Abstract
Background:
Although sepsis is one of the leading causes of mortality in hospitalized patients, information regarding early predictive factors for mortality and morbidity is limited.
Materials and Methods:
Patients fulfilling the Infectious Disease Society of America criteria of sepsis within the medical intensive care unit (ICU) were included over two years. Apart from baseline hematological, biochemical, and metabolic parameters, Acute Physiology and Chronic Health Evaluation II (APACHE II), Simplified Acute Physiology Score II and III (SAPS II and SAPS III), and Sequential Organ Function Assessment (SOFA) scores were calculated on day 1 of admission. Patients were followed till death or discharge from the ICU.
Results:
One hundred patients were enrolled over two years (54% males). The overall mortality was 53%, (69.5% in females, 38.8% in males (P < 0.01). Mortality was 65.7%, 55.7%, and 33.3% in patients with septic shock, severe sepsis, and sepsis, respectively. Patients who died were significantly older than the survivors (mean age, 57.37 ± 20.42 years and 44.29 ± 15.53 years respectively, P < 0.01). Nonsurvivors were significantly more anemic and had higher APACHE II, SAPS II, SAPS III, and SOFA scores. The presence of acute respiratory distress syndrome and renal dysfunction were associated with higher mortality (75% and 70.2%, respectively). There was no significant difference in the duration of mechanical ventilation or ICU stay between survivors and nonsurvivors. On multivariate analysis, significant predictors of mortality with odds ratio greater than 2 included the presence of anemia, SAPS II score greater than 35, SAPS III score greater than 47, and SOFA score greater than 6 at day 1 of admission.
Conclusion:
Several demographic and laboratory parameters as well as composite critical illness scoring systems are reliable early predictors of mortality in sepsis. A sepsis mortality prediction formula (AIIMS Sepsis Score) based on SAPS II, SAPS III, and SOFA scores and hemoglobin has greater predictive power than these scoring methods individually. Routine use of critical illness scoring systems and a composite mortality prediction formula may provide useful early prognostic information in sepsis/severe sepsis.
KEY WORDS: Mortality, scoring, sepsis
INTRODUCTION
Sepsis is one of the leading causes of inhospital mortality and morbidity among medical and surgical patients. Severe sepsis accounts for one in five admissions to intensive care units (ICUs) and is the leading cause of death in the noncoronary ICU.[1] Unfortunately, the outcome of sepsis has remained unacceptably high to the tune of 30%–40% despite the development and availability of an increasing array of higher-generation antibiotics with broader spectrum of coverage and advances in intensive supportive measures.[2] Although well recognized as an important health issue globally, most of the epidemiological data regarding the incidence and mortality of sepsis have emerged from western countries and puts the overall incidence of sepsis ranging from 10% to 30% with mortality ranging from 10% to 56%.[2,3] Available data from India suggest that the overall mortality of all septic patients is approximately 14% and that of severe sepsis alone is higher than 50%.[4]
Several laboratory and clinical variables have been found to be independent predictors of mortality in sepsis/severe sepsis. However, these vary widely according to the type of ICU setting, patient population, and quality of medical care provided. The presence of pre-existing disease and organ dysfunction and severity of illness scores have been associated with poorer outcome in majority of reports.[2,5] This information, along with a knowledge of early and reliable prognostic markers, is essential for optimum clinical management. This study was thus carried out to determine the incidence of sepsis/severe sepsis within a closed ICU and to identify early and reliable prognostic variables for severe disease.
MATERIALS AND METHODS
The study was a prospective observational study conducted between June 2010 and June 2012 in the Medical ICU of a tertiary care referral hospital in Northern India. All the patients who were admitted in ICU either with existing sepsis or those who developed new episode of sepsis/severe sepsis/septic shock within the ICU were enrolled. Excluded were those who died within 24 h of admission and those with systemic inflammatory response syndrome (SIRS) without any definite focus of infection.
Sepsis was defined as clinical evidence of infection plus presence of SIRS; Severe sepsis as the presence of sepsis plus evidence of organ dysfunction or tissue hypoperfusion,[6] and septic shock as the presence of sepsis induced hypotension, which persisted despite adequate fluid resuscitation.[7]
After obtaining informed consent, detailed demographic, clinical, and laboratory data were recorded, including arterial blood gas analysis and relevant cultures of blood, urine, sputum, tracheal aspirates, or other samples as indicated. Acute Physiologic Assessment and Chronic Health Evaluation II (APACHE II),[8] Simplified Acute Physiological Score II (SAPS II),[9] SAPS III,[10] and Sequential Organ Failure Asssessment (SOFA)[11] indices were calculated at baseline to assess the severity of illness. The total duration of ICU stay, mechanical ventilation, and hospital stay were recorded. All patients recruited in the study were monitored until death or discharge, whichever occurred earlier.
