggested that robotic distal pancreatectomy could be a feasible approach but were lacking in detailed oncologic and comparative data.
In a study comparing rates of splenic preservation in robotic distal pancreatectomy (n=20) and laparoscopic distal pancreatectomy (n=25), the success of spleen preservation was higher in the robotic group (95% vs. 64%, P=0.027) (68); however, in the case of pancreatic adenocarcinoma, splenic preservation is not recommended. A recent single-institution US study compared consecutive robotic resections (n=30) to an earlier cohort of laparoscopic (n=94) distal pancreatectomies (73). There were no differences in length of hospital stay, pancreatic fistula formation, rate of blood transfusion, or readmission between the two groups. The study included 27 cases of pancreatic adenocarcinoma representing 43% of the robotic and 15% of the laparoscopic patients (P<0.05). For the pancreatic adenocarcinoma cases, the rate of R1 resections was lower in the robotic group (0% vs. 36%; P<0.05), and the robotic procedure yielded more LNs (19 vs. 9; P<0.01) (73). Though this study offers promising short-term oncologic results, studies on long-term outcomes are warranted.
Data from a single institutional study suggest that robotic surgery may further shorten hospital length of stay, resulting in lower total hospital cost compared to open and laparoscopic approaches (LOS: 4 vs. 8 vs. 6 days, P<0.05; $10,588 vs. $16,059 vs. $12,986, P<0.05) (74). Though this offers insight into a single hospital’s experience, it does not reflect financial outcomes universally or the monetary investment in the robotic technology and its upkeep. Further studies are needed.
Not enough data exist to evaluate the safety and long-term outcomes of robotic distal pancreatectomy for pancreatic adenocarcinoma. The robotic approach to distal pancreatectomy does offer the advantage of increasing the surgeon’s ability to preserve the spleen, yet this is contraindicated in the case of pancreatic adenocarcinoma. Therefore, at this time, robotic surgery for distal pancreatic adenocarcinoma does not offer a definitive benefit.
Fluorescence-guided intraoperative tumor localization
Another emerging technology in oncologic surgery is fluorescence-guided tumor localization to aid in complete tumor resection. In this technique, tumor-specific fluorescent particles are administered to the patient that bind tumor. These particles can then be visualized or detected with an instrument, which allows surgeons to more easily distinguish between cancer cells and normal tissue during resection. In mouse models of pancreatic cancer, this technique has allowed for improved margins of resection, decreased local and distant recurrence, and longer disease-free survival after open and laparoscopic resections (75,76). In another study of a mouse model, a fluorescence-detecting device showed promise for use in the inspection of surgical margins for residual disease, which could increase rates of attaining negative margins (77). This technology could represent the next step to improving treatment of pancreatic cancer in open and laparoscopic resections.
Conclusions
Over the last two decades, laparoscopic distal pancreatectomy for pancreatic adenocarcinoma has become more common, though there are no randomized trials comparing this technique to open surgical technique. Data primarily from retrospective studies suggest that post-operative complication rates between open and laparoscopic distal pancreatectomies are similar. In exploring short-term oncologic outcomes after laparoscopic resection of distal pancreatic adenocarcinoma, there are no differences in the rate of achieving negative margins or in the number of LNs resected when compared to open surgery. There are limited recurrence and survival data on laparoscopic compared to open distal pancreatectomy for pancreatic adenocarcinoma, but in the few studies that assess long term outcomes, recurrence rates and survival outcomes appear similar; the need for randomized trials remains. Most recent studies have suggested that though laparoscopic distal pancreatectomy incurs a greater operative cost, the associated shorter length of hospital stay leads to decreased overall cost compared to open procedures.
Multiple new technologies are emerging to improve treatment of pancreatic cancer. Robotic pancreatectomy is feasible, but there are limited data on resection of pancreatic adenocarcinoma, and outcomes appear similar to laparoscopic approaches. Additionally fluorescence-guided surgery represents a new technology on the horizon that could improve oncologic outcomes after resection of pancreatic adenocarcinoma. Overall, laparoscopic distal pancreatectomy appears safe and reasonable, though additional studies of long-term oncologic outcomes are merited.
