Abstract
Thyroid orbitopathy causes a unique form of strabismus with adult-onset diplopia. Prisms can be a temporizing measure, but many patients require strabismus surgery, which can be challenging. In this article, we highlight clinical guidelines for addressing strabismus due to thyroid orbitopathy and review our surgical experience.
Keywords: Diplopia, Graves’ Disease, Restrictive Strabismus, Strabismus Surgery, Thyroid Orbitopathy
INTRODUCTION/BACKGROUND
Enlargement and dysfunction of the extraocular muscles is characteristic of thyroid orbitopathy and can cause strabismus with diplopia that is difficult to manage and significantly affects the quality of life.1,2 When posterior extraocular muscle thickening is severe, compressive optic neuropathy can occur. Conjunctival injection, lid retraction, and proptosis are other well-recognized signs of thyroid orbitopathy.
Lid retraction and lid lag, both suggesting involvement of the superior rectus/levator complex, are the earliest sign of myopathy.3,4 As it progresses, involvement of individual muscles is unpredictable, asynchronous and often asymmetric, thus making management problematic. The behavior of the levator muscle affected by this myopathy provides insight into the muscle dysfunction: The increased resting tone of the muscle, or “spasm”, results in lid retraction. The failure to relax, or restriction, is evident as lid lag, and lagophthalmos. A parallel process is seen in the extraocular muscles where the hallmark of the myopathy is restriction.5
Clinically, there is a predilection for the inferior and the medial rectus muscles although any extraocular muscle may be affected.5 Involvement of a muscle with relative sparing of its antagonist causes the eye to deviate toward the affected muscle. The tendency to misalignment in the resting position is counteracted by the patient's fusional range, which increases with time. Initially, patients may avoid diplopia by adopting a compensatory head posture; early involvement may cause a type of muscle spasm characterized by discomfort on movement and restriction in the opposite field of gaze. At this stage, injection of botulinum toxin into the affected muscle can readily cause a significant overcorrection, suggesting that the muscle itself is free of the secondary fibrosis which is noted later and which prevents muscle relaxation.6
Sparing of the contralateral yoke muscle may cause a significant overshoot. Because the strabismus is usually incomitant, prisms may not help as the field of binocularity is often too small.5 However, Fresnel prisms can help a patient with the symmetrical disease and a reasonably comitant deviation. Fresnel prisms are inexpensive and can be adjusted periodically until the deviation stabilizes.
Since the inferior rectus muscles are most often involved, patients with thyroid orbitopathy frequently have difficulty using bifocal reading glasses. Patients complain that the power of the near add segment is “either out of reach” or within the diplopic field. Fresnel prisms may be cut to cover or spare the near segment of bifocal glasses. Separate reading and distance glasses may be the best alternative for these patients.
Any individual muscle can become involved and the disease progresses asynchronously in each involved muscle; it may be difficult to determine if a particular muscle is involved or not. Although the active inflammatory (spasm) phase is often accompanied by vascular injection of the overlying conjunctiva,7 this is variable and may be absent altogether, particularly in elderly patients who sometimes appear un-inflamed and whose duction deficit in the context of “silent” thyroid orbitopathy can occasionally be mistaken for a cranial nerve palsy. Magnetic resonance imaging and computed tomography are useful in assessing which muscles are involved and to what extent.8
At each visit, the patient should undergo an orthoptic evaluation with Hess chart documentation and prism cover test measurement in all positions of gaze, both at a distance and near. The field of binocular single vision (BSV) and a BSV score may help documentation and provide a graphic description of the patient's current limitation.9,10 It also helps to illustrate the surgical goals.
For surgical planning, the primary, secondary and tertiary actions of each muscle are important to take into account. For instance, the inferior rectus is primarily a depressor, but secondarily an adductor (as well as an excyclorotator). A patient may be esotropic due to inferior rectus involvement alone while the medial rectus muscles (which would have been the expected cause of the esotropia) are actually spared! The involvement of individual muscles should be assessed by careful duction (both spontaneous and forced) testing.