Data analysis
Statistical analysis was done using STATA 11 software. Mean (standard deviation) or median (min–max) were calculated for continuous variables and frequency (%) for categorical variables. To compare the continuous and categorical variables with the primary outcome, that is, death/survival, paired t-test/Wilcoxon rank-sum test, and Chi-square/Fischer's exact test were used, respectively. In the case of two continuous variables, Spearman's/Pearson's correlation coefficient was calculated. Univariate and stepwise multivariate logistic regression analysis was done to see the predictors of ICU death and duration of mechanical ventilation, respectively. Odds ratios (95% CI) were calculated by logistic regression model for ICU deaths and duration of mechanical ventilation. Odds ratio was then converted to relative risk ratio using appropriate conversion formula. P < 0.05 is considered as significant. We then devised the weighted prediction score for predicting mortality using β-coefficient of these variables from logistic mode, and using the goodness-of-fit test, multiplied by 10 and divided by the least regression coefficient among these variables. Discrimination power and calibration of predicting score were evaluated using the area under the receiver operating characteristic) curve and Hosmer–Lemeshow goodness-to-fit test, respectively. We also calculated the optimal cutoff for weighted score using the ROC curve and estimated the sensitivity, specificity, and positive likelihood ratio of mortality for this prediction score.
RESULTS
A total of 170 patients were screened during the study period, of whom 23 died within 24 h of admission and hence were excluded; the remaining 47 did not fulfill the definition of sepsis. Consequently, 100 patients were finally included and analyzed (54% males). Significant comorbidities such as diabetes, hypertension, previous coronary heart disease, and chronic obstructive pulmonary disease were present in 20%, 34%, 13%, and 22%, respectively. The overall in-ICU mortality of the study group was 53%. The baseline comparison of survivors and nonsurvivors is depicted in Table 1.
Table 1.
Comparison of baseline characteristics between survivors and nonsurvivors*
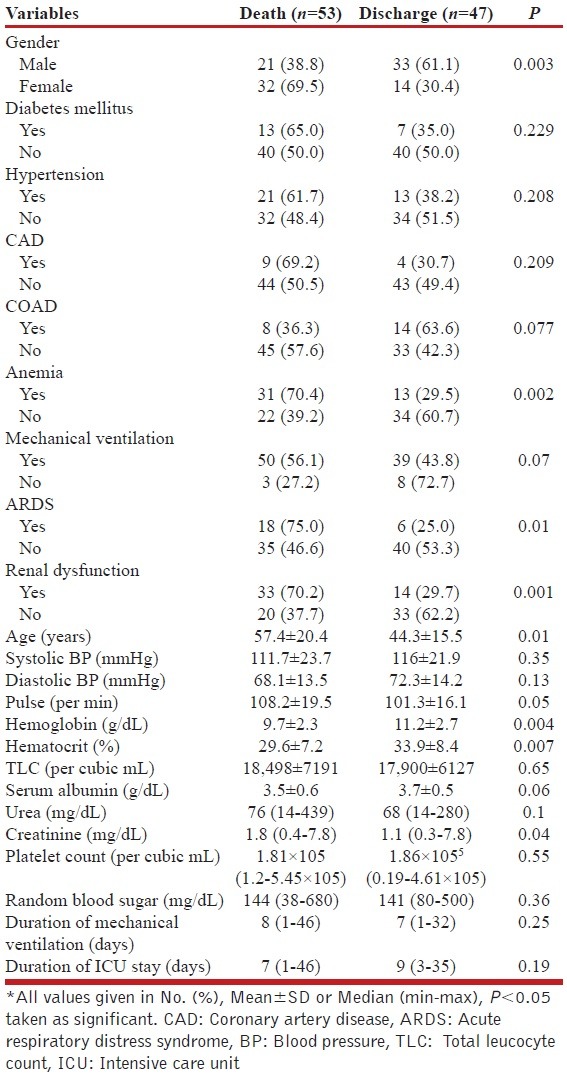
Mortality rate was significantly higher among females compared with males, (69.5% and 38.8%, respectively). Older age, presence of anemia (defined as hemoglobin less than 12 g/dL in males and 10 g/dL in females), renal dysfunction (creatinine level more than 1.3 g/dL), and acute respiratory distress syndrome (ARDS) were associated with higher mortality. Almost 90% patients were admitted with features of lower respiratory tract infection and 89% patients required mechanical ventilation at the day of admission. At the time of ICU admission, of the total 100 patients, sepsis alone, severe sepsis, and septic shock were present in 27%, 38%, and 35%, respectively. Mortality was highest in patients with septic shock (65.7%), followed by severe sepsis (55.3%) and sepsis (33.3%). The total duration of hospital stay was higher among survivors (mean, 15 days (range, 8–65) and 12 days (range, 2–46 days), respectively. However, no difference was observed in the total duration of mechanical ventilation and the duration of ICU stay.
