Abstract
The rectus extraocular muscles (EOMs) and inferior oblique muscle have paths through the orbit constrained by connective tissue pulleys. These pulleys shift position during contraction and relaxation of the EOMs, dynamically changing the biomechanics of force transfer from the tendon onto the globe. The paths of the EOMs are tightly conserved in normal patients and disorders in the location and/or stability of the pulleys can create patterns of incomitant strabismus that may mimic oblique muscle dysfunction and cranial nerve paresis. Developmental disorders of pulley location can occur in conjunction with large, obvious abnormalities of orbital anatomy (e.g., craniosynostosis syndromes) or subtle, isolated abnormalities in the location of one or more pulleys. Acquired disorders of pulley location can be divided into four broad categories: Connective tissue disorders (e.g., Marfan syndrome), globe size disorders (e.g., high myopia), senile degeneration (e.g., sagging eye syndrome), and trauma (e.g., orbital fracture or postsurgical). Recognition of these disorders is important because abnormalities in pulley location and movement are often resistant to standard surgical approaches that involve strengthening or weakening the oblique muscles or changing the positions of the EOM insertions. Preoperative diagnosis is aided by: (1) Clinical history of predisposing risk factors, (2) observation of malpositioning of the medial canthus, lateral canthus, and globe, and (3) gaze-controlled orbital imaging using direct coronal slices. Finally, surgical correction frequently involves novel techniques that reposition and stabilize the pulley and posterior muscle belly within the orbit using permanent scleral sutures or silicone bands without changing the location of the muscle's insertion.
Keywords: Extraocular Muscles, Incomitant Strabismus, Pulleys
INTRODUCTION
The location and composition of the rectus extraocular muscle (EOM) pulleys have been defined by an extensive series of studies utilizing noninvasive in vivo imaging1,2,3 and cadaveric orbit gross anatomy and histology.4,5,6,7 The key findings of these and other investigations can be summarized into a few key biomechanical concepts:
Normal EOM paths through the orbit are highly stereotypic between individuals2
Normal EOM paths are constrained (resist displacement) by connective tissue pulleys during changes in position of the muscle's insertion, either after routine gaze changes2 or after surgical transposition of the insertion8,9
Normal pulley positions are important in facilitating neural control of eye movements10,11,12,13,14
Normal pulley positions shift anteriorly and posteriorly during muscular contraction and relaxation to facilitate implementation of Listing's Law12
Abnormal pulley positions introduce unbalanced muscle forces within the orbit that destabilize neural control of eye position and help create incomitant strabismus15,16,17,18,19
Traumatic scarring of the orbital connective tissue can impede normal pulley movement, tethering the pulley and restricting movement toward and away from the affected EOM's field of action20,21
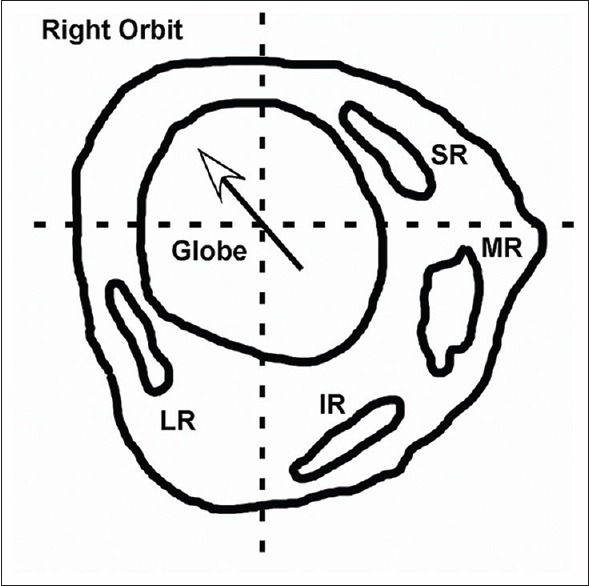
The precise effects of abnormal pulley positions can be modeled mathematically, but such calculations are beyond the scope of this review. Conceptually, the normal pulley positions are necessary to maintain each EOM in its proper location with respect to its insertion, simplifying neural control of eye movement and balancing the forces of antagonist EOMs [Figure 1]. Conversely, abnormal pulley positions destabilize control of eye movements by both changing the direction of the EOM force applied to the globe and unbalancing the forces of the abnormally placed EOM with its antagonist [Figure 2]. The resulting abnormality may manifest clinically as strabismus affecting primary gaze, from the unbalanced forces between agonist and antagonist, which becomes incomitant in eccentric gaze because of the abnormally directed contractile forces applied to the globe by the misdirected EOM. In addition, entrapped pulley tissue restricts normal EOM contraction and relaxation, acting as a leash or tether that prevents a full range of gaze toward or away from the affected EOM even though the muscle belly itself is not entrapped. The final result of either mechanism is an incomitant strabismus that mimics common motility problems and thus is misdiagnosed by clinicians unfamiliar with abnormalities of EOM pulleys. This review will describe the known types of pulley abnormalities that can create incomitant strabismus and discuss current and future diagnostic and therapeutic options.