Ductions in the field of the vertical rectus muscle should be tested with the eye in abduction. Limitation of any single duction usually implicates the antagonist to that movement (e.g. limited elevation in abduction implicates the ipsilateral inferior rectus). Myasthenia gravis should be kept in mind as an important exception to this rule, co-existing in approximately 5% of patients with thyroid orbitopathy.11
As the dose-response expectation for a recession in these cases is variable we almost always use adjustable recessions for thyroid strabismus.12 When multiple muscles are involved, three prism diopters of correction can be expected per mm of recession. If a single muscle is involved, the yield per millimeter may be much greater. In the absence of ipsilateral antagonist involvement recession of a medial or vertical rectus muscle of more than 6–7 mm results in a duction deficit. Beyond this, ductions reduce rapidly. Nevertheless greater amounts of recession are frequently necessary to obtain the best field of BSV in primary position and downgaze. In order to achieve this goal, new duction deficits with diplopia in eccentric gaze may occur; these are acceptable as a “collateral drawback” but it is essential to thoroughly counsel the patient of this risk preoperatively.
OUR SURGICAL EXPERIENCE
We retrospectively reviewed the hospital records of all the patients with thyroid strabismus undergoing strabismus surgery under our care over a 10 years period at the University of British Columbia Ophthalmology Department. The indication for surgery was diplopia in primary or reading position, where the use of prisms was impossible or unsatisfactory.
Surgical correction was performed once the measurements were stable for at least 6 months. Vicryl 6/0 (Ethicon) absorbable adjustable sutures were used for all cases. A surgical result was considered successful when the patient achieved a satisfactory field of BSV in the primary position at a distance and near. Success rates for horizontal and vertical muscle surgeries were calculated and their significance verified with a sign test. The Chi-squared test was used to verify if the success rates differed between surgeries for horizontal and vertical rectus muscles. A (P < 0.05) was statistically significant.
A total of 141 patients were identified. Two were excluded due to co-existing myasthenia gravis. Of the remaining 139 patients, 93 were female, and 46 were males. The median age of the study sample was 54 years (range, 22 years to 88 years).
Of the 139 patients, 96 underwent vertical muscle surgery and 61 underwent horizontal muscle surgery (the totals exceed 139 as some patients underwent combined surgery). The overall success was 75% and the success rates were similar for subsequent surgeries leading to a cumulative success rate of 99% [Table 1].
Table 1.
Success rates of consecutive surgeries in TED

There was a notable difference in the success rates between the vertical and horizontal surgeries following a single surgery. The success rate for the horizontal component for patients undergoing horizontal or combined surgery was 84% (51/61, P < 0.001). Success rates for the vertical surgery and vertical component of the combined surgery produced less favorable outcomes with a success rate of 67% (64/96, P = 0.001) [Figure 1]. There was a statistically significant difference between success rates for horizontal and vertical surgeries (Chi-squared = 5.46, P < 0.05). In the vertical muscle surgery group, there was no difference between success and repeat surgery groups in terms of age (median 56.5 years; range 22 years to 88 years; median 55.5 years; range 29 years to 81 years; respectively) (P = 0.847) or gender (44 females and 20 male, 21 females and 11 males, respectively). One-third (32/96, 33%) of the patients who underwent vertical surgery required another operation to correct their deviation [Table 2]. Four of the patients had their repeat surgery over 2 years after the primary surgery, likely due to the progression of disease. Disease progression was demonstrated by imaging or by clinical signs of the inflammation. 21 of the remaining 28 patients had a consecutive deviation following their initial surgery and 7 patients had a residual deviation [Table 2].
Figure 1.

Repeat operation rates for vertical and horizontal strabismus surgery in thyroid Eye disease
Table 2.
Classification of surgical outcomes in patients with vertical strabismus from TED
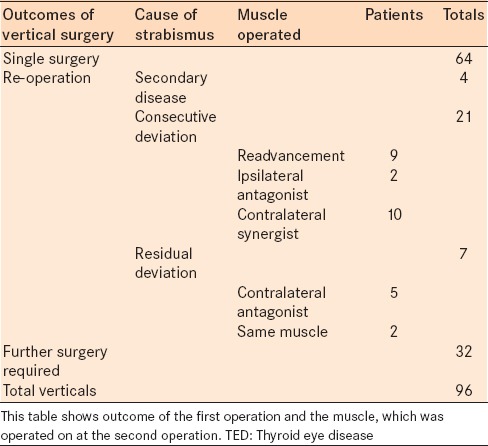
Of the 21 patients with a consecutive deviation, 9 had re-advancement of the previously recessed muscle and 12 required surgery to either the ipsilateral antagonist (2) or the contralateral synergist (10).
