Abstract
Context:
Mycobacterium tuberculosis (MTB), the human pathogen causes Tuberculosis (TB). It is a highly infectious and globally pandemic disease. The severity increases when the MTB becomes resistant to antituberculosis drugs. India is reported to be in the second place, with the highest number of drug-resistant TB cases. The treatment of drug-resistant TB is even more complicated.
Materials and Methods:
The present study comprises of 159 TB patients, in which 88 are reported to have drug-resistant TB (55.3%). All the patients are in the age group of 18–70 years. Patients having extrapulmonary TB and diabetes were excluded from the study. The collected samples were processed and stained for acid fastness and smear positivity. They were subjected to inoculation on Lowenstein–Jensen (LJ) slants.
Results:
The results showed that out of the four drugs — Streptomycin, Isoniazid, Rifampicin, and Ethambutol — the resistant cases reported in Streptomycin were 45 (24.9%), whereas, in Isoniazid, Rifampicin, and Ethambutol, the resistant cases were 62 (34.2%), 27 (14.9%), and 47 (26.0%), respectively. Isoniazid showed the highest percentage of resistance among the patients.
Conclusion:
Effective measures such as convincing the patients to take the prescribed drugs and follow the five major strategies under the Directly Observed Treatment, Short Course (DOTS), could help in managing such cases.
KEY WORDS: Anti-TB drugs, drug resistance, isoniazid, Mycobacterium, tuberculosis
INTRODUCTION
Tuberculosis (TB) is a fatal contagious disease. It is mainly an infection of the lungs, but can affect almost any part of the body. Although TB is a preventable and treatable disease, yet it still poses a significant threat globally. On account of the constant increase in the number of TB cases, the World Health Organization (WHO), in 1993, had declared TB as a serious public health emergency.[1] Approximately, nine million new cases and one-and-a-half million TB-related deaths occur each year; the incidence may vary. In some countries like Sub-Saharan Africa and Asia, the annual incidence is several hundred per 100,000 population, whereas, in the United States the annual incidence is <4 per 100,000 population (http://www.who.int/tb/en/). In 2012, an estimated 8.6 million people developed TB and 1.3 million died from the disease. The new cases of TB mostly occur in a population aged between 15 and 49 years (http://www.tbcindia.nic.in/).
Tuberculosis is caused by the human pathogen Mycobacterium tuberculosis (MTB) — an infectious human agent.[2] MTB may have killed more persons than any other microbial pathogen. The inadequacy of awareness and treatment results in major health problems, where the results are complicated. TB is second only to the human immunodeficiency virus (HIV).[3] The amelioration of Latent Tuberculosis Infection (LTBI) to active TB is hastened by the most powerful risk factor, which is, HIV, as it weakens the immune system of the host.[4] HIV not only facilitates LTBI to active TB, but also serves to bring about the progression of spread of Multi-drug Resistant Tuberculosis (MDR-TB).[1]
The two most important drugs that have helped in eradicating nearly 80% of the MTB cells primarily from the cavity are rifampicin (RIF) and isoniazid (INH). RIF is bactericidal and has a sterilizing activity for MTB. INH is also bactericidal against the replicating bacteria and is the most widely used first-line anti-TB drug. Pyrazinamide (PZA) is used along with INH and RMP. It helps in clearing the MTB cells in the initial phase only. It is weakly bactericidal, but effective against bacteria in acidic environments. On the other side, ethambutol (EMB) is bacteriostatic and is used in combination with INH, RMP, and PZA. This combination prevents the emergence of drug resistance.[5] The moment an MTB isolate becomes resistant to RIF and INH; it takes the shape of a serious health hazard for the public. This is because the patients with MDR-TB are a constant source of transmission of MDR MTB.[6]
After China, India has the second highest burden of MDR-TB cases. Although, MDR-TB is less common than drug-susceptible TB, each year about 440,000 new cases of MDR-TB are diagnosed. In October, 2011, a total of 77 countries reported cases of drug-resistant tuberculosis (XDR-TB). The reported MDR-TB cases, among the notified pulmonary TB cases, in 2011, were approximately 66,000 (http://apps.who.int/iris/).