Figure 1.
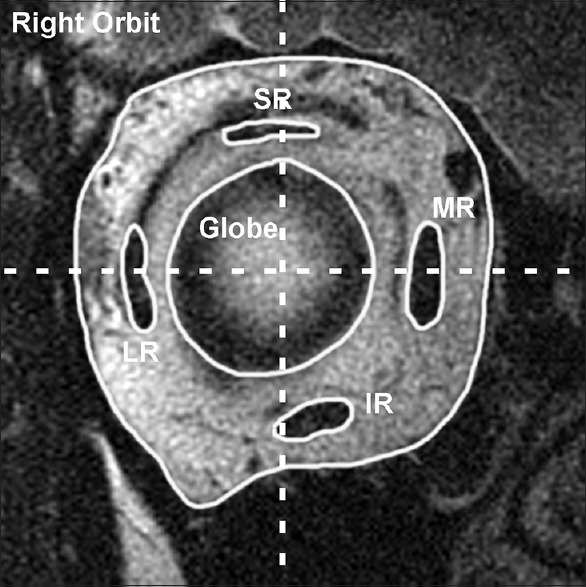
Coronal magnetic resonance imaging of a normal right orbit posterior to the equator shows the vertical and horizontal rectus muscles symmetrically arrayed around the globe. Horizontal and vertical lines that bisect the globe center should intersect at least a portion of each muscle belly. MR = Medial rectus, SR = Superior rectus, LR = Lateral rectus, IR = Inferior rectus
Figure 2.

Schematic of excyclorotation of the right orbit from a craniosynostosis syndrome shows each extraocular muscle displaced counterclockwise from its normal location with respect to the center of the globe. The abnormal pulling directions for the rectus muscles can create primary gaze misalignments and simulate oblique muscle dysfunction. MR = Medial rectus, SR = Superior rectus, LR = Lateral rectus, IR = Inferior rectus
DEVELOPMENTAL DISORDERS OF PULLEY POSITION
Craniosynostosis syndromes
Premature fusion of the cranial sutures can create a variety of abnormal pulley positions. The most common patterns involve incyclorotation or excyclorotation of the entire orbit [Figure 2].22,23,24 With incyclorotation, on imaging the connective tissue pulleys are systematically displaced clockwise for the right orbit and counterclockwise for the left orbit, while with excyclorotation the pulleys are displaced counterclockwise for the right orbit and clockwise for the left orbit. Pulley displacements as small as 2 mm from normal can create unbalanced EOM forces and incomitant patterns of strabismus that often imitate superior oblique (SO) overaction (excessive depression during adduction) and/or A pattern strabismus with incyclorotation of the orbit and inferior oblique (IO) overaction (excessive elevation in adduction) and/or V-pattern strabismus with excyclorotation of the orbit.15,22,24,25 The mechanical effects of the abnormal pulley positions on motility can be divided into two parts:
Primary gaze misalignment because agonist/antagonist EOMs are no longer symmetrically opposed across the globe's horizontal and vertical meridians
Asymmetric eye movements because EOM contraction toward the pulley results in misdirected eye movement (i.e., contraction of the superior rectus [SR] results in both elevation and either abduction or adduction of the globe because of a medially or laterally displaced SR pulley)
In an excyclorotated orbit, when excess elevation occurs in the adducting eye, commonly labeled “IO overaction,” the fixing, abducting eye is pulled downward by the inferiorly displaced lateral rectus (LR) pulley, while the nonfixing, adducting eye is pulled upward by the superiorly displaced medial rectus (MR) pulley. The SR in the abducting eye activates to resist the downward displacement of the globe and maintain fixation, causing the SR in the adducting eye to also activate (Hering's Law). The combination of the elevated MR pulley and activated SR creates excess elevation in the adducting eye without any involvement from the IO15
Similarly, in an incyclorotated orbit, when excess depression occurs in the adducting eye, commonly labeled “SO overaction,” the fixing, abducting eye is pulled upward by the superiorly displaced LR pulley, while the nonfixing, adducting eye is pulled downward by the inferiorly displaced MR pulley. The inferior rectus (IR) in the abducting eye activates to resist the upward displacement of the globe and maintain fixation, causing the IR in the adducting eye to also activate. The combination of the depressed MR pulley and activated IR creates excess depression in the adducting eye without any involvement from the SO15
The lack of oblique muscle involvement in “IO overaction” and “SO overaction” in craniosynostosis syndromes is readily apparent by the lack of substantial change in the pattern of incomitant eye movement after oblique muscle weakening procedures.26 Instead, surgical correction of the incomitant strabismus typically involves recessions of the EOM insertions to correct the primary gaze deviation combined with transpositions to correct the pattern strabismus.27 Because of the abnormal anatomy, those transpositions may not completely correct the incomitance and may also induce unwanted torsion.28
Currently, there are no proven treatments to reposition most of the pulleys into their correct anatomic locations. Surgical procedures designed to normalize the bony abnormalities do not cause any significant change in the strabismus.29 Several procedures have been advocated to correct the positions of the SR and LR after acquired dehiscence and destabilization,30,31,32,33,34,35,36 but only equatorial myopexy of the LR,33,34,35 described below, has been used to reposition the LR pulley in its intact state. The LR pulley normally has the least amount of connective tissue5 and thus may be most amenable to repositioning when the orbital connective tissue is intact, but malrotated.
