ABSTRACT
Most animal-microbe mutualistic associations are characterized by nutrient exchange between the partners. When the host provides the nutrients, it can gain the capacity to shape its microbial community, control the stability of the interaction, and promote its health and fitness. Using the bioluminescent squid-vibrio model, we demonstrate how a single host-derived glycan, chitin, regulates the metabolism of Vibrio fischeri at key points in the development and maintenance of the symbiosis. We first characterized the pathways for catabolism of chitin sugars by V. fischeri, demonstrating that the Ccr-dependent phosphoenolpyruvate-pyruvate phosphotransferase system (PTS) prioritizes transport of these sugars in V. fischeri by blocking the uptake of non-PTS carbohydrates, such as glycerol. Next, we found that PTS transport of chitin sugars into the bacterium shifted acetate homeostasis toward a net excretion of acetate and was sufficient to override an activation of the acetate switch by AinS-dependent quorum sensing. Finally, we showed that catabolism of chitin sugars decreases the rate of cell-specific oxygen consumption. Collectively, these three metabolic functions define a physiological shift that favors fermentative growth on chitin sugars and may support optimal symbiont luminescence, the functional basis of the squid-vibrio mutualism.
IMPORTANCE
Host-derived glycans have recently emerged as a link between symbiont nutrition and innate immune function. Unfortunately, the locations at which microbes typically access host-derived glycans are inaccessible to experimentation and imaging, and they take place in the context of diverse microbe-microbe interactions, creating a complex symbiotic ecology. Here we describe the metabolic state of a single microbial symbiont in a natural association with its coevolved host and, by doing so, infer key points at which a host-controlled tissue environment might regulate the physiological state of its symbionts. We show that the presence of a regulatory glycan is sufficient to shift symbiont carbohydrate catabolism, acetate homeostasis, and oxygen consumption.
INTRODUCTION
Metabolic coordination between partners is a central factor in the evolution of beneficial symbiotic associations (1–3); in particular, the provision of nutrients by the symbiont and/or host can drive coevolution, codevelopment, and ecological scaffolding in the symbiosis (2, 3). Well-known examples of metabolic coordination are found in endosymbiotic associations, common among insects, in which the combined metabolic activity of host and microbe compensate for nutritional deficiencies of both partners (4). Host-derived nutrition also structures surface-associated microbial communities. For example, milk oligosaccharides (5), and later mucin-derived oligosaccharides (6), shape the composition of the mammalian gut microbiota. Vertebrate microbiota are complex and variable, which complicates the study of the effect of host-derived nutrition on the physiology of any one microbial constituent. Natural, yet less complex microbial communities are maintained by invertebrate hosts, such as the honeybee (7), the medicinal leech (8), or the bobtail squid (9). Thus, invertebrates present tractable animal models to elucidate the core principles by which host-derived nutrition impacts symbiont metabolism and physiology.
Vibrio fischeri can exist both free-living in seawater and as the specific symbiont of the squid, Euprymna scolopes (10). In the latter, V. fischeri colonizes epithelium-lined crypt spaces in the squid’s light-emitting organ, a structure anatomically designed to use bacterium-produced luminescence during the host’s nocturnal activities (11). Each newly hatched squid must obtain an inoculum of V. fischeri from the ambient seawater; these bacteria quickly proliferate in the crypts, where the symbiont population reaches a high density, and luminescence is induced by quorum signaling (12). Luminescence is essential for symbiosis, as strains of V. fischeri that have lost the ability to produce light fail to persist in this association (13–15). Because the symbionts provide only luminescence and not any known nutrient, this binary model is ideal for studying the role of the host in driving the association’s underlying metabolism (15, 16).
Chitin, a polymeric glycan of N-acetylglucosamine, and its derivatives are common host-derived nutrients and signal factors in diverse plant and animal symbioses (17–20). Chitin-like oligosaccharides are also the most abundant source of nitrogen in marine surface waters (21). As the major structural component of many invertebrates, chitin is also a potential nutrient for microbial symbionts. Moreover, the ability to degrade and metabolize chitin is widespread within the Vibrionaceae (22) and other marine bacteria. The principal chitin breakdown products are the disaccharide chitobiose (GlcNAc)2 and the monosaccharide N-acetylglucosamine (GlcNAc). Deacetylated chitin may yield dimeric or monomeric glucosamine. In Vibrio cholerae and Vibrio furnisii, the chitobiose is taken into the cell by ABC-type transport, while the monosaccharide GlcNAc is transported by the phosphoenolpyruvate-pyruvate phosphotransferase system (PTS) (23, 24). The PTS transport of GlcNAc contributes to catabolite repression in V. cholerae, which targets physiological processes like natural transformation (25). PTS transport also regulates biofilm formation and colonization of the germ-free mouse intestine by V. cholerae (26). Thus, PTS sugars represent an important class of host-derived nutrients in Vibrio physiology.
In the mutualism between E. scolopes and V. fischeri, chitin-derived sugars play several roles. First, a concentration gradient of host-derived chitobiose facilitates the establishment of symbiosis by guiding motile V. fischeri cells into the juvenile E. scolopes nascent light organ (17, 27). Second, as the symbionts initially colonize the crypt space, it is free of chitin sugars; in fact, induction of chitin catabolism-dependent transcription actually destabilizes the symbiont population (28). However, as the host matures, chitin sugars begin to be provided as a nutrient for the symbionts, but only during the night (29). The catabolism of these sugars by V. fischeri then supports the bacterium’s nocturnal luminescence by acidifying the crypts and liberating oxygen, a substrate of bacterial luciferase (29, 30).