Critical care scoring systems
All the four scoring systems as depicted in Table 2 were significantly higher among patients who died as compared with those who survived.
Table 2.
Comparison of critical care scoring systems
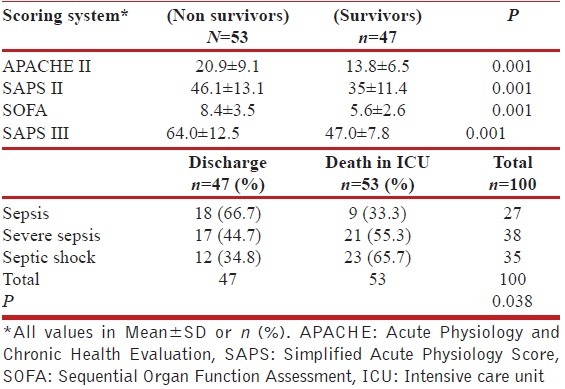
Source of infection and microbiological characteristics
Out of 100, 84 patients had clinical/radiological with/without microbiological features of lower respiratory tract infections. Fifty-two positive culture isolates were obtained from 46 patients. Of these, tracheal aspirates accounted for 76.9%, blood (15.3%) and urine (7.1%). Acinetobacter baumannii was the most common organism isolated (63.4%), followed by Kliebsella (9.5%), and Escherichia coli (8%).
Predictors of mortality in sepsis/severe sepsis/septic shock
All the variables which were significantly different between patients who died and those who survived were further tested with univariate and multivariate regression models. The odds ratio obtained after regression models were converted to relative risks to determine the predictors of mortality.
Table 3 depicts the logistic regression of various parameters that were significantly different between survivors and nonsurvivors. It was observed that the risk of death was 1.8 times higher in females compared with males. Similarly, age was also strong predictors of death; the risk of dying was doubled (relative risk of 2.1) in patients who were above 60 years of age as compared with patients who were below 30 years of age. The presence of anemia was associated with 1.8 times higher chance of dying as compared to those in whom anemia was not present. The risk of death was 2.1 times higher when hemoglobin level was less than 12.0 gm/dL as compared with those in whom hemoglobin level was more than 12.0 gm/dL. The renal dysfunction was also associated with higher probability of death. The risk of mortality was 1.8 times higher in patients with renal dysfunction. ARDS was associated with 1.6 times higher risk of dying as compared with those patients in whom ARDS was not present at the time of admission. Patients who presented with septic shock were having almost double the risk of dying (relative risk of 1.9) as compared with those patients who had features of sepsis only. The APACHE II score more than 14, SAPS II score more than 35, SAPS III score more than 47 and SOFA score more than 6 at the day 1 of admission were associated with more than double the risk of death.
Table 3.