Acknowledgements
Funding: This work was funded in part by the Katz Foundation.
Disclosure: The authors declare no conflict of interest.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians 2014;64:9-29. [DOI] [PubMed] [Google Scholar]
- 2.Niederhuber JE, Brennan MF, Menck HR. The National Cancer Data Base report on pancreatic cancer. Cancer 1995;76:1671-7. [DOI] [PubMed] [Google Scholar]
- 3.Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg 1999;189:1-7. [DOI] [PubMed] [Google Scholar]
- 4.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473-81. [DOI] [PubMed] [Google Scholar]
- 5.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77. [DOI] [PubMed] [Google Scholar]
- 6.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 2008;299:1019-26. [DOI] [PubMed] [Google Scholar]
- 7.Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modolell I, Guarner L, Malagelada JR. Vagaries of clinical presentation of pancreatic and biliary tract cancer. Ann Oncol 1999;10 Suppl 4:82-4. [PubMed] [Google Scholar]
- 9.Porta M, Fabregat X, Malats N, et al. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol 2005;7:189-97. [DOI] [PubMed] [Google Scholar]
- 10.Rosales-Velderrain A, Bowers SP, Goldberg RF, et al. National trends in resection of the distal pancreas. World J Gastroenterol 2012;18:4342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeh HJ, 3rd, Udelsman R. One hundred laparoscopic adrenalectomies: a single surgeon's experience. Ann Surg Oncol 2003;10:1012-7. [DOI] [PubMed] [Google Scholar]
- 12.Gagner M, Pomp A, Heniford BT, et al. Laparoscopic adrenalectomy: lessons learned from 100 consecutive procedures. Ann Surg 1997;226:238-46; discussion 246-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs JK, Goldstein RE, Geer RJ. Laparoscopic adrenalectomy. A new standard of care. Ann Surg 1997;225:495-501; discussion 501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo PC, Johnson LB, Sitzmann JV. Laparoscopic donor nephrectomy with a 23-hour stay: a new standard for transplantation surgery. Ann Surg 2000;231:772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLeod R. Long-term results of laparoscopic-assisted colectomy are comparable to results after open colectomy. Ann Surg 2008;248:8-9. [DOI] [PubMed] [Google Scholar]
- 16.Kooby DA, Gillespie T, Bentrem D, et al. Left-sided pancreatectomy: a multicenter comparison of laparoscopic and open approaches. Ann Surg 2008;248:438-46. [DOI] [PubMed] [Google Scholar]
- 17.Fernández-Cruz L, Cosa R, Blanco L, et al. Curative laparoscopic resection for pancreatic neoplasms: a critical analysis from a single institution. J Gastrointest Surg 2007;11:1607-21; discussion 1621-2. [DOI] [PubMed] [Google Scholar]
- 18.D'Angelica M, Are C, Jarnagin W, et al. Initial experience with hand-assisted laparoscopic distal pancreatectomy. Surg Endosc 2006;20:142-8. [DOI] [PubMed] [Google Scholar]
- 19.Mabrut JY, Fernandez-Cruz L, Azagra JS, et al. Laparoscopic pancreatic resection: results of a multicenter European study of 127 patients. Surgery 2005;137:597-605. [DOI] [PubMed] [Google Scholar]
- 20.Gagner M, Pomp A, Herrera MF. Early experience with laparoscopic resections of islet cell tumors. Surgery 1996;120:1051-4. [DOI] [PubMed] [Google Scholar]
- 21.Fisichella PM, Shankaran V, Shoup M. Laparoscopic distal pancreatectomy with or without splenectomy: how I do it. J Gastrointest Surg 2011;15:215-8. [DOI] [PubMed] [Google Scholar]
- 22.Robinson S, Saif R, Charnley RM, et al. Surgical adjuncts to laparoscopic distal pancreatectomy. Minim Invasive Ther Allied Technol 2011;20:369-73. [DOI] [PubMed] [Google Scholar]