The physiology of V. fischeri during growth on chitin sugars led to predictions regarding the potential nodes at which host-derived chitin sugars might regulate symbiont catabolism. Even under aerobic conditions, chitin sugar catabolism is highly acidogenic and leads to acetate excretion (29) (see Fig. S1 in the supplemental material). The excretion is reversed under certain physiological conditions, such as occur in the immature light organ (31). This alternation between excretion and uptake is called the “acetate switch” and results from the activity of acetyl coenzyme A (CoA) synthase (encoded by the acs gene) (32), whose transcription is regulated by the AinSR quorum-signaling circuit (31) (see Fig. S1). Thus, provision of chitin sugars by the host might represent a counterbalance to the AinSR-regulated acetate switch. In addition, the correlation between chitin-sugar acidification and the presence of oxygen in the context of symbiosis suggests that this host-derived nutrient might participate in defining a balance between the oxygen-consuming activities of light-producing luciferase and energy-conserving cytochrome oxidases (see Fig. S1).
In the present work, we show how chitin sugar catabolism shifts the symbiont’s metabolic state during the immature association by our (i) defining the pathways of chitin-sugar transport in V. fischeri, (ii) describing the physiological consequences of chitin-sugar catabolism on the acetate switch and respiratory oxygen consumption, and (iii) examining the contribution of chitin sugar-driven acetate homeostasis to the colonization of the immature light organ.
RESULTS
The EIIAcrr-dependent PTS prioritizes transport of chitin sugars.
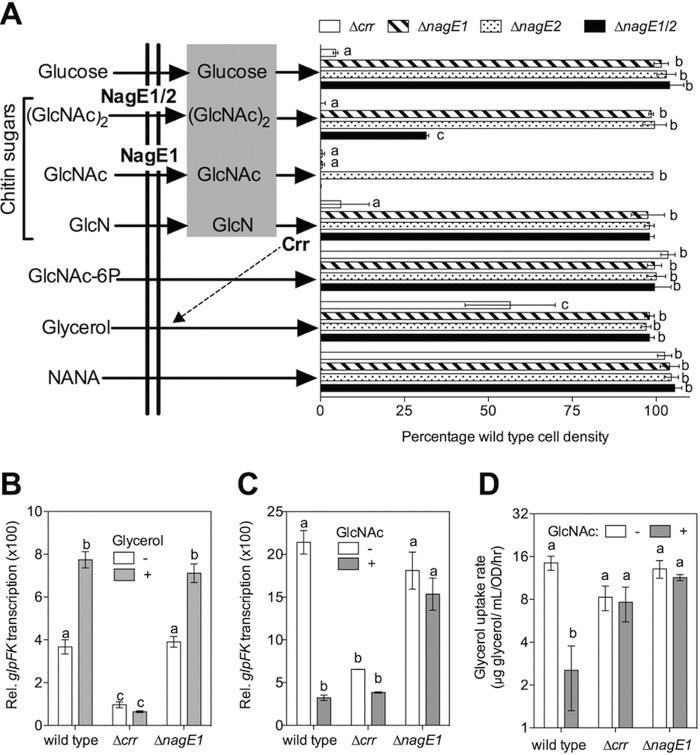
During PTS transport, the enzyme II complex completes the phosphotransfer chain that extracts the phosphoryl group from the glycolytic intermediate phosphoenolpyruvate to support sugar uptake (33). Enzyme II contains transmembrane B and C domains and a kinase domain, A. A subset of PTS enzyme II complexes lack an A domain and instead rely on the cytoplasmic phosphotransferase Ccr (referred to here as EIIAcrr) (see Fig. S1 in the supplemental material). Glucose, sucrose, trehalose, and GlcNAc have been identified as EIIAcrr-dependent PTS sugars in V. cholerae (26). We identified two homologs of the putative GlcNAc EIIBC transporter NagE (nagE1 [VF_0808] and nagE2 [VF_A0438]) and one homolog of EIIAcrr (crr [VF_1897]). We constructed strains carrying individual deletions of crr, nagE1, and nagE2 and a double mutant (nagE1/2). We evaluated the ability of these mutants to grow on a minimal medium containing a selection of single carbon sources (Fig. 1A). Like V. cholerae, growth of the V. fischeri Δcrr mutant was impaired on glucose and GlcNAc. Surprisingly, we also observed that the mutant was unable to grow on chitobiose or glucosamine (GlcN) as a sole carbon source. The Δcrr mutant only grew comparably to wild type on N-acetylneuraminic acid (NANA), an aminosugar not derived from chitin. Collectively, these observations indicate that, unlike V. cholerae and V. furnisii (24, 26), the catabolism of chitin breakdown products, including the dimer chitobiose and the monomers GlcNAc and GlcN, relies upon transport by the crr-dependent PTS. Future studies will be needed to determine whether chitotriose (GlcNAc)3 is also a substrate of the EIIAcrr-dependent PTS. However, previous evidence of abundant host exo- and endochitinase activities (27, 29) suggests that the chitin sugars presented to V. fischeri by the host are likely to include monomeric and dimeric GlcNAc. Thus, we used GlcNAc as a representative chitin sugar to explore PTS-dependent regulation in subsequent experiments.
FIG 1 .
Prioritization of carbohydrate catabolism in V. fischeri. (A) The effects of deletions of genes encoding PTS proteins (NagE1, NagE2, and crr) on growth yield with chitin sugars, aminosugars, or non-PTS carbohydrates as substrates. The percent cell yield (based on the culture OD600), relative to that for the wild type, was calculated following 24 h of growth in a minimal medium containing 30 mM of one of seven sole carbon sources (n = 2): (GlcNAc)2, chitobiose; GlcNAc, N-acetylglucosamine; GlcN, glucosamine; NANA, N-acetylneuraminic acid. Sugars shown in the gray box are PTS-transported sugars that require EIIAcrr (Ccr). (B and C) Relative promoter activity for the glpFK locus during growth in a tryptone-based medium in the absence or presence of glycerol (B) or a glycerol- and tryptone-containing medium in the absence or presence of GlcNAc (C) (n = 2; results shown are representative of two independent experiments). (D) Effect of GlcNAc addition on the rate of glycerol consumption by V. fischeri wild-type and mutant strains (n = 3). a, b, and c indicate statistically different mean values, determined by a two-way analysis of variance with post hoc Bonferroni t tests. Error bars indicate standard errors.