Determination of mortality predictors by univariate and multivariate analyses
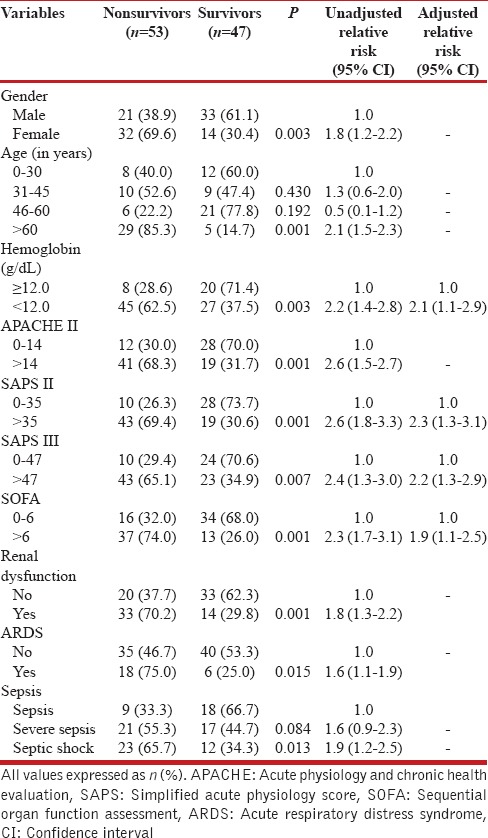
The relative risk of death in APACHE II > 14, SAPS II > 35, SAPS III > 47, and SOFA > 6 were 2.6, 2.6, 2.4, and 2.3, respectively. The cutoff value of critical care scores were the mean score of the patients who survived in ICU. Among these, the variables which were associated with higher risk of death, after multivariate regression analysis were the presence of anemia, SAPS II more than 35, SAPS III more than 47, and SOFA score more than 6.
Derivation of mortality predictor equation for sepsis
By using the β-coefficient of the variables, which were statistically significant in multivariate regression model of analysis a weighted mortality prediction formula was devised. The optimal cutoff for weighted score was calculated using the ROC curve and estimated the sensitivity, specificity and positive likelihood ratio. The mortality prediction formula (AIIMS Sepsis Score) was devised as: 10.5 × SAPS II category + 10 × Hb category + 12 × SAPS III category + 10.5 × SOFA score category.
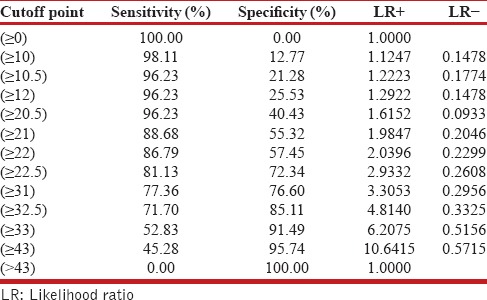
The category score for the above variables with their individual sensitivity and specificity are given in Table 4. The hemoglobin category has the highest sensitivity of 84.9% in predicting the mortality but it has got specificity of 42.5%, which is very low. Similarly, SAPS II and SAPS III score category have got good sensitivity of 81.1% but their specificities are low. SOFA score category has got the best specificity of 72.3% but its sensitivity of 69.8% is lowest among all the categories. The ROC analysis of the AIIMS Sepsis Score is given in Figure 1. Using the ROC analysis, a score more than 22.5, when used as a predictor of mortality had a sensitivity of 81.1%, specificity of 72.3%, positive likelihood ratio (LR+) of 2.9, and negative likelihood (LR−) of 0.26.
Table 4.
Category scores of individual variables used in mortality prediction formula
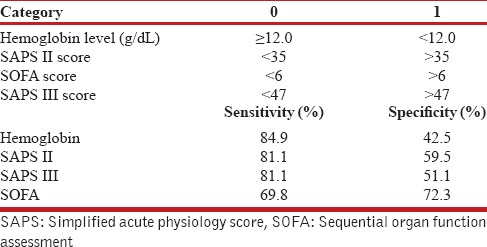
Figure 1.
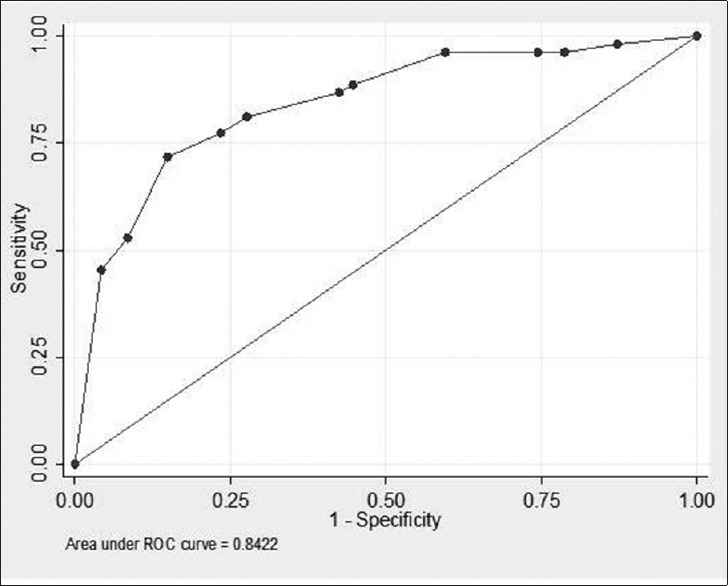
Receiver operating characteristic curve analysis of the predicting score
Table 5 shows the cutoff points for the various levels of the AIIMS Sepsis Score with their respective sensitivity and specificity values determined by the ROC analysis. A score more than 22.5, when used as a predictor of mortality had a sensitivity of 81.1%, specificity of 72.3%, which is better than overall sensitivity and specificity of individual category (shown in Figure 1) used in mortality predicting formula.