Of the 81 patients undergoing inferior rectus surgery as part of their first surgery 8 required re-advancement of the muscle resulting a slippage rate of 9% (8/81). Of the 14 requiring a superior rectus muscle recession 1 required a re-advancement, resulting in a slippage rate of 7% (1/14) (P = 0.864).
DISCUSSION/RECOMMENDATIONS
Our experience shows that surgery for horizontal deviations is more successful than surgery for vertical strabismus (84% vs. 66% success after the primary surgery). Unfortunately, strabismus due to thyroid orbitopathy has a predilection for affecting the inferior rectus resulting in a greater preponderance of vertical strabismus.13 Recession of this muscle is frequently complicated by consecutive hypertropia. Volpe et al., reported 65% of their 54 patients with vertical strabismus from thyroid orbitopathy had a successful result after a single adjustable surgery which concurs with the outcomes of our study.14
Slippage of the inferior rectus has led some to question the use of hang-back or adjustable sutures in this situation.15 Some have recommended the use of nonabsorbable sutures for inferior rectus surgery.16 Although we agree that slippage is a cause of overcorrection,13,15 it was implicated in <10% of cases in our study and re-exploration frequently showed the inferior rectus was positioned correctly.
Several factors may contribute to consecutive hypertropia. The resting ocular alignment is the sum result of vector forces from the twelve extraocular muscles. Although an eye will deviate toward the strongest vectors, all the forces acting on the eye are not evident preoperatively. Computed tomography or orbital magnetic resonance imaging may add to the results of duction and forced duction testing to determine each muscle's involvement in the disorder. Progressive overcorrection after inferior rectus muscle recessions has been reported in the literature. Sprunger and Helveston17 reviewed 18 cases, of which 50% had late overcorrection. Hudson and Feldon18 reported 5 of 12 patients (42%) undergoing inferior rectus muscle recessions for thyroid eye disease (TED) had marked overcorrection within weeks to months after unilateral surgery.
The causes of consecutive hypertropia may include:
Increased force from the ipsilateral superior rectus. The involvement of this muscle in the myopathy process may be masked by the severity of the restriction of the inferior rectus. Once the inferior rectus is recessed, the superior rectus “takes up the slack” and the myopathic spasm and restriction becomes evident as a consecutive hypertropia outcome
The contralateral inferior rectus is affected, producing fixation duress. In order to fixate with this eye, more stimulation is sent to its superior rectus muscle. By Hering's law, equal innervation is sent to both superior rectus muscles. Thus, after recession of one inferior rectus, the increased tone in both superior rectus muscles results in consecutive hypertropia on the surgical side. Because of this, bilateral inferior rectus recessions or simultaneous recession of the ipsilateral inferior and superior recti should be considered in a patient presenting with a hypotropia who has not previously undergone surgery. The decision should be based on forced duction testing at the start of surgery, repeated as the inferior rectus of the hypotropic eye has been dis-inserted in preparation for recession.19
In a patient with consecutive hypertropia, readvancement of the inferior rectus alone is often of limited value and is best combined with surgery to the contralateral inferior rectus or ipsilateral superior rectus (or both) depending on duction, forced duction, and computed tomography scans.
The following twelve thyroid strabismus surgery guidelines may be helpful to optimize surgical results and to reduce the risk of re-operation:
Surgery should not be performed before the alignment has been documented to be stable for 6 months5
Surgical goals and expectations should be defined and clearly discussed with the patient. Diplopia is a frustrating symptom, which may interfere with a patient's ability to work, read, drive, and function day-to-day.1 Restrictive strabismus, particularly in this context, is challenging surgically. The goal should be to restore BSV in the primary position at distance and near, as well as the reading position5 It is important to ensure that the patient understands the possibility of residual double vision and new double vision in certain gaze directions. It is important to stress the fact that multiple surgeries may be necessary to achieve this goal.