The function of the two annotated GlcNAc-specific EI transporters, nagE1 and nagE2, was found to be more complex. First, growth on GlcNAc required nagE1 but not nagE2. Second, while individual mutation of either of these two genes did not impact growth on chitobiose, deletion of both putative transporters reduced the growth yield by 50%. Third, growth on glucosamine required crr but needed neither of the nagE paralogs (Fig. 1A). Collectively, these data suggest that certain chitin sugars, such as GlcNAc, are transported by a specific EIIBC transporter (nagE1), while others, such as chitobiose, may be transported by at least two distinct EIIBC transporters (nagE1 and nagE2), in addition to previously annotated ABC transporters conserved among Vibrio spp. (22).
We also observed that the growth yield of the crr mutant was significantly reduced on glycerol, a 3-carbon carbohydrate not expected to require PTS transport for catabolism (Fig. 1A). The Escherichia coli EIIAcrr homolog apparently activates the transport of glycerol, and other non-PTS carbohydrates, indirectly through a mechanism called inducer exclusion (33). During inducer exclusion, the EIIAcrr-dependent transport of a PTS sugar blocks the transport of a subset of non-PTS carbohydrates, like glycerol. These carbon sources all require the presence of their phosphorylated, intracellular form to activate the transcription factor that regulates their catabolism. For example, glycerol catabolism genes in E. coli, including glpF and glpK, are targets of inducer exclusion (33). Because glpK is required for catabolism of glycerol by V. fischeri (M. Mandel, personal communication), we reasoned that the V. fischeri EIIAcrr homolog, encoded by crr, might regulate the transcriptional activity of the promoter associated with the V. fischeri glpFK operon (VF_0235 and -6), in a manner similar to inducer exclusion in E. coli. To determine whether this operon is indeed induced by the presence of glycerol, we measured the activity of the V. fischeri glpFK promoter in tryptone medium, with and without added glycerol. The resulting glpFK promoter activity was significantly increased by the presence of glycerol in the tryptone-based medium, in a crr-dependent manner (Fig. 1B). Notably, in tryptone medium without glycerol, glpFK promoter activity was also significantly lower in the Δcrr mutant than in either wild-type or ΔnagE1 V. fischeri, consistent with activation of glpFK promoter activity by an EIIAcrr-dependent mechanism, such as inducer exclusion (Fig. 1B). When the chitin monomer, GlcNAc, was added to medium containing both glycerol and tryptone, glpFK promoter activity decreased 7-fold (Fig. 1C). Similarly, glpFK promoter activity also significantly decreased in the presence of GlcN and the GlcNAc dimer, chitobiose, in a crr-dependent manner (see Fig. S2 in the supplemental material). This result is particularly interesting, given previous reports that, in V. cholerae, chitobiose does not contribute to regulation resulting from PTS transport, such as carbon catabolite repression (34).
We also monitored glycerol uptake in the presence and absence of GlcNAc (Fig. 1D), and we found that this substrate significantly decreased the nagE1-dependent transport of glycerol. Glycerol uptake was slightly, but not significantly, decreased in the crr mutant, consistent with the partial growth defect of this mutant during growth on glycerol minimal medium (Fig. 1A). Thus, chitin sugar substrates such as GlcNAc, GlcN, and chitobiose inhibit transcription of glycerol catabolism-associated genes by a crr-dependent mechanism that is indistinguishable from inducer exclusion, suggesting that the V. fischeri EIIAcrr-dependent PTS prioritizes chitin sugar catabolism ahead of non-PTS carbohydrates, such as glycerol.
The acs-dependent acetate switch is repressed by chitin sugars.
Acetate is a major excretion product of chitin sugar catabolism by V. fischeri (29) (see Fig. S1 in the supplemental material). To better understand the physiological conditions associated with this excretion, we measured the accumulation of acetate in culture supernatants during oxic and anoxic growth, either with or without chitin sugars, on a complex medium containing glycerol and peptides. Not surprisingly, we observed a >2-fold-higher accumulation of acetate in anoxic cultures than in oxic cultures (Fig. 2A). Further, cultures containing chitin sugars (GlcNAc) also accumulated ~2-fold more acetate, indicating that chitin-sugar catabolism promotes extracellular acetate buildup, even during aerobic growth.
FIG 2 .
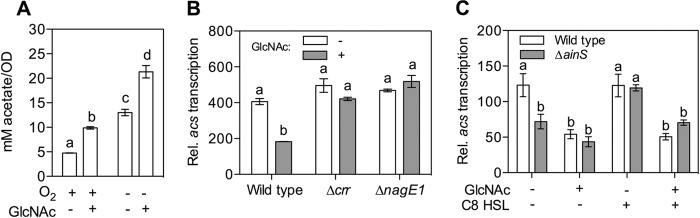
Repression of the acs-dependent acetate switch by chitin sugars. (A) Detection of acetate in the culture supernatant after growth in LBS under anoxic and oxic growth conditions, either with or without 20 mM GlcNAc (n = 2; results shown are representative of two independent experiments). Activity of the acs promoter region during growth in a tryptone-based medium, either with or without 20 mM GlcNAc added, of either PTS mutants (n = 2; results are representative of two independent experiments) (B) or in the presence or absence of 20 mM GlcNAc and/or 100 nM C8-HSL (n = 3). a and b indicate groups of statistically similar means, assessed as described for Fig. 1.