Table 5.
Sensitivity and specificity levels of various cutoff levels of the predicting score
DISCUSSION
In this observational, prospective ICU-based study, we assessed the predictors of mortality and morbidity of patients admitted with sepsis/severe sepsis/septic shock in a medical ICU.
Our patients comprised an almost equal distribution of males and females, however, the mean age of the entire group was almost a decade less than most reports, especially from the Western countries.[3] Among our patients, 73% had either severe sepsis/septic shock, indicating the greater severity of illness among majority of patients compared with some previous studies such as by Padkin et al.,[12] in 2003 (27.1% severe sepsis), but comparable to the figure of 76.5% reported by Todi et al.[4] The higher proportion of severe sepsis in our patients is likely due to the tertiary referral bias leading to sicker patients being admitted.
Consequently, our overall mortality figures of 53% probably reflect the higher proportion of patients with severe sepsis. This is underlined by the fact that 89% of our patients needed mechanical ventilation at the very outset. Generally speaking, a wide variation in sepsis mortality has been observed, ranging between 18% and 56% depending on patient type, admission policies, and local facilities.[2,5,13] By the same analogy, our mortality rate was higher in patients with severe sepsis and septic shock (55.3% and 65.7%, respectively).
Blanco et al.,[14] in a large Spanish study reported a mortality of 54.3% in severe sepsis, comparable to the figure of 54.1% reported by Todi et al.[4] in an Indian cohort. Expectedly, the mortality rate in patients with septic shock was much higher at 65.7%, which is higher than mortality figures observed in two previous European studies, which reported a crude ICU mortality rate of 60.1% and 60%, respectively.[15,16]
Variables associated with increased mortality in sepsis
We observed that mortality was significantly higher in females than males (69.6% and 38.9%, respectively) probably as a result of higher proportion of severe sepsis/septic shock (76.2% compared with 70.1% in males). In addition, non survivors were significantly older than survivors with a 2.1-fold higher risk of death in patients older than 60 years compared with those younger than 30 years. Age as an independent risk factor for increased mortality had been highlighted in several previous studies.[1,12,17] Lower immune response, greater associated comorbidities, higher chances of health care–related complications, and nutritional deficiencies may be likely explanations for this association.
Similarly, the mean hemoglobin level was significantly lower in non survivors (9.7 g/dL) compared with survivors (11.2 g/dL) with a 2.1 times higher risk of death in patients with hemoglobin levels less than 12.0 g/dL than those above this value.
ARDS, a dreaded complication of sepsis, was present in 24% of our patients and associated with higher risk of death. Rates of ARDS have varied widely from 28% to up to 59% leading to higher requirement of mechanical ventilation and associated complications.[4,15,16] Acute kidney injury (AKI) affects 1%–25% of ICU patients causing mortality to the tune of 15%–60%.[18,19,20] Renal dysfunction was present in 47% of our patients with 70.2% mortality. The reasons for this higher kidney-related adverse outcome could be associated hypoxic damage due to the high proportion of patients with septic shock, as well as direct kidney damage from infectious diseases such as malaria or leptospirosis.
Critical care scoring systems
Presence of higher SOFA, SAPS II, and SAPS III baseline scores were independent predictors of increased mortality. A higher SOFA score was associated with 1.9 times higher risk of death. The SAPS II score more than 35 and SAPS III score more than 47 were associated with higher mortality (odds ratio 2.3 and 2.2, respectively). The prognostic value of critical scoring systems has been validated previously as well[21,22,23,24,25]; however, none of the studies used a comprehensive panel of multiple scoring systems and compared them head-to-head among the same group of patients. Furthermore, no consensus exists regarding the most efficient predictive mortality scoring system specifically for sepsis/severe sepsis. Older age and higher APACHE II score were associated with increase in total duration of mechanical ventilation. Similarly, higher APACHE II score at the time of admission was also associated with increased duration of ICU stay in our study, a result that has been reported previously as well.[26] Interestingly, APACHE II was not an independent predictor of mortality among this group.