Large esotropias can require large recessions of both medial rectus muscles. Postoperatively, convergence may be limited, interfering with reading and near work; if so, base-in prisms inserted into the patient's reading glasses may help to treat this convergence insufficiency
Recession of the inferior rectus may result in lower lid retraction and the patient should be counseled preoperatively. To reduce the retraction, the lower lid retractor fibers should be carefully dissected from the inferior surface of the inferior rectus muscle, optimizing surgical exposure with a Desmarres retractor
Large inferior rectus recessions result in limitation of depression. With very large recession the eye's other depressor, the superior oblique, increasingly acts in downgaze. However, whereas the inferior rectus is an adductor, the superior oblique is an abductor period20 Thus, the eye abducts in downgaze postoperatively, resulting in an A-pattern with incyclotorsion, and diplopia in downgaze. Transposing the inferior rectus medially by an insertion's width at the time of recession surgery can reduce this abduction effect, but increases the tendency for incyclotorsion. Posterior tenotomy (sparing torsion) or full weakening of the superior oblique muscles can be considered (although further limitation in downgaze will result)
As in all forms of restrictive strabismus, muscle resection should be avoided. Apart from being technically challenging, the restriction is likely to be aggravated if a muscle is shortened, inducing gaze limitation and any recurrence of the disease will result in further restriction
A guarded strabismus hook is useful when operating on very tight muscles; it may avoid complications such as inadvertent globe perforation or inadequate capture of the muscle tendon. Bishop or Kowal hooks are commercially available for this purpose
Careful duction testing should be performed preoperatively. Intraoperative forced ductions should be checked at the beginning of surgery and after each muscle is dis-inserted
Large muscle recessions may need to be accompanied by conjunctival recession to avoid postoperative restriction through conjunctival tethering. To ensure this has not occurred, the forced duction test should be repeated after conjunctival closure
Patients with vertical strabismus develop a large fusional range for their deviation. Thus, a patient with right hypotropia will have developed the ability to remain binocular despite a large right hypophoric deviation.21 Conversely, the fusional range for left hypophoria will be virtually nonexistent, and the deviation will become manifest and cause diplopia as soon as reversal occurs. Surgery and adjustment should be aimed at retaining the preoperative pattern, planning to leave the previously hypotropic eye with a small hypo-deviation, which can readily be fused. Similarly, esotropic eyes should be left with a small esodeviation, allowing the patient to fuse easily.
-
Patients with hypotropia secondary to inferior rectus involvement are liable to develop a secondary hypertropia postoperatively
They should be carefully evaluated for involvement of the contralateral inferior rectus. Testing should include the Hess chart, assessment of limitation of elevation in the abduction, and forced duction. Evidence of contralateral inferior rectus involvement should prompt bilateral (asymmetric) inferior rectus recession or ipsilateral superior rectus recession depending on the findings of forced duction testing
Scrupulous attention should be paid to dissection of Tenon's capsule, particularly around the inferior rectus since the incidence of slipped muscle after large recessions is high, partly due to this muscle's short arc of contact with the globe but also the presence of Tenon's capsule which may predispose to nonadhesion to the globe.
SUMMARY/CONCLUSIONS
Strabismus is a disabling aspect of thyroid orbitopathy.1,2 Prisms may be useful as a temporizing measure since surgery should be delayed until a stable deviation is observed. The use of prisms is limited by the degree of incomitance noted in this group of patients.
Surgery is very helpful but more complex in TED than other classes of strabismus.22 Careful preoperative patient counseling of the goals and limitations of strabismus surgery is of paramount importance since a degree of limitation remains postoperatively, even after two or even three surgeries. Other issues such as lid retraction following vertical rectus recession should also be discussed preoperatively.
Horizontal deviations can be corrected relatively simply and the success rate of a single surgery is comparable to that of other forms of strabismus surgery. Because the orbitopathy often affects the inferior rectus, vertical deviations are common. We have emphasized preoperative, intraoperative, and postoperative measures to optimize outcomes in this complex group of patients.