We reasoned that chitin sugar catabolism could promote accumulation and excretion of acetate through two metabolic mechanisms: (i) a classic Crabtree effect, which favors the flow of acetate through glycolysis rather than into the tricarboxylic acid (TCA) pathway (see Fig. S1 in the supplemental material), or (ii) the repression of the acetate switch (32). To address the latter possibility, we measured the transcription of acs, which encodes the key acetate switch enzyme, acetyl-CoA synthase. We observed that acs promoter activity was significantly decreased by the presence of the chitin monomer, GlcNAc, and that this repression was dependent on a functional PTS; specifically, acs promoter activity was insensitive to GlcNAc in both the Δcrr and ΔnagE1 mutants (Fig. 2B). Transcription of acs is also activated by the presence of octanoyl homoserine lactone (C8-HSL), the quorum signal synthesized by AinS (31). We next determined the relative strengths of GlcNAc and C8-HSL as negative and positive transcriptional effectors, respectively, by comparing acs promoter activity in the presence of none, either, or both of these signals. Maximum acs activation was achieved with the addition of 100 nM C8-HSL for the wild type, and 20 mM GlcNAc was sufficient to overcome this activation (Fig. 2C). Interestingly, GlcNAc addition was unable to further decrease acs promoter activity below the baseline level of an ainS knockout mutant, suggesting that the mechanism of regulation by GlcNAc (and perhaps other chitin sugars) is more of a deactivation than a repression. Thus, while the regulatory element targeted by chitin sugar catabolism remains unknown, our results indicate that catabolism of chitin sugars prevents AinSR-dependent activation of the acetate switch (31).
Regulation of cytochrome oxidase activity by chitin sugar catabolism.
Acetate catabolism typically generates chemical energy through respiration; therefore, we predicted that, in V. fischeri, chitin sugar-driven suppression of the acetate switch, and thus suppression of acetate utilization, would decrease cytochrome-based oxygen consumption (see Fig. S1 in the supplemental material). V. fischeri expresses two cytochrome-based terminal oxidases: a cbb3-type cytochrome oxidase encoded by ccoNOQP (VF1299 to -1302), and a bd-type quinol oxidase encoded by cydAB (VF_0953 and -4). Both enzyme complexes are produced in culture and during at least the first few days of light organ colonization, and their relative oxygen affinities have been estimated to be in the nanomolar range (16). However, mutation of cydAB severely impairs growth under aerobic culture conditions, and genetic suppressor mutations arise rapidly in such strains (35). Thus, we focused our efforts on characterizing the regulation and activity of the ΔccoNOQP (designated Δcco) mutant strain. The Δcco strain had a small but reproducible decrease in growth rate relative to wild type (Fig. 3A) when grown under micro-oxic conditions on a peptide-based medium predicted to support aerobic respiration. The divergence in rate became detectable within 2 h of growth under moderate aeration. However, the mutant’s decreased growth rate was not apparent during growth with a PTS carbohydrate such as GlcNAc (Fig. 3A) or with glucose (see Fig. S3A in the supplemental material) as the sole source of carbon. Moreover, the addition of GlcNAc or glucose to growing cultures of the Δcco strain rapidly restored the mutant to a growth rate comparable to wild type (Fig. 3A). In contrast, the addition of N-acetylneuraminic acid, a non-EIIAcrr-dependent aminosugar catabolized by V. fischeri (Fig. 1A), failed to resolve the difference in optical density (OD) between mutant and wild-type strains (see Fig. S3B). The difference in growth rates between the Δcco mutant strain and wild-type V. fischeri was accompanied by a smaller average cell size of the Δcco strain during mid-log-phase growth (Fig. 3B). We reasoned that the decrease in cell size might be correlated with a decrease in respiration at this growth stage, due to the lack of the CcoNOQP cytochrome oxidase (see Fig. S1 in the supplemental material). To address this hypothesis, we determined the respiratory rate for the cytochrome oxidase mutant and its wild-type parent during oxic growth on peptide-based medium. Wild-type V. fischeri and the Δcco derivative consumed comparable amounts of oxygen at ODs between 0.05 and 0.3 (see Fig. S3C and D), but when the OD was between 0.3 and 0.8, the Δcco mutant developed a pronounced decrease in the relative rate of oxygen consumption (Fig. 3C). However, the presence of GlcNAc reduced the level of consumption by wild type to that of the Δcco strain. Interestingly, the timing of this transition was coincident with the appearance of the growth rate and cell size phenotypes observed in this mutant (Fig. 3A and B).
FIG 3 .