Isolated abnormalities in pulley position
Isolated abnormal pulley positions are much more difficult to detect using the standard clinical exam. A systematic attempt to correlate facial asymmetry and relative positions of the medial and lateral canthi with pulley heterotopy on orbital imaging has demonstrated that a substantial percentage of patients with pulley heterotopy have facial asymmetry, but the location and type of the asymmetry does not consistently correlate with the abnormal pulley position.37 Thus, the presence of facial asymmetry provides a clue that abnormal pulley positions might be present, but orbital imaging is required to confirm the presence and type of pulley abnormalities.
A more useful clinical finding is the atypical presentation of a common strabismus entity. For example, a patient with acquired childhood esotropia (ET) presenting with low hyperopia or especially myopia can often be found to have an inferiorly displaced LR pulley,33 similar to the position found in sagging eye syndrome [Figure 3].24,34,38 Other clinical findings include the presence of a pattern strabismus, either A or V pattern, and apparent oblique muscle overaction and underaction. Abnormal pulley positions can mimic these clinical entities by changing the effective pulling direction of the EOMs and should be included in the differential diagnosis of those clinical presentations of incomitant strabismus.15
Figure 3.
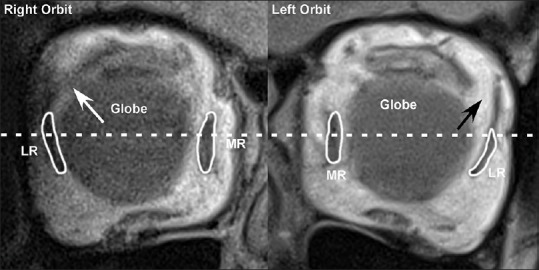
Coronal magnetic resonance imaging near the equator of the globe in an elderly patient with age-related divergence insufficiency esotropia shows an intact lateral rectus-superior rectus band in the right orbit (white arrow) with a normal lateral rectus pulley position and a dehisced lateral rectus-superior rectus band in the left orbit (black arrow) with an inferiorly displaced lateral rectus pulley. MR = Medial rectus, LR = Lateral rectus
Unfortunately, because orbital imaging is not standard in the preoperative workup of strabismus patients, these pulley abnormalities are often detected only after a poor response to the standard strabismus surgical techniques.33 If the primary cause of an acquired ET is an inferiorly displaced LR, for example, simple recession of the MR insertions can result in a substantial undercorrection. The anomalous LR pulley is only identified during the subsequent reoperation.
The surgical correction of these types of anomalous pulley positions is entirely dependent on the EOM involved. Just as in craniosynostosis patients, often the treatment involves transposition of the insertions of the affected EOMs, with some risk of inducing torsion,28 especially if the transposition is in the opposite direction of the displaced pulley, e.g., superiorly transposing the LR insertion to correct the effects of an inferiorly displaced LR pulley might inadvertently create excess excyclotorsion.15
If only the LR pulley is involved, a more elegant approach uses permanent sutures to restore the normal LR pulley position. This technique, labeled equatorial myopexy,33,34,35 begins with isolation of the LR insertion through a standard conjunctival incision, either fornix or limbal. With the insertion secured on a muscle hook, the anomalous inferotemporal posterior muscle path toward the equator of the globe can be clearly identified [Figure 4]. Any planned recession or resection of the insertion is performed first, followed by the equatorial myopexy procedure. Because the LR pulley is relatively deformable, the posterior LR belly can be repositioned by rotating the muscle hook until the midpoint of the muscle belly at the equator lies along an imaginary line connecting the medial and lateral canthi. The LR is locked into this position using a single permanent suture at the equator through the sclera and adjacent ¼ muscle belly [Figure 5] and then the conjunctiva is closed in the standard fashion. The procedure can be repeated bilaterally if both LR are involved.
Figure 4.
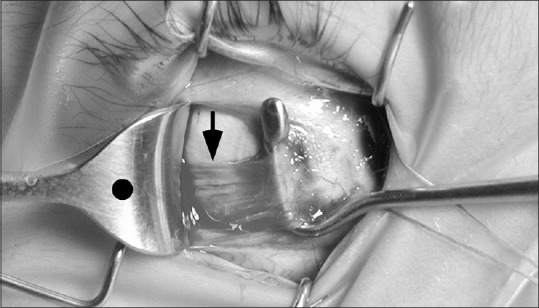
Intraoperative photo shows the lateral rectus muscle insertion is in the correct temporal anatomic location, but the posterior muscle belly is inferiorly displaced by one-half tendon-width near the equator (black arrow). The posterior muscle should be centered on the retractor, marked with a black dot
Figure 5.