- 23.Postlewait LM, Kooby DA. Key operative steps in laparoscopic distal pancreatectomy and splencetomy for pancreatic adenocarcinoma. Asvide 2015;2:050. Available online: http://www.asvide.com/articles/506
- 24.Kooby DA. Laparoscopic surgery for cancer: historical, theoretical, and technical considerations. Oncology (Williston Park) 2006;20:917-27; discussion 927-8, 931-2. [PubMed]
- 25.Cuschieri A. Laparoscopic hand-assisted surgery for hepatic and pancreatic disease. Surg Endosc 2000;14:991-6. [DOI] [PubMed] [Google Scholar]
- 26.Laxa BU, Carbonell AM, 2nd, Cobb WS, et al. Laparoscopic and hand-assisted distal pancreatectomy. Am Surg 2008;74:481-6; discussion 486-7. [PubMed] [Google Scholar]
- 27.Kneuertz PJ, Patel SH, Chu CK, et al. Laparoscopic distal pancreatectomy: trends and lessons learned through an 11-year experience. J Am Coll Surg 2012;215:167-76. [DOI] [PubMed] [Google Scholar]
- 28.Warshaw AL. Conservation of the spleen with distal pancreatectomy. Arch Surg 1988;123:550-3. [DOI] [PubMed] [Google Scholar]
- 29.Shoup M, Brennan MF, McWhite K, et al. The value of splenic preservation with distal pancreatectomy. Arch Surg 2002;137:164-8. [DOI] [PubMed] [Google Scholar]
- 30.Lillemoe KD, Kaushal S, Cameron JL, et al. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg 1999;229:693-8; discussion 698-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiNorcia J, Schrope BA, Lee MK, et al. Laparoscopic distal pancreatectomy offers shorter hospital stays with fewer complications. J Gastrointest Surg 2010;14:1804-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strasberg SM, Drebin JA, Linehan D. Radical antegrade modular pancreatosplenectomy. Surgery 2003;133:521-7. [DOI] [PubMed] [Google Scholar]
- 33.Strasberg SM, Linehan DC, Hawkins WG. Radical antegrade modular pancreatosplenectomy procedure for adenocarcinoma of the body and tail of the pancreas: ability to obtain negative tangential margins. J Am Coll Surg 2007;204:244-9. [DOI] [PubMed] [Google Scholar]
- 34.Mitchem JB, Hamilton N, Gao F, et al. Long-term results of resection of adenocarcinoma of the body and tail of the pancreas using radical antegrade modular pancreatosplenectomy procedure. J Am Coll Surg 2012;214:46-52. [DOI] [PubMed] [Google Scholar]
- 35.Park HJ, You DD, Choi DW, et al. Role of radical antegrade modular pancreatosplenectomy for adenocarcinoma of the body and tail of the pancreas. World J Surg 2014;38:186-93. [DOI] [PubMed] [Google Scholar]
- 36.Jamieson NB, Foulis AK, Oien KA, et al. Positive mobilization margins alone do not influence survival following pancreatico-duodenectomy for pancreatic ductal adenocarcinoma. Ann Surg 2010;251:1003-10. [DOI] [PubMed] [Google Scholar]
- 37.Butturini G, Stocken DD, Wente MN, et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg 2008;143:75-83; discussion 83. [DOI] [PubMed] [Google Scholar]
- 38.Cho CS, Kooby DA, Schmidt CM, et al. Laparoscopic versus open left pancreatectomy: can preoperative factors indicate the safer technique? Ann Surg 2011;253:975-80. [DOI] [PubMed] [Google Scholar]
- 39.Weber SM, Cho CS, Merchant N, et al. Laparoscopic left pancreatectomy: complication risk score correlates with morbidity and risk for pancreatic fistula. Ann Surg Oncol 2009;16:2825-33. [DOI] [PubMed] [Google Scholar]
- 40.Boutros C, Ryan K, Katz S, et al. Total laparoscopic distal pancreatectomy: beyond selected patients. Am Surg 2011;77:1526-30. [PubMed] [Google Scholar]