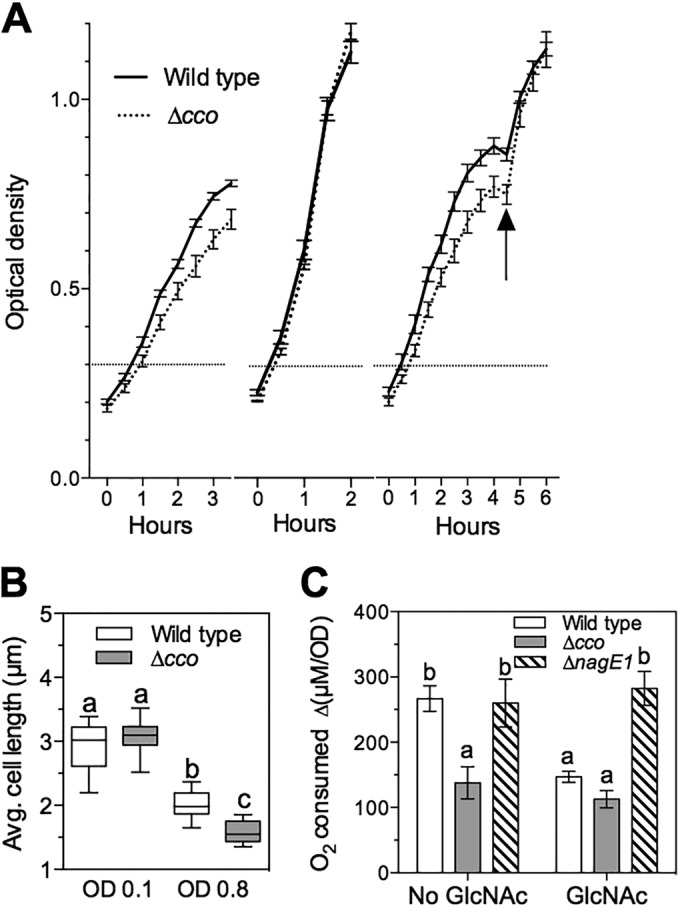
Repression of aerobic respiration by chitin sugars. (A) Growth of wild-type V. fischeri (solid lines) and a cytochrome oxidase mutant strain (Δcco; dashed lines) under moderate aeration in LBS medium (left panel), LBS containing 20 mM GlcNAc (center panel), or LBS with 20 mM GlcNAc added at the arrow (right panel) (n = 3). (B) Average cell length of wild-type (open) and Δcco strain (gray) cells during growth in LBS medium at two stages of growth: early phase (OD600, 0.1) and mid-log phase (OD600, 0.8). (C) Oxygen consumption of wild-type (open), Δcco mutant (gray), and ΔnagE1 mutant (hatched) cells, expressed as the rate per OD600 unit, that takes place between OD values of 0.3 and 0.8 during growth in a peptide-based medium, either with or without 20 mM GlcNAc added. Error bars indicate standard errors (n = 3). Statistical comparisons were performed as described for Fig. 1.
Chitin sugar catabolism destabilizes colonization of the immature light organ by V. fischeri.
Acetyl-CoA synthase, encoded by acs, has been shown to help V. fischeri maintain a normal colonization of the light organ during the first 48 h of symbiosis (31), indicating that acetate uptake is a determinant of symbiotic stability. Moreover, we observed that a mutant deficient in the glyoxylate shunt, the metabolic pathway through which acetate is converted into biomass to support anaplerosis, was as fit as wild-type V. fischeri during cocolonization of the light organ (see Fig. S4A in the supplemental material). This result suggested that the benefit of acetate uptake to symbiosis is not as a carbon source, but for its contribution to respiratory energy generation (see Fig. S1 in the supplemental material). It is hypothesized that the light organ is oxic during the first 48 h of colonization (16) and that cytochrome oxidases contribute to metabolism at this stage of the symbiosis; thus, we reasoned that symbiont metabolism might rely on aerobic respiration of ambient acetate. Adding chitin sugars to such an environment should repress acs transcription, thereby reversing the advantage in acetate utilization that wild-type cells normally have during competition with an acs mutant. To test this idea, we cocolonized squid with wild-type and mutant strains of V. fischeri defective in either acetate uptake (acs Tn:erm) or the GlcNAc-specific PTS (ΔnagE1 or Δcrr), and we compared how well they competed when GlcNAc was present or absent. As predicted, the disadvantage of the acs mutant at 48 h was eliminated by the addition of GlcNAc (Fig. 4A). In addition to this “chemical complementation,” genetic complementation of the acs mutation also restored competitive fitness to the strain (see Fig. S4B). We next assessed the competitive fitness of the ΔnagE1 mutant, a strain unable to metabolize GlcNAc, under the same conditions. Both this transporter mutant and wild-type V. fischeri were equally competitive after 48 h of colonization in the absence of GlcNAc (Fig. 4A). However, the fitness of the ΔnagE1 strain increased significantly relative to the wild type when GlcNAc was present, consistent with the inability of this PTS sugar to repress acs in this mutant (Fig. 2B).
FIG 4 .
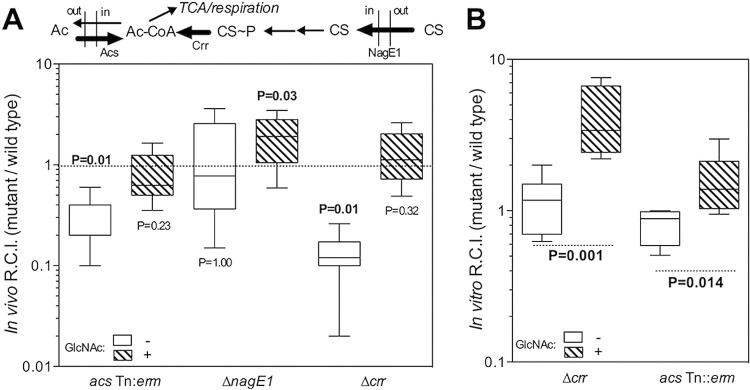
Chemical complementation of chitin sugar-modulated metabolic processes. (A) Cocolonization of squid for 48 h with wild-type V. fischeri and one of three isogenic strains carrying a mutation in either a PTS or an acetate switch protein (pathways are noted above plots). Cocolonization was performed for 48 h in the absence (open) or presence (hatched) of 20 mM GlcNAc. CS, chitin sugars. (B) Competition in cocultures of wild-type V. fischeri and the indicated mutants growing in SWT medium either without (open) or with (hatched) 20 mM GlcNAc. RCI, relative competitive index. The P value above the box-and-whisker plot represents the statistical similarity to a theoretical mean of 1.0, calculated by a one-sample t test (n = 20 squid per condition). Inner fences were determined by Tukey’s method. For comparisons in which either the wild type or the mutant performed significantly (>95%) better, P values are given in bold. Error bars indicate standard errors.