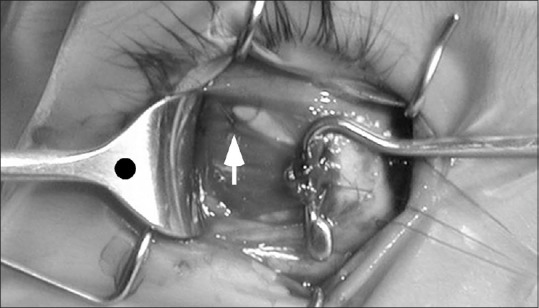
Intraoperative photo of the lateral rectus equatorial myopexy procedure shows a single 6–0 polyester monofilament suture is placed through the equatorial sclera and adjacent superior lateral rectus muscle belly (white arrow) to lock the posterior muscle belly into its correct temporal location, centered on the black dot marking the center of the retractor
Although similar in both concept and execution to retro equatorial myopexy, also known as the fadenoperation,39,40 equatorial myopexy differs in both the location of the permanent suture and its biomechanical effect. For the LR, the faden operation places the permanent suture 17–19 mm posterior to the LR insertion,41 where the LR enters its pulley sleeve. In this location, the suture collides with the LR pulley during contraction and resists posterior displacement, creating a mild restriction to abduction.42 Equatorial myopexy places the permanent suture only 8–10 mm posterior to the LR insertion, near the globe equator. In this anterior location, there is no collision between the suture and the LR pulley and thus no restriction to abduction. Instead, the suture creates its biomechanical effect by restoring the normal pulling direction of the LR and thus restoring its normal abducting force. Equatorial myopexy on the LR has been shown to be effective in both developmental and acquired LR pulley position abnormalities,33,34,35 although the numbers of patients treated with this technique are small compared to the clinical experience with the faden operation.
Acquired disorders of pulley position
Connective tissue pulley disorders
Because the EOM pulleys are connective tissue structures with distinct mechanical properties, it has been proposed that connective tissue disorders like Marfan syndrome and Ehlers-Danlos syndrome that weaken the strength and rigidity of collagen might lead to unstable pulleys. Limited imaging studies have confirmed greater EOM sideslip in patients with Marfan syndrome, combined with atypical globe translation during changes of gaze.19,43 The combination of movement of the functional origin of the EOM, the pulley, plus movement of the functional insertion, the globe, can create unusual patterns of incomitant strabismus such as X pattern misalignments where an exotropia deviation increases in both upgaze and downgaze compared with primary gaze. The misalignment pattern can be exacerbated by the enlarged globe size and high myopia that often accompany these disorders. Over time, the pulley may completely dehisce from its normal position, leading to large static displacements of pulley position similar to those found in highly myopic strabismus36,44,45 or sagging eye syndrome.25,34
Pulley instability versus pulley displacements cannot be determined using static techniques like orbital imaging in a single gaze position. Images must be obtained in multiple controlled positions of gaze to demonstrate gaze-dependent changes in the globe and pulley positions within the bony orbit. Intraoperative instability of the posterior EOMs, particularly of the LR and SR with their limited connective tissue support, may also help make the diagnosis.
The optimum treatment for pulley instability has yet to be established. In these cases, simple recessions, resections, and transpositions of the EOM insertions are not likely to correct the motility problems caused by the unstable orbital connective tissue structures. Techniques like equatorial myopexy have promise as a method to simultaneously stabilize the pulley and constrain abnormal globe translation with respect to the pulley. For large, static displacements of the globe and EOM pulleys, more aggressive techniques like loop myopexies, permanently binding the posterior borders of the SR and LR together with sutures or silicone bands with or without scleral sutures, might be indicated to repair the pulley locations and restore normal motility.30,31,32,36
Globe size pulley disorders
Highly myopic strabismus, also known as myopic strabismus fixus and heavy eye syndrome, is the best characterized of the acquired pulley position disorders. Previously thought to be caused by a large, heavy globe that resists rotation, it is now known that the expanded globe size associated with high myopia can cause a dehiscence of the LR-SR band with superotemporal herniation of the globe [Figure 6].30,31,32,35,36,45,46 The LR pulley is forced inferiorly, reducing its abducting force and converting some LR force toward infraduction, and the SR pulley is forced nasally, reducing its supraducting force and converting some SR force toward adduction. The net result is a highly myopic eye that is esotropic and hypotropic with impaired elevation and abduction.
Figure 6.