A survey was conducted by the Central Tuberculosis Division through National Institute for Research in Tuberculosis (NIRT), in Gujarat. A total of 1,571 isolates from new patients were taken, in which, 1,236 (78.7%) were susceptible to all first-line drugs, 173 (11%) had Isoniazid (INH) resistance and MDR-TB was found in 37 (2.35%).[7] Inadequate treatment leads to a serious and life-threatening illness, that is, it results in a constant increase in the proportion of MDR-TB as well as XDR-TB. Therefore, the aim of the present study is to check the pattern of drug resistance to first-line antituberculosis drugs among the new pulmonary cases.
MATERIALS AND METHODS
Collection of Mycobacterium isolates
The samples were collected during the months of June to December, 2013. One hundred and fifty-nine (159) susceptible TB patients of both sexes, aged between 18 and 70 years were taken. Out of these, 88 were found to be smear-positive cases. All of these subjects were newly diagnosed, HIV-negative cases, collected from the King George Medical University, Lucknow (U.P). Patients who were immunocompromised and had extrapulmonary tuberculosis (EPTB) were excluded and also their clinical history was taken.
After obtaining informed consent, all the individuals were personally interviewed for information on food habits, occupation, and tobacco usage.
The patients were advised to collect their sputum samples every morning for three subsequent days, in a labeled sterile disposable container, and they were also instructed to rinse their mouths with plain water in order to avoid false-positive results. The collected samples were processed and stained for acid fastness, as MTB is an acid fast bacillus. The samples were tested for smear positivity, and thus, were subjected to inoculation on Lowenstein-Jensen (LJ) slants. The samples were allowed to incubate at 37°C for six weeks. The visible growth on the LJ slants helped in the identification of the Mycobacterium isolates.
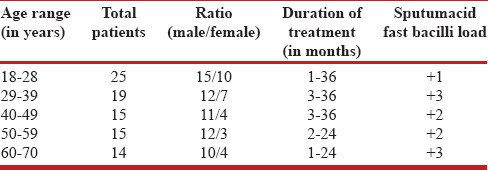
The history, duration of infection, and all other relevant data were recorded in the data sheet as follows [Table 1].
Table 1.
Clinical profile of tuberculosis patients
Drug susceptibility testing
The MTB clinical isolates were isolated and identified by the conventional methods and subjected to drug susceptibility testing against the first-line anti-tuberculosis drugs by the Proportion Method — against isoniazid (0.2 μg/ml), ethambutol (2 μg/ml), streptomycin (SM) (4 μg/ml), and rifampicin (40 μg/ml).[8] The control media were incubated at 37˚C for three weeks. Prior to the growth recording, examination of the media was done at 48 hours, weekly, and the reading for drug susceptibility was done at three weeks.
RESULTS
One hundred fifty nine (159) samples were studied. Out of 159, the number of cases resistant to SM, INH, RIF, and EMB were 45 (24.9%), 62 (34.2%), 27 (14.9%), and 47 (26.0%), respectively. The number of males and females resistant to SM were 34 and 11, respectively. In INH, the resistant cases were 43 males and 19 females; whereas, the number of males and females resistant to RIF were eighteen and nine, respectively. Also, in EMB, the male resistant cases were 32 and the female resistant cases were 15. All the patients were in the age group of 18–70 years [Table 1].
Mostly patients came from the rural areas. These patients had never experienced extrapulmonary tuberculosis (EPTB) or diabetes mellitus. All the patients had a history of previous infection of MTB. First, the patients were treated with the WHO Category 1 therapy, which included treatment with INH, PZA, and EMB for two months, followed by treatment with INH and RIF or INH, RIF, and PZA for a further four months. As soon as the patients were diagnosed with MDR-TB, they were immediately shifted to a different treatment, based on their treatment history, drug susceptibility, and so on.
DISCUSSION
Anti-TB drugs have proved to be a boon in the treatment of TB, but on the other side they have also some negative points. If a patient consumes his anti-TB drugs on a routine basis without any gap, he can be sure that these drugs will help him in combating the infection, but if the drugs are taken in a discontinuous manner the treatment can become more difficult and sometimes fatal.[9]
The present study shows the pattern of drug resistance to first-line antituberculosis drugs among new pulmonary cases. In this particular study the drug resistance of MTB was observed in all the cases. This shows that cases of drug resistance are increasing at a high speed. Thus, we can state that, day by day, the increasing rates of resistance to Isoniazid lead to life-threatening problems among the population.[10] Also, the sex factor was significantly noticeable in our study, as males showed more resistant behavior to antituberculosis drugs than females. Forty-three males and 19 females were found to be resistant to INH [Table 2].