With careful planning and meticulous surgery, a satisfactory central field of BSV can be achieved in the primary position and in downgaze, returning these patients to a comfortable binocular status for the vast majority of their day-to-day life.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Yeatts RP. Quality of life in patients with Graves ophthalmopathy. Trans Am Ophthalmol Soc. 2005;103:368–411. [PMC free article] [PubMed] [Google Scholar]
- 2.Coulter I, Frewin S, Krassas GE, Perros P. Psychological implications of Graves’ orbitopathy. Eur J Endocrinol. 2007;157:127–31. doi: 10.1530/EJE-07-0205. [DOI] [PubMed] [Google Scholar]
- 3.Feldon SE, Levin L. Graves’ ophthalmopathy: V. Aetiology of upper eyelid retraction in Graves’ ophthalmopathy. Br J Ophthalmol. 1990;74:484–5. doi: 10.1136/bjo.74.8.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rootman J, Patel S, Berry K, Nugent R. Pathological and clinical study of Müller's muscle in Graves’ ophthalmopathy. Can J Ophthalmol. 1987;22:32–6. [PubMed] [Google Scholar]
- 5.Nardi M. Squint surgery in TED - Hints and fints, or why Graves’ patients are difficult patients. Orbit. 2009;28:245–50. doi: 10.1080/01676830903104603. [DOI] [PubMed] [Google Scholar]
- 6.Lyons CJ, Vickers SF, Lee JP. Botulinum toxin therapy in dysthyroid strabismus. Eye (Lond) 1990;4(Pt 4):538–42. doi: 10.1038/eye.1990.74. [DOI] [PubMed] [Google Scholar]
- 7.Smith B, Soli DB. Strabismus associated with thyroid disease. Am J Ophthalmol. 1960;50:473–8. doi: 10.1016/0002-9394(60)90688-7. [DOI] [PubMed] [Google Scholar]
- 8.Nugent RA, Belkin RI, Neigel JM, Rootman J, Robertson WD, Spinelli J, et al. Graves orbitopathy: Correlation of CT and clinical findings. Radiology. 1990;177:675–82. doi: 10.1148/radiology.177.3.2243967. [DOI] [PubMed] [Google Scholar]
- 9.Fitzsimons R, White J. Functional scoring of the field of binocular single vision. Ophthalmology. 1990;97:33–5. doi: 10.1016/s0161-6420(90)32631-3. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan TJ, Kraft SP, Burack C, O’Reilly C. A functional scoring method for the field of binocular single vision. Ophthalmology. 1992;99:575–81. doi: 10.1016/s0161-6420(92)31931-1. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson DM. Acetylcholine receptor antibodies in patients with Graves’ ophthalmopathy. J Neuroophthalmol. 1995;15:166–70. [PubMed] [Google Scholar]
- 12.Schotthoefer EO, Wallace DK. Strabismus associated with thyroid eye disease. Curr Opin Ophthalmol. 2007;18:361–5. doi: 10.1097/ICU.0b013e32827038f2. [DOI] [PubMed] [Google Scholar]
- 13.Pratt-Johnson J, Tillson G. Management of Strabismus and Amblyopia: A Practical Guide. 2nd ed. New York: Thieme; 2001. p. 221. [Google Scholar]
- 14.Volpe NJ, Mirza-George N, Binenbaum G. Surgical management of vertical ocular misalignment in thyroid eye disease using an adjustable suture technique. J AAPOS. 2012;16:518–22. doi: 10.1016/j.jaapos.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Buckley EG, Plager DA, Plager DA, Repka MX, Wilson ME, Parks MM, et al. Strabismus surgery: Basic and advanced strategies. Am Orthopt J. 2005;55:166–7. [Google Scholar]
- 16.Kerr NC. The role of thyroid eye disease and other factors in the overcorrection of hypotropia following unilateral adjustable suture recession of the inferior rectus (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2011;109:168–200. [PMC free article] [PubMed] [Google Scholar]
- 17.Sprunger DT, Helveston EM. Progressive overcorrection after inferior rectus recession. J Pediatr Ophthalmol Strabismus. 1993;30:145–8. doi: 10.3928/0191-3913-19930501-04. [DOI] [PubMed] [Google Scholar]
- 18.Hudson HL, Feldon SE. Late overcorrection of hypotropia in Graves ophthalmopathy. Predictive factors. Ophthalmology. 1992;99:356–60. doi: 10.1016/s0161-6420(92)31965-7. [DOI] [PubMed] [Google Scholar]
- 19.Black BC. Treatment of incomitant hypertropia and diplopia with recession of the inferior rectus and superior rectus muscles of the same eye. J AAPOS. 2007;11:262–5. doi: 10.1016/j.jaapos.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Flanders M, Hastings M. Diagnosis and surgical management of strabismus associated with thyroid-related orbitopathy. J Pediatr Ophthalmol Strabismus. 1997;34:333–40. doi: 10.3928/0191-3913-19971101-04. [DOI] [PubMed] [Google Scholar]
- 21.Fells P, Kousoulides L, Pappa A, Munro P, Lawson J. Extraocular muscle problems in thyroid eye disease. Eye (Lond) 1994;8(Pt 5):497–505. doi: 10.1038/eye.1994.125. [DOI] [PubMed] [Google Scholar]
- 22.Ormrod JN. Management of squint in dysthyroid disease. Trans Ophthalmol Soc U K. 1981;101(Pt 2):284–9. [PubMed] [Google Scholar]