- 41.Kooby DA, Hawkins WG, Schmidt CM, et al. A multicenter analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate? J Am Coll Surg 2010;210:779-85. [DOI] [PubMed] [Google Scholar]
- 42.Magge D, Gooding W, Choudry H, et al. Comparative effectiveness of minimally invasive and open distal pancreatectomy for ductal adenocarcinoma. JAMA Surg 2013;148:525-31. [DOI] [PubMed] [Google Scholar]
- 43.Rehman S, John SK, Lochan R, et al. Oncological feasibility of laparoscopic distal pancreatectomy for adenocarcinoma: a single-institution comparative study. World J Surg 2014;38:476-83. [DOI] [PubMed] [Google Scholar]
- 44.Hu M, Zhao G, Wang F, et al. Laparoscopic versus open distal splenopancreatectomy for the treatment of pancreatic body and tail cancer: a retrospective, mid-term follow-up study at a single academic tertiary care institution. Surg Endosc 2014;28:2584-91. [DOI] [PubMed] [Google Scholar]
- 45.Velanovich V. Case-control comparison of laparoscopic versus open distal pancreatectomy. J Gastrointest Surg 2006;10:95-8. [DOI] [PubMed] [Google Scholar]
- 46.Vijan SS, Ahmed KA, Harmsen WS, et al. Laparoscopic vs open distal pancreatectomy: a single-institution comparative study. Arch Surg 2010;145:616-21. [DOI] [PubMed] [Google Scholar]
- 47.Marangos IP, Buanes T, Rosok BI, et al. Laparoscopic resection of exocrine carcinoma in central and distal pancreas results in a high rate of radical resections and long postoperative survival. Surgery 2012;151:717-23. [DOI] [PubMed] [Google Scholar]
- 48.Jayaraman S, Gonen M, Brennan MF, et al. Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg 2010;211:503-9. [DOI] [PubMed] [Google Scholar]
- 49.Choi SH, Kang CM, Lee WJ, et al. Multimedia article. Laparoscopic modified anterior RAMPS in well-selected left-sided pancreatic cancer: technical feasibility and interim results. Surg Endosc 2011;25:2360-1. [DOI] [PubMed] [Google Scholar]
- 50.Lee SH, Kang CM, Hwang HK, et al. Minimally invasive RAMPS in well-selected left-sided pancreatic cancer within Yonsei criteria: long-term (>median 3 years) oncologic outcomes. Surg Endosc 2014;28:2848-55. [DOI] [PubMed] [Google Scholar]
- 51.House MG, Gönen M, Jarnagin WR, et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg 2007;11:1549-55. [DOI] [PubMed] [Google Scholar]
- 52.Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol 2008;15:165-74. [DOI] [PubMed] [Google Scholar]
- 53.Mehta SS, Doumane G, Mura T, et al. Laparoscopic versus open distal pancreatectomy: a single-institution case-control study. Surg Endosc 2012;26:402-7. [DOI] [PubMed] [Google Scholar]
- 54.Baker MS, Bentrem DJ, Ujiki MB, et al. A prospective single institution comparison of peri-operative outcomes for laparoscopic and open distal pancreatectomy. Surgery 2009;146:635-43; discussion 643-5. [DOI] [PubMed] [Google Scholar]
- 55.Eom BW, Jang JY, Lee SE, et al. Clinical outcomes compared between laparoscopic and open distal pancreatectomy. Surg Endosc 2008;22:1334-8. [DOI] [PubMed] [Google Scholar]
- 56.Abu Hilal M, Hamdan M, Di Fabio F, et al. Laparoscopic versus open distal pancreatectomy: a clinical and cost-effectiveness study. Surg Endosc 2012;26:1670-4. [DOI] [PubMed] [Google Scholar]
- 57.Fox AM, Pitzul K, Bhojani F, et al. Comparison of outcomes and costs between laparoscopic distal pancreatectomy and open resection at a single center. Surg Endosc 2012;26:1220-30. [DOI] [PubMed] [Google Scholar]
- 58.Limongelli P, Belli A, Russo G, et al. Laparoscopic and open surgical treatment of left-sided pancreatic lesions: clinical outcomes and cost-effectiveness analysis. Surg Endosc 2012;26:1830-6. [DOI] [PubMed] [Google Scholar]