Schematic of globe herniation (white arrow) between the superior rectus and lateral rectus from high myopia. A portion of the superior rectus and inferior rectus contractile force is converted to adduction and a portion of the lateral rectus and medial rectus force is converted to infraduction, resulting in esotropia and hypotropia with an impaired range of ductions. MR = Medial rectus, SR = Superior rectus, LR = Lateral rectus, IR = Inferior rectus
The disorder should be suspected in all patients with higher levels of myopia and ET, particularly with impaired abduction or elevation, and has been identified in children as young as 3 years of age.33 Patients with the classic triad of high myopia, ET, and hypotropia may not require orbital imaging to confirm the diagnosis, although imaging is helpful to determine the magnitude of the pulley displacements.
The principles of surgical repair involve repairing the dehiscence of the LR-SR band, restoring the globe to its normal position within the intramuscular cone and restoring the LR and SR pulleys to their normal positions. Current techniques involve loop myopexies, binding the temporal border of the SR and superior border of the LR together with permanent sutures or silicone bands 10 mm or more posterior to their insertions, with or without permanent scleral sutures to lock the myopexy to the globe, to repair the globe herniation and improve the LR and SR pulley locations.30,31,32,36 Some studies also advocate augmenting the myopexy with standard recession of the MR to provide more correction of the ET.31 All of the cited studies utilize small numbers of subjects and report good results, but larger studies with longer follow-up are required to determine the optimum procedure.
Senile degeneration pulley disorders
Like connective tissue throughout the body, the orbital connective tissue loses strength and rigidity with age, resulting in a small inferior displacement of the horizontal rectus pulleys in the normal elderly population.47 If the LR pulley, in particular, with the weakest connective tissue support, sags too far inferiorly because of weakening or dehiscence of the LR-SR band [Figure 3], the loss of LR abducting force can create the excess distance ET that is the hallmark of the clinical entity previously known as “divergence palsy” and now known as “age-related divergence insufficiency ET.” Rather than a neurologic condition that affects the divergence mechanism or a partial paresis of the LR, the mechanical effects of an inferiorly displaced LR pulley can cause the distance ET that typically affects patients 50 years or older.25,34,35,38
Clinically, these patients often present with other signs of periocular connective tissue laxity, such as ptosis, deepening of the superior eyelid sulcus and loss of lower eyelid tensile strength.24,25,34 Unlike patients with developmental isolated pulley position abnormalities, however, these patients typically do not have signs of facial asymmetry. Furthermore, unlike patients with highly myopic strabismus, the globe size is normal and there is no limitation to abduction because the magnitude of the LR displacement is not typically as severe. Instead, these older patients present with an acquired distance ET more than 10 prism diopters greater than near ET with a full range of ductions.24,25,34
The treatment depends on the magnitude of the distance ET. For deviations <10 prism diopters, temporary or permanent base-out prisms may relieve the diplopia.48 For deviations >10 prism diopters or when spectacle-free correction of the strabismus is desired, three surgical approaches can be used: (1) Augmented MR recessions,49(2) LR resections using surgical dosages for the distance ET,50 and (3) LR equatorial myopexies, with or without LR resections.34,35 MR recessions can correct the deviation, but with potentially less predictable long-term results48 because the underlying anatomic problem, the sagging LR pulley, has not been addressed. In addition, augmenting the MR surgical dosages to compensate for diminished LR force might cause an overcorrection at near.24,48 LR resection both augments LR force and decreases the LR sag by shortening the muscle length from origin to insertion, although further inferior sag may occur over time. Finally, LR equatorial myopexy directly corrects the LR sag by permanently fixing the LR into its proper temporal location near the equator.33,34,35 Unilateral LR equatorial myopexy can correct up to 10 prism diopters of distance ET, bilateral LR equatorial myopexies up to 20 prism diopters, and bilateral LR equatorial myopexies plus small LR resections can correct deviations >20 prism diopters.34 Short term (<1-year) follow-up results for LR equatorial myopexy have revealed excellent control of the distance ET without overcorrection at near, but longer follow-up with larger numbers of patients are required to determine if this approach should supplant MR recessions or LR resections as the treatment of choice.34
Traumatic pulley disorders
These disorders can be divided into two broad categories, orbital trauma, and surgical (iatrogenic) trauma. In orbital trauma, the well-known entity is an entrapped EOM from a blowout fracture. On imaging, the entire EOM-pulley complex is displaced into one of the periorbital sinuses and definitive treatment involves repair of the orbital fracture with release and repositioning of the EOM-pulley complex back into the orbit. Less well-known, however, is the fact that herniation and entrapment of just the pulley tissue, without including the EOM, can create a similar pattern of restricted movement toward and away from the involved muscle.20 Because the pulleys normally shift positions posteriorly and anteriorly during contraction and relaxation,12 an entrapped pulley resists contraction and relaxation of its EOM and in effect, acts as a tether to restrict globe movement into and out of its field of gaze.