To learn more about what carbon sources are available to symbionts in the juvenile light organ, we performed the same type of cocolonization experiments, this time with the Δcrr strain. It was observed previously that a Δcrr mutant is less competitive than wild type during the first 48 h of light organ colonization (36). There are two alternative hypotheses to explain this phenotype. Either (i) an EIIAcrr-dependent PTS sugar or (ii) a carbohydrate that is subject to inducer exclusion is an important nutrient present in the light organ environment, and this nutrient cannot be catabolized by the Δcrr mutant. In the first case, the addition of chitin sugars to the seawater would be predicted to aggravate the competitive disadvantage of the Δcrr strain, because even more of an EIIAcrr-dependent PTS substrate would be available to the wild type but not the Δcrr strain. In the second case, the addition of chitin sugars would be expected to ameliorate the competitive disadvantage of the Δcrr strain because the presence of chitin sugars would lead to an inducer exclusion response in the wild type, which phenocopies the constitutive inducer exclusion that occurs in the Δcrr strain. We observed the latter case, i.e., the addition of GlcNAc to the seawater around the squid ameliorated the competitive disadvantage of the Δcrr strain (Fig. 4A), consistent with the presence in the symbiont’s environment of a non-PTS carbohydrate substrate that is subject to inducer exclusion.
To further predict the nutrient conditions in the juvenile light organ that lead to the initial, GlcNAc-independent decrease of fitness in acs and Δcrr strains, and thereby test our interpretations of the symbiotic effect of chitin sugars, we performed coculture experiments in nutrient media. During semiaerated growth on a modified SWTO (high-osmolarity seawater-tryptone) medium containing 1/10 of the normal levels of glycerol and peptides, we observed a GlcNAc-dependent shift in competitive fitness (Fig. 4B) comparable to that seen with these mutants in the light organ cocolonization assays (Fig. 4A). In contrast, in the absence of GlcNAc, there was no difference in competitive fitness between either of the two mutants and wild type. We reasoned that, unlike in the light organ environment, the batch culture conditions might not favor induction of the acetate switch. When we repeated the coculturing experiment with the acs mutant and wild-type V. fischeri in full-strength SWTO, we observed a decrease in competitive fitness comparable to that observed in the squid (see Fig. S4C in the supplemental material). As in the squid, this loss of competitive fitness could be reversed by the presence of GlcNAc in the culture medium. Together, these results suggest that the immature light organ environment has a balance of carbon and oxygen availability not unlike that of aerated, full-strength SWTO; in addition, the carbon substrates in this environment (e.g., glycerol) support an active acetate switch and may be targets of inducer exclusion. The metabolic and regulatory strategies available to V. fischeri in such an environment, compared to those in an environment containing chitin sugars, are quite distinct.
DISCUSSION
In this study, we have shown that chitin sugars, which can be provided by the host squid E. scolopes (29), serve as regulatory molecules whose catabolism by the PTS in V. fischeri leads to a shift from respiratory to fermentative metabolism. In the symbiotic state, the provision of chitin sugars is linked to the developmental stage of the host, i.e., while chitin sugars apparently serve only a nonnutritional, signaling role in the juvenile symbiosis (17, 27), in the mature association they are provided each night in higher concentrations as a nutrient (29). Thus, while losing the ability to catabolize chitin sugars actually promotes a bacterium’s initial colonization of the light organ (28), the ability to ferment chitin sugars becomes critical for the symbiont to maintain its association in the adult host (29). Oxygen limitation is also likely to accompany the transition from the immature to mature symbiotic state (16); therefore, during this transition, it is possible that the provision of chitin sugars may function to change the relative levels of symbiont growth, oxygen consumption, and luminescence (Fig. 5). In the present study, we highlight three areas of V. fischeri physiology that are both subject to regulation associated with EIIAcrr-dependent PTS transport and contribute to light organ colonization: (i) prioritization of chitin sugar transport, (ii) modulation of metabolic functions regulated by quorum sensing, and (iii) repression of aerobic respiration (Fig. 5).
FIG 5 .
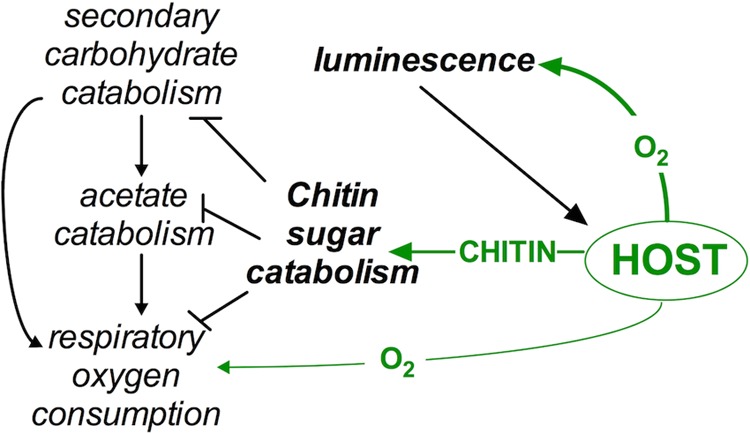
Impact of chitin sugars on symbiont physiology. Host-derived chitin is hydrolyzed to form chitin sugars. Chitin sugar catabolism by V. fischeri inhibits secondary (i.e., non-PTS) carbohydrate catabolism, acetate uptake, and respiratory oxygen consumption. The result of this physiological shift is an increased availability of host-provided oxygen to the symbiont’s luminescence-producing enzyme, luciferase. Luminescence, which is used in the squid’s behavior, is required to support a sustained colonization of the light organ by V. fischeri.