Table 2.
Number and percentage of antituberculosis drug resistance

Deaths due to TB (1.4 million) were recorded worldwide, in 2011. An estimated 8.6 million new cases of TB and 1.3 million TB-related deaths have been recorded worldwide; 6.2 million cases of TB were notified by the National TB Control Programs and reported to the WHO, in 2011, out of these, 5.8 million were individuals who were newly diagnosed in 2011, and 0.4 million were the previously diagnosed TB patients, whose treatment regimen was changed. A wide variation in the prevalence rate of MDR has been reported from different Asian countries. Different surveys done by the WHO using standardized guidelines showed levels of primary resistance to antituberculosis drugs (http://www.who.int/topics/tuberculosis/en/). For example, INH ranged from 0–16.9%; SM from 0.1–23%; EMB was low ranging from 0–4.2%, and RMP had a rate ranging from 0–3%. Prevalence of initial MDR-TB reported from India varied between 0 and 5%. To know about the current TB Control Program, one may get it from the drug-resistant program of any country. Countries such as India and Kenya reported high rates of primary resistance to Isoniazid. On the other hand, countries such as Melbourne and Argentina showed low rates for primary resistance to INH.[11]
Thus, it becomes important to look into the case findings, convincing patients to take their prescribed medicines, and also strengthen the smear-positive patients; under the five main strategies of DOTS.
CONCLUSION
For a better and healthy population, all the effective measures should be adopted, to manage both the fresh and drug-resistant TB cases by following the five major strategies under DOTS and convince the patients to take the prescribed drugs on a routine basis, without any gap.
ACKNOWLEDGMENT
The authors are thankful to the Department of Zoology, University of Lucknow (U.P), for providing the necessary facilities and guidance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Raviglione MC, Snider DE, Jr, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–6. [PubMed] [Google Scholar]
- 2.Krutzik SR, Modlin RL. The role of Toll-like receptors in combating mycobacteria. SeminImmunol. 2004;16:35–41. doi: 10.1016/j.smim.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: Global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–21. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 4.Mokrousov I, Otten T, Filipenko M, Vyazovaya A, Chrapov E, Limeschenko E, et al. Detection of isoniazid-resistant mycobacterium tuberculosis strains by a multiplex allele-specific PCR assay targeting katG codon 315 variation. J Clin Microbiol. 2002;40:2509–12. doi: 10.1128/JCM.40.7.2509-2512.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. American Thoracic Society, Centers for Disease Controland Prevention and the Infectious Diseases Society. American Thoracic Society / Centers for Disease Control and Prevention /Infectious Diseases Society of America: Treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–62. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 6.Mokrousov I, Narvskaya O, Limeschenko E, Otten T, Vyshnevskiy B. Detection of ethambutol - resistant Mycobacterium tuberculosis strains by multiplex allele-specific PCR assay targeting embB306 mutations. J Clin Microbiol. 2002;40:1617–20. doi: 10.1128/JCM.40.5.1617-1620.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: Estimated incidence, prevalence, and mortality by country. WHO global surveillance and monitoring project. JAMA. 1999;282:677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 8.Menon S, Dharmshale S, Chande C, Gohil A, Lilani S, Mohammad S, et al. Drug resistance profiles of Mycobacterium tuberculosis isolates to first line anti-tuberculous drugs: A five years study. Lung India. 2012;29:227–31. doi: 10.4103/0970-2113.99104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson R, Streicher EM, Louw GE, Warren RM, van Helden PD, Victor TC. Drug resistance in mycobacterium tuberculosis. Curr Issues Mol Biol. 2006;8:97–111. [PubMed] [Google Scholar]
- 10.Falzon D, Jaramillo E, Schünemann HJ, Arentz M, Bauer M, Bayona J, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J. 2011;38:516–28. doi: 10.1183/09031936.00073611. [DOI] [PubMed] [Google Scholar]
- 11.Prasad R. Multidrug and extensively drug-resistant TB (M/XDR-TB): Problems and solutions. Indian J Tuberc. 2010;57:180–91. [PubMed] [Google Scholar]