Because these orbital fractures are smaller than the fractures that entrap the entire EOM-pulley complex, the mechanism of injury is often not as severe and may not be recalled by the patient unless specifically prompted. The orbital fractures are readily visible on high-resolution orbital imaging, however, as well as a slight displacement in the EOM position in proximity to the fracture.20 Treatment depends on the length of time from the original orbital injury. Within the first few weeks after injury, repair of the fracture with repositioning of the orbital contents should relieve the restriction to EOM movement. For older injuries, standard surgical techniques repositioning the EOM insertions may need to be performed based on the location of the restriction and the size of the deviation.
Surgical trauma can also be divided into well-known complications from orbital surgeries, such as decompression surgery for dysthyroid orbitopathy, and lesser-known complications from more anterior oculoplastic surgeries. Decompression surgery has the goal of creating more space for the orbital contents, but that goal necessarily involves expansion and repositioning of the orbital connective tissue with the possible iatrogenic creation of abnormal pulley positions. With eyelid surgery, on the other hand, care must be taken to avoid incorporating the vertical EOMs and their pulleys within the surgical field.21 Just as strabismus surgeons must take care to separate the lid retractors from the vertical EOMs during recessions and resections to avoid advancing or retracting the eyelids, the oculoplastic surgeons must take care to avoid incorporating the pulley tissue when repositioning orbital fat or placing mechanical inserts into the anterior or mid-orbital regions.21 Any scarring or mechanical device that resists the anteroposterior movement of the pulley will also restrict the anteroposterior movement of its EOM, similar in effect to the placement of posterior fixation sutures. Surgical correction of this type of pulley scarring is challenging, typically involving the release of the scar tissue or removal of the mechanical device with periocular steroid injection to reduce the reformation of additional scarring, plus advancing or recessing the EOM insertion to balance the primary gaze deviation.
DISCUSSION
The connective tissue EOM pulleys have static and dynamic roles within the bony orbit to facilitate neurologic control of normal eye movement and alignment. Disorders in the location and/or stability of these pulleys can create incomitant strabismus without invoking cranial nerve paresis or oblique muscle overaction and underaction. Developmental disorders of pulley location include large abnormalities involving the entire orbit and subtle abnormalities in the location of one or more pulleys. Acquired disorders of pulley location include instability from connective tissue disorders, dehiscence from globe size disorders, displacement from senile degeneration, and displacement or entrapment from orbital trauma. Knowledge of these disorders is important because abnormalities in pulley location and movement may be difficult to correct using standard surgical techniques. Instead, surgical correction may optimally utilize new techniques to release, reposition, and stabilize the pulley and posterior EOM within the orbit without changing the location of the EOM's insertion.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Clark RA, Miller JM, Demer JL. Location and stability of rectus muscle pulleys. Muscle paths as a function of gaze. Invest Ophthalmol Vis Sci. 1997;38:227–40. [PubMed] [Google Scholar]
- 2.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–97. [PubMed] [Google Scholar]
- 3.Miller JM. Functional anatomy of normal human rectus muscles. Vision Res. 1989;29:223–40. doi: 10.1016/0042-6989(89)90126-0. [DOI] [PubMed] [Google Scholar]
- 4.Demer JL, Miller JM, Glasgow BJ, Rabiah PK, Vinters HV. Location and composition of the rectus extraocuolar muscle (EOM) “pulleys” in humans. Invest Ophthalmol Vis Sci. 1994;35:2199. [Google Scholar]
- 5.Demer JL, Miller JM, Poukens V, Vinters HV, Glasgow BJ. Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci. 1995;36:1125–36. [PubMed] [Google Scholar]
- 6.Demer JL, Poukens V, Micevych PE. Nitroxidergic and catecholaminergic innervation of the smooth muscle of the medial rectus pulley in humans. ARVO abstracts. Invest Ophthalmol Vis Sci. 1995;36:S959. [Google Scholar]
- 7.Demer JL, Poukens V, Miller JM, Micevych P. Innervation of extraocular pulley smooth muscle in monkeys and humans. Invest Ophthalmol Vis Sci. 1997;38:1774–85. [PubMed] [Google Scholar]