- 59.Rutz DR, Squires MH, Maithel SK, et al. Cost comparison analysis of open versus laparoscopic distal pancreatectomy. HPB (Oxford) 2014;16:907-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanly EJ, Talamini MA. Robotic abdominal surgery. Am J Surg 2004;188:19S-26S. [DOI] [PubMed] [Google Scholar]
- 61.Kang CM, Kim DH, Lee WJ, et al. Initial experiences using robot-assisted central pancreatectomy with pancreaticogastrostomy: a potential way to advanced laparoscopic pancreatectomy. Surg Endosc 2011;25:1101-6. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen NT, Hinojosa MW, Finley D, et al. Application of robotics in general surgery: initial experience. Am Surg 2004;70:914-7. [PubMed] [Google Scholar]
- 63.Ntourakis D, Marzano E, Lopez Penza PA, et al. Robotic distal splenopancreatectomy: bridging the gap between pancreatic and minimal access surgery. J Gastrointest Surg 2010;14:1326-30. [DOI] [PubMed] [Google Scholar]
- 64.Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc 2010;24:1646-57. [DOI] [PubMed] [Google Scholar]
- 65.Choi SH, Kang CM, Hwang HK, et al. Robotic anterior RAMPS in well-selected left-sided pancreatic cancer. J Gastrointest Surg 2012;16:868-9. [DOI] [PubMed] [Google Scholar]
- 66.Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003;138:777-84. [DOI] [PubMed] [Google Scholar]
- 67.Kim DH, Kang CM, Lee WJ, et al. The first experience of robot assisted spleen-preserving laparoscopic distal pancreatectomy in Korea. Yonsei Med J 2011;52:539-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang CM, Kim DH, Lee WJ, et al. Conventional laparoscopic and robot-assisted spleen-preserving pancreatectomy: does da Vinci have clinical advantages? Surg Endosc 2011;25:2004-9. [DOI] [PubMed] [Google Scholar]
- 69.Ntourakis D, Marzano E, De Blasi V, et al. Robotic left pancreatectomy for pancreatic solid pseudopapillary tumor. Ann Surg Oncol 2011;18:642-3. [DOI] [PubMed] [Google Scholar]
- 70.Suman P, Rutledge J, Yiengpruksawan A. Robotic distal pancreatectomy. JSLS 2013;17:627-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhan Q, Deng XX, Han B, et al. Robotic-assisted pancreatic resection: a report of 47 cases. Int J Med Robot 2013;9:44-51. [DOI] [PubMed] [Google Scholar]
- 72.Melvin WS, Needleman BJ, Krause KR, et al. Robotic resection of pancreatic neuroendocrine tumor. J Laparoendosc Adv Surg Tech A 2003;13:33-6. [DOI] [PubMed] [Google Scholar]
- 73.Daouadi M, Zureikat AH, Zenati MS, et al. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg 2013;257:128-32. [DOI] [PubMed] [Google Scholar]
- 74.Waters JA, Canal DF, Wiebke EA, et al. Robotic distal pancreatectomy: cost effective? Surgery 2010;148:814-23. [DOI] [PubMed] [Google Scholar]
- 75.Metildi CA, Kaushal S, Hardamon CR, et al. Fluorescence-guided surgery allows for more complete resection of pancreatic cancer, resulting in longer disease-free survival compared with standard surgery in orthotopic mouse models. J Am Coll Surg 2012;215:126-35; discussion 135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Metildi CA, Kaushal S, Luiken GA, et al. Advantages of fluorescence-guided laparoscopic surgery of pancreatic cancer labeled with fluorescent anti-carcinoembryonic antigen antibodies in an orthotopic mouse model. J Am Coll Surg 2014;219:132-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mohs AM, Mancini MC, Singhal S, et al. Hand-held spectroscopic device for in vivo and intraoperative tumor detection: contrast enhancement, detection sensitivity, and tissue penetration. Anal Chem 2010;82:9058-65. [DOI] [PMC free article] [PubMed] [Google Scholar]