The integration of chitin sugar transport with the EIIAcrr-dependent PTS ensures that catabolism of these sugars is favored in V. fischeri. We demonstrated that one of the outcomes of EIIAcrr-dependent transport is a decrease in the uptake of non-PTS carbohydrates, such as glycerol (Fig. 1A). This phenotype is consistent with the inducer-exclusion mechanism described for other Gram-negative organisms (33). Notably, homologs of the V. fischeri EIIAcrr in E. coli and Salmonella enterica serovar Typhimurium posttranslationally regulate adenylate cyclase activity, which contributes to cyclic AMP-mediated transcriptional regulation by Crp (33). However, due to a highly active cyclic AMP phosphodiesterase in V. fischeri, evidence for conservation of such a function in this species’ EIIAcrr remains elusive (E. Stabb and D. Colton, personal communication). The transport of GlcNAc by the EIIAcrr-dependent PTS is a shared trait of the Vibrionaceae (25); however, our studies suggest an expansion of this route of transport in V. fischeri, relative to that in V. furnisii and V. cholerae. Specifically, the chitin sugars chitobiose and glucosamine require EIIAcrr for transport. In the context of the squid-vibrio symbiosis, the expanded repertoire of PTS-dependent chitin sugars presents a flexible strategy for a single type of host glycan, chitin, to regulate symbiont physiology: if chitin sugars are present in the environment, inducer exclusion will ensure that they will be preferentially used by symbionts before other carbohydrate nutrients, such as glycerol.
A second example of how this class of host-derived glycans exerts metabolic control over the bacterial partner is the ability of chitin sugars to override quorum-induced group behaviors of symbionts. Specifically, transcriptional activation of the V. fischeri acs gene by AinSR integrates the acetate switch into the quorum-signaling network (31) and indicates the important link between acetate homeostasis and the symbiont’s activities in the light organ. Indeed, deletion of acs, which encodes the cell’s key acetate uptake enzyme, results in decreased representation of this mutant during cocolonization with wild-type V. fischeri (31). Here, we demonstrated that, even at high cell density, the repression of acs transcription by chitin sugar catabolism can fully counteract the activation of acs by quorum signaling (Fig. 2). Previous studies have demonstrated that the ainSR locus is, itself, regulated by the transcription factor Crp (37), a transcription factor that is responsive to EIIAcrr-dependent PTS transport in Gram-negative bacteria (33). Chitin sugars are a prominent nutrient provided by the adult host each night (29, 38); thus, while AinSR quorum signaling may have a dominant effect on the symbiont population in a juvenile organ (39), its contribution to acetate homeostasis by symbionts in the adult may be confined to that part of the diel cycle when chitin sugars are not present (29). This finding illustrates how a host can override quorum-signaled events between bacteria by redirecting their metabolism.
Finally, host-derived chitin sugar utilization by V. fischeri results in a metabolic strategy that reduces respiratory activity by cytochrome oxidase (Fig. 3C). We predict that this reduction increases the availability of key substrates for bacterial luciferase, such as oxygen and reducing power (Fig. 5). Like its respiratory oxidases, the luciferase of V. fischeri has an affinity for oxygen in the nanomolar range (16), suggesting that these oxidases directly compete for this reactant. Because symbionts must produce luminescence to maintain their colonization of the light organ (13, 15), within this environment their physiology must favor light emission, at least at night. We predict that host provision of chitin sugars to symbionts will both decrease respiratory activity and promote luminescence (Fig. 5), thereby supporting the functional basis of the squid-vibrio mutualism (11).
Taken as a whole, our work highlights the contribution that the presence of specific glycans in the host environment can make to the physiology of a coevolved symbiont. Glycans are emerging as a class of key regulatory nutrients in diverse host-microbe interactions. For example, the uptake and metabolism of PTS carbohydrates is a central component of the microbial ecology within the human small intestine (40); similarly, the prioritization of host-derived polysaccharide and glycan catabolism by the Bacteroides thetaiotaomicron starch utilization system is a crucial regulatory point in the primary colonization of the mammalian gut (41). The fatty acids produced by Bacteroides spp. (e.g., butyrate and acetate) during starch fermentation are potent immunomodulators of host macrophage function (42). PTS transport also contributes to the regulation of virulence traits in several enteric pathogens (43), including Vibrio cholerae (25, 44). Notably, in both the mouse gut (45) and the squid light organ (29), cells of the innate immune system contribute to the regulation of host glycan delivery to symbionts. Thus, host-derived chitin sugars, like other nutrients, may be key to elucidating the mechanisms governing stability and dysbiosis of symbiotic microbiota in general.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Strains and plasmids used in this work are listed in Table S1 in the supplemental material. V. fischeri strains were derived from strain MJM1100 (46, 47). Unless otherwise noted, cultures were grown at 28°C, with shaking at 225 rpm, in 17- by 100-mm test tubes containing 3 ml of either seawater-tryptone medium (SWT) (46), high-osmolarity SWT (SWTO) medium (48), or Luria-Bertani salt medium (LBS) (49), as noted. Escherichia coli strains were grown in Luria-Bertani (LB) medium (50) containing 1.5% agar for solid media. When appropriate, antibiotics were added to LB and LBS media at the following concentrations: chloramphenicol (Cam), 5 µg/ml for V. fischeri and 25 µg/ml for E. coli; erythromycin (Erm), 5 µg/ml for V. fischeri; kanamycin (Kan), 100 µg/ml for V. fischeri. Medium reagents were purchased from Thermo, Fisher Scientific (Waltham, MA, United States), and Sigma-Aldrich (St. Louis, MO, United States).
β-Galactosidase assay.