Positive cultures could be obtained from various sites in only 46% of patients, which is considerably lower than most previous figures that range from 62% to 81%.[27,28,29] Similarly, the blood culture positivity of 8% is low compared with earlier reported rates of 20%-40%. One possible reason for this lower microbiological yield could be the fact that virtually all patients had received higher generation antibiotics for variable periods before samples were cultured. The spectrum of sepsis etiology was remarkably distinct in our group. As with most other studies,[3,28] a pulmonary focus of sepsis was the most commonly identified, comprising 84% of the cohort, followed by neurological and urinary tract (3% each). This pulmonary predominance is probably related to admission policies and nature of referrals in our area.
The category score for these variables are given in Table 4, whereas the sensitivity and specificity of individual categories used in this formula are depicted in Table 5. Using the ROC analysis on the mortality prediction formula devised above, a score more than 22.5 had a sensitivity of 81.1%, specificity of 72.3%, positive likelihood ratio (LR+) of 2.9, and negative likelihood (LR−) of 0.26, implying thereby an 81.1% chances of mortality compared with 27.7% if the score is less than 22.5. In practical terms, a score more than 22.5 in a patient with sepsis at day 1 has a 2.9 times greater likelihood of dying than those with the score less than 22.5. The performance of this formula was better compared with the individual variables used in the mortality score.
This study had some limitations. In spite of being designed as a prospective observational study, the sample size was relatively small, although adequately powered to determine the primary objective of predicting mortality in ICU. In addition, there is likely to be a tertiary referral bias that affects the patient profile, especially severity of illness and microbiological yield due to multiplicity of antibiotics elsewhere. Thus, this score needs to be tested and validated on a much larger and heterogenous patient population to assess reliability and reproducibility before it can be applied in routine clinical practice. Thirdly, patients were evaluated till they were discharged from the ICU, hence a complete profile beyond ICU stay was not analyzed. However, in spite of these shortcomings, we feel this data adds significant knowledge to the hospital-based outcomes of patients with sepsis/severe sepsis and merits further evaluation.
CONCLUSION
To conclude, severe sepsis continues to be a syndrome with high fatality. Several critical scoring systems are useful for early prediction of mortality. A sepsis mortality prediction formula based on SAPS II, SAPS III, SOFA scores and hemoglobin has greater predictive power than these scoring methods individually.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidiker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome and associated cost of care. Crit Care Med. 2001;29:1309–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Alejandria MM, Ann M, Lansang D, Fonbuena GE, Fadreguilan E, Timbreza F, et al. Epidemiology and predictors of mortality from sepsis in medical patients at UP-PGH. Phil J Microbiol Infect Dis. 2000;29:23–32. [Google Scholar]
- 3.Moss M. Epidemiology of sepsis: Race, sex, and chronic alcohol abuse. Clin Infect Dis. 2005;41(Suppl 7):S490–7. doi: 10.1086/432003. [DOI] [PubMed] [Google Scholar]
- 4.Todi S, Chattarjee S, Bhattacharyya M. Epidemiology of severe sepsis in India. Crit Care. 2007;11(Suppl 2):65. [Google Scholar]
- 5.Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA. 1995;274:968–74. [PubMed] [Google Scholar]
- 6.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definition Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 7.Dellinger R, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. International Surviving Sepsis Campaign Guidelines Committee; American Association of Critical-Care Nurses; American College of Chest Physicians; American College of Emergency Physicians; Canadian Critical Care Society; European Society of Clinical Microbiology and Infectious Diseases, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. [Google Scholar]