- 8.Clark RA, Rosenbaum AL, Demer JL. Magnetic resonance imaging after surgical transposition defines the anteroposterior location of the rectus muscle pulleys. J AAPOS. 1999;3:9–14. doi: 10.1016/s1091-8531(99)70088-1. [DOI] [PubMed] [Google Scholar]
- 9.Clark RA, Demer JL. Rectus extraocular muscle pulley displacement after surgical transposition and posterior fixation for treatment of paralytic strabismus. Am J Ophthalmol. 2002;133:119–28. doi: 10.1016/s0002-9394(01)01264-8. [DOI] [PubMed] [Google Scholar]
- 10.Miller JM, Demer JL. New orbital constraints on eye rotation. In: Fetter M, Misslisch H, Tweed D, editors. Three-Dimensional Kinematic Principles of Eye-, Head-, and Limb Movements in Health and Disease. Tubingen: University of Tubingen; 1995. p. 40. [Google Scholar]
- 11.Demer JL, Oh SY, Poukens V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 2000;41:1280–90. [PubMed] [Google Scholar]
- 12.Kono R, Clark RA, Demer JL. Active pulleys: Magnetic resonance imaging of rectus muscle paths in tertiary gazes. Invest Ophthalmol Vis Sci. 2002;43:2179–88. [PubMed] [Google Scholar]
- 13.Demer JL. Pivotal role of orbital connective tissues in binocular alignment and strabismus: The Friedenwald lecture. Invest Ophthalmol Vis Sci. 2004;45:729–38. doi: 10.1167/iovs.03-0464. [DOI] [PubMed] [Google Scholar]
- 14.Demer JL. Current concepts of mechanical and neural factors in ocular motility. Curr Opin Neurol. 2006;19:4–13. doi: 10.1097/01.wco.0000198100.87670.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotopic muscle pulleys or oblique muscle dysfunction? J AAPOS. 1998;2:17–25. doi: 10.1016/s1091-8531(98)90105-7. [DOI] [PubMed] [Google Scholar]
- 16.Demer JL, Clark RA, Miller JM. Role of orbital connective tissue in the pathogenesis of strabismus. Am Orthopt J. 1998;48:56–64. [Google Scholar]
- 17.Demer JL, Clark RA, Miller JM. Heterotopy of extraocular muscle pulleys causes incomitant strabismus. In: Lennerstrand G, editor. Advances in Strabismology. Amsterdam: Swets; 1999. pp. 91–4. [Google Scholar]
- 18.Demer JL. The orbital pulley system: A revolution in concepts of orbital anatomy. Ann N Y Acad Sci. 2002;956:17–32. doi: 10.1111/j.1749-6632.2002.tb02805.x. [DOI] [PubMed] [Google Scholar]
- 19.Oh SY, Clark RA, Velez F, Rosenbaum AL, Demer JL. Incomitant strabismus associated with instability of rectus pulleys. Invest Ophthalmol Vis Sci. 2002;43:2169–78. [PubMed] [Google Scholar]
- 20.Ortube MC, Rosenbaum AL, Goldberg RA, Demer JL. Orbital imaging demonstrates occult blow out fracture in complex strabismus. J AAPOS. 2004;8:264–73. doi: 10.1016/j.jaapos.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Pirouzian A, Goldberg RA, Demer JL. Inferior rectus pulley hindrance: A mechanism of restrictive hypertropia following lower lid surgery. J AAPOS. 2004;8:338–44. doi: 10.1016/j.jaapos.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Weiss AH, Phillips J, Kelly JP. Crouzon syndrome: Relationship of rectus muscle pulley location to pattern strabismus. Invest Ophthalmol Vis Sci. 2014;55:310–7. doi: 10.1167/iovs.13-13069. [DOI] [PubMed] [Google Scholar]
- 23.Tan KP, Sargent MA, Poskitt KJ, Lyons CJ. Ocular overelevation in adduction in craniosynostosis: Is it the result of excyclorotation of the extraocular muscles? J AAPOS. 2005;9:550–7. doi: 10.1016/j.jaapos.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Demer JL. The Apt lecture. Connective tissues reflect different mechanisms of strabismus over the life span. J AAPOS. 2014;18:309–15. doi: 10.1016/j.jaapos.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhuri Z, Demer JL. Sagging eye syndrome: Connective tissue involution as a cause of horizontal and vertical strabismus in older patients. JAMA Ophthalmol. 2013;131:619–25. doi: 10.1001/jamaophthalmol.2013.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coats DK, Paysse EA, Stager DR. Surgical management of V-pattern strabismus and oblique dysfunction in craniofacial dysostosis. J AAPOS. 2000;4:338–42. doi: 10.1067/mpa.2000.110337. [DOI] [PubMed] [Google Scholar]
- 27.Rosenbaum AL, Santiago AP. Clinical Strabismus Management: Principles and Surgical Techniques. Philadelphia: W.B. Saunders Company; 1999. pp. 401–3. [Google Scholar]
- 28.Kushner BJ. Torsion and pattern strabismus: Potential conflicts in treatment. JAMA Ophthalmol. 2013;131:190–3. doi: 10.1001/2013.jamaophthalmol.199. [DOI] [PubMed] [Google Scholar]
- 29.Diamond GR, Katowitz JA, Whitaker LH, Quinn GE, Schaffer DB. Ocular alignment after craniofacial reconstruction. Am J Ophthalmol. 1980;90:248–50. doi: 10.1016/s0002-9394(14)74862-7. [DOI] [PubMed] [Google Scholar]
- 30.Durnian JM, Maddula S, Marsh IB. Treatment of “heavy eye syndrome” using simple loop myopexy. J AAPOS. 2010;14:39–41. doi: 10.1016/j.jaapos.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Kekunnaya R, Shenoy HB, Sachdeva V. Silicone band loop myopexy in treatment of myopic strabismus fixus: Surgical outcome of a novel modification. J AAPOS. 2013;17:E6. doi: 10.1136/bjophthalmol-2014-305166. [DOI] [PubMed] [Google Scholar]
- 32.Wong I, Leo SW, Khoo BK. Loop myopexy for treatment of myopic strabismus fixus. J AAPOS. 2005;9:589–91. doi: 10.1016/j.jaapos.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Clark RA, Choy AE, Demer JL. Inferiorly displaced lateral rectus pulley causes recurrent esotropia after strabismus surgery. J AAPOS. 2007;11:86–7. [Google Scholar]
- 34.Demer JL, Chaudhuri Z, Clark RA. Apt lecture workshop: Cutting no slack for sagging eye syndrome. J AAPOS. 2013;17:E33. [Google Scholar]
- 35.Fresina M, Sapigni L, Benedetti C, Giannaccare G, Campos EC. Equatorial loop myopexy in “Sagging Eye” syndrome: A case report. Clin Exp Ophthalmol. 2014;5:1000337. [Google Scholar]
- 36.Yamaguchi M, Yokoyama T, Shiraki K. Surgical procedure for correcting globe dislocation in highly myopic strabismus. Am J Ophthalmol. 2010;149:341–6.e2. doi: 10.1016/j.ajo.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 37.Velez FG, Clark RA, Demer JL. Facial asymmetry in superior oblique muscle palsy and pulley heterotopy. J AAPOS. 2000;4:233–9. doi: 10.1067/mpa.2000.105277. [DOI] [PubMed] [Google Scholar]
- 38.Rutar T, Demer JL. “Heavy Eye” syndrome in the absence of high myopia: A connective tissue degeneration in elderly strabismic patients. J AAPOS. 2009;13:36–44. doi: 10.1016/j.jaapos.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuppers C. The so-called “fadenoperation” (surgical corrections by well-defined changes of the arc of contact) In: Fells P, editor. The 2nd Congress of the International Strabismological Association. Marseille: Diffusion Generale's Bookstore; 1976. pp. 395–400. [Google Scholar]
- 40.Shuckett EP, Hiles DA, Biglan AW, Evans DE. Posterior fixation suture operation (fadenoperation) Ophthalmic Surg. 1981;12:578–85. [PubMed] [Google Scholar]
- 41.Holmes JM, Hatt SR, Leske DA. Lateral rectus posterior fixation suture. J AAPOS. 2010;14:132–6. doi: 10.1016/j.jaapos.2009.12.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark RA, Isenberg SJ, Rosenbaum AL, Demer JL. Posterior fixation sutures: A revised mechanical explanation for the fadenoperation based on rectus extraocular muscle pulleys. Am J Ophthalmol. 1999;128:702–14. doi: 10.1016/s0002-9394(99)00356-6. [DOI] [PubMed] [Google Scholar]
- 43.Demer JL, Kono R, Wright W, Oh SY, Clark RA. Gaze-related orbital pulley shift: A novel cause of incomitant strabismus. In: De Faber JT, editor. Progress in Strabismology. Lisse: Swets and Zeitlinger; 2003. pp. 207–10. [Google Scholar]
- 44.Krzizok TH, Schroeder BU. Measurement of recti eye muscle paths by magnetic resonance imaging in highly myopic and normal subjects. Invest Ophthalmol Vis Sci. 1999;40:2554–60. [PubMed] [Google Scholar]
- 45.Krizok TH, Kaufmann JM. Elucidation of the restrictive motility disorders in high myopia by MRI. Arch Ophthalmol. 1997;115:1019–27. doi: 10.1001/archopht.1997.01100160189008. [DOI] [PubMed] [Google Scholar]
- 46.Krizok TH, Kaufmann H, Traupe H. New approach in strabismus surgery in high myopia. Br J Ophthalmol. 1997;81:625–30. doi: 10.1136/bjo.81.8.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark RA, Demer JL. Effect of aging on human rectus extraocular muscle paths demonstrated by magnetic resonance imaging. Am J Ophthalmol. 2002;134:872–8. doi: 10.1016/s0002-9394(02)01695-1. [DOI] [PubMed] [Google Scholar]
- 48.Repka MX, Downing E. Characteristics and surgical results in patients with age-related divergence insufficiency esotropia. J AAPOS. 2014;18:370–3. doi: 10.1016/j.jaapos.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Chaudhuri Z, Demer JL. Medial rectus recession is as effective as lateral rectus resection in divergence paralysis esotropia. Arch Ophthalmol. 2012;130:1280–4. doi: 10.1001/archophthalmol.2012.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thacker NM, Velez FG, Bhola R, Britt MT, Rosenbaum AL. Lateral rectus resections in divergence palsy: Results of long-term follow-up. J AAPOS. 2005;9:7–11. doi: 10.1016/j.jaapos.2004.11.014. [DOI] [PubMed] [Google Scholar]