- 8.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 9.Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 10.Metnitz PG, Moreno RP, Almeida E, Jordan B, Bauer P, Campos RA, et al. SAPS 3 Investigators. SAPS 3-From evaluation of the patient to evaluation of the intensive care unit. Part 1: Objectives, methods and cohort description. Intensive Care Med. 2005;31:1336–44. doi: 10.1007/s00134-005-2762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA scores to predict outcome in critically ill patients. JAMA. 2001;286:1754–8. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 12.Padkin A, Goldfrad C, Brady AR, Young D, Black N, Rowan K. Epidemiology of severe sepsis occurring in the first 24 hrs in Intensive care units in England, Wales and northern Ireland. Crit Care Med. 2003;31:2332–8. doi: 10.1097/01.CCM.0000085141.75513.2B. [DOI] [PubMed] [Google Scholar]
- 13.Martin GS, Mannino DM, Eaton S, Moss M. The Epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 14.Blanco J, Muriel-Bombín A, Sagredo V, Taboada F, Gandía F, Tamayo L, et al. Grupo de Estudios y Análisis en Cuidados Intensivos. Incidence, organ dysfunction and mortality in severe sepsis: A Spanish multicentre study. Crit Care. 2008;12:R158. doi: 10.1186/cc7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–9. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 16.Salvo I, de Cian W, Musicco M, Langer M, Piadena R, Wolfler A, et al. The Italian SEPSIS study: Preliminary results on the incidence and evolution of SIRS, sepsis, severe sepsis and septic shock. Intensive Care Med. 1995;21(Suppl 2):S244–9. doi: 10.1007/BF01740762. [DOI] [PubMed] [Google Scholar]
- 17.Angus DC, Wax RS. Epidemiology of sepsis: An update. Crit Care Med. 2001;29(Suppl):S109–16. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 18.Dennen P, Douglas IS, Anderson R. Acute kidney injury in the intensive care unit: An update and primer for the intensivist. Crit Care Med. 2010;38:261–75. doi: 10.1097/CCM.0b013e3181bfb0b5. [DOI] [PubMed] [Google Scholar]
- 19.Clec’h C, Gonzalez F, Lautrette A, Nguile-Makao M, Garrouste-Orgeas M, Jamali S, et al. Multiple-center evaluation of mortality associated with acute kidney injury in critically ill patients: A competing risks analysis. Crit Care. 2011;15:R128. doi: 10.1186/cc10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandelbaum T, Scott DJ, Lee J, Mark RG, Malhotra A, Waikar SS, et al. Outcome of critically ill patients with acute kidney injury using the Acute Kidney Injury Network criteria. Crit Care Med. 2011;39:2659–64. doi: 10.1097/CCM.0b013e3182281f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno R, Vincent JL, Matos R, Mendonça A, Cantraine F, Thijs L, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care: Results of a prospective, multicentre study. Working group on sepsis related problems of the ESICM. Intensive Care Med. 1999;25:686–96. doi: 10.1007/s001340050931. [DOI] [PubMed] [Google Scholar]
- 22.Stevens V, Lodise TP, Tsuji B, Stringham M, Butterfield J, Dodds Ashley E, et al. The utility of acute physiology and chronic health evaluation II scores for prediction of mortality among intensive care unit (ICU) and non-ICU patients with methicillin-resistant Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol. 2012;33:558–64. doi: 10.1086/665731. [DOI] [PubMed] [Google Scholar]
- 23.Chen SJ, Chao TF, Chiang MC, Kuo SC, Chen LY, Yin T, et al. Prediction of patient outcome from Acinetobacter baumannii bacteremia with Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation (APACHE) II scores. Intern Med. 2011;50:871–7. doi: 10.2169/internalmedicine.50.4312. [DOI] [PubMed] [Google Scholar]
- 24.Richards G, Levy H, Laterre PF, Feldman C, Woodward B, Bates BM, et al. CURB-65, PSI, and APACHE II to assess mortality risk in patients with severe sepsis and community acquired pneumonia in PROWESS. J Intensive Care Med. 2011;26:34–40. doi: 10.1177/0885066610383949. [DOI] [PubMed] [Google Scholar]
- 25.Khwannimit B, Bhurayanontachai R. The performance of customised APACHE II and SAPS II in predicting mortality of mixed critically ill patients in a Thai medical intensive care unit. Anaesth Intensive Care. 2009;37:784–90. doi: 10.1177/0310057X0903700515. [DOI] [PubMed] [Google Scholar]
- 26.Abraham E, Anzueto A, Gutierrez G, Tessler S, San Pedro G, Wunderink R, et al. Double-blind randomized controlled trial of monoclonal antibody to human tumor necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998;351:929–33. [PubMed] [Google Scholar]
- 27.Annane D, Sébille V, Charpentier C, Bollaert PE, François B, Korach JM, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;21:862–71. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 28.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Recombinant Human Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) Study Group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 29.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]