The β-galactosidase activity assays were preformed as described previously (31). Briefly, cells were grown overnight in LBS medium with antibiotic and then subcultured (1:200) in duplicate tubes of SWTO medium. To collect samples, 1 ml of culture was pelleted for 15 min at 1,300 rpm and 4°C in a refrigerated tabletop microcentrifuge. Supernatants were decanted, and cell pellets were stored at −20°C. β-Galactosidase activity was determined using a microtiter dish method modified from that of Slauch and Silhavy (51). Briefly, the SDS-chloroform step was omitted, and the A420 values of the microtiter wells were read every 30 s for 1 h using a GENiosPro 96-well plate reader (Tecan, Research Triangle Park, NC). Three replicates of two dilutions were tested for each cell pellet. The relative units of β-galactosidase activity were calculated using the following formula: Vmax/(OD × volume). Blank vector controls revealed no nonspecific β-galactosidase activity under any condition tested.
Contribution of glycerol to target promoter activity.
Strains carrying either the PglpFK LacZ transcriptional reporter plasmid pJAS223 or the empty parent vector (pADK701) were cultured in SWTO to an optical density at 600 nm (OD600) of 0.5 ± 0.1 (mean ± standard deviation; equivalent to 107 CFU per ml), then subcultured once more after dilution (1:100) into fresh SWTO made either with no glycerol or with 3.8 g/liter glycerol. Cultures were incubated for 2 h under these experimental conditions prior to sample collection.
Contribution of chitin sugars to target promoter activity.
Duplicate cultures of the LacZ transcriptional reporters pJAS223 (PglpFK), pJAS224 (Pacs), or a blank parent vector (pAKD701) were grown to an OD of 0.5 ± 0.1. Then, 20 mM GlcNAc was added to one of the two sets of cultures. Cells were then incubated 1 h prior to sample collection.
Contribution of chitin sugars and C8-HSL quorum sensing to target promoter activity.
Cultures of either the Pacs LacZ transcriptional reporter plasmid, pJAS224, or a blank parent vector (pAKD701) were grown to an OD of 0.5 ± 0.1. Each culture was then diluted (1:100) into four tubes of SWTO medium, each containing separate additions: (i) 20 mM GlcNAc, (ii) 100 nM C8-HSL, diluted from a stock in dimethyl sulfoxide, (iii) GlcNAc and C8-HSL, or (iv) no addition. The cultures were incubated for 2 h prior to sample collection.
Growth curve assays.
Cells were grown in LBS medium to an OD of 0.5 ± 0.1 and then diluted to an OD of 0.02 in fresh experimental medium. Depending on the experimental condition, cells were diluted into 0.1× LBS (1/10 the concentration of yeast extract and tryptone and the normal concentrations of salts and buffer), or MSM buffered at pH 7.5 with piperazine-N,N'-bis(2-ethanesulfonic acid) (PIPES). Where indicated, cultures were supplemented with 20 mM of either glucose, GlcNAc, or NANA. To monitor growth in test tubes or flasks, either 3-ml cultures were placed in a 17- by 100-mm test tube or 10-ml cultures were added to a 125-ml flask; the tubes or flasks were incubated at 28°C with agitation at 225 rpm in an Innova 4080 shaker (New Brunswick, CT, United States), and the OD was measured at 600 nm with a Biophotometer (WPA CO 8000 cell density meter; Biochrom, United Kingdom). Where indicated, samples of the cultures were plated for enumeration of CFU by serial dilution in 70% Instant Ocean (United Pet Group, Blacksburg, VA) and plated onto LBS agar. To monitor growth in 1 ml of medium in 24-well plates, absorbance at 600 nm was monitored using a GENiosPro 24-well plate reader (Tecan, Research Triangle Park, NC). The plates were shaken continuously, and absorbance readings were corrected for a nonstandard path length by linear transformation. Each test was done in triplicate. Further methodological details can be found in Text S1 in the supplemental material.
Cell size determination.
Wild-type and Δcco mutant V. fischeri cells were cultured in 3 ml LBS overnight and then subcultured by 1:100 dilution into fresh LBS medium and grown to an OD of 0.5 ± 0.1, to obtain a homogenous population of cells growing in steady state. The cells were subcultured again into LBS containing either 20 mM GlcNAc, 20 mM glucose, or no addition. As the cultures grew, 5-µl aliquots were spotted onto a glass slide and visualized with an Axio Imager M2 epifluorescence scope (Zeiss Intl., Germany). The cell length was measured at 100× magnification using Axio Vision 4.8 software (Zeiss). Three replicates, each consisting of 40 cell length measurements, were tested for the strains under each growth condition.
Respirometry.
Cultures growing in LBS at steady state (OD, 0.5 ± 0.1) were subcultured (1:100) into 3 ml of either LBS or 20 mM GlcNAc MSM buffered at pH 7.5 with 50 mM PIPES in an 18-mm test tube. Two milliliters of this culture was transferred to a respirometry vial, and the oxygen concentration and OD were measured over time by using an oxygen probe (oxygen microsensor; Unisense, Denmark) and a biophotometer (WPA CO 8000 cell density meter; Biochrom, United Kingdom), respectively. Three biological replicates were tested for each condition. The rate of oxygen consumption was calculated by the following formula: ΔO2(t0 − tn)/ΔOD(tn − t0), where t0 is the time the measurements started, and tn is the time the measurements ended.
Squid colonization assays.
V. fischeri strains were cultivated as overnight cultures in 3 ml LBS containing antibiotic selection, as appropriate. Strains were subcultured (1:100) into SWT medium and grown to an OD of 0.5 ± 0.1 (equivalent to 107 CFU per ml) for use as seawater inocula. To colonize squid, newly hatched animals were exposed for 16 h to 3,000 CFU/ml of V. fischeri in filter-sterilized instant ocean (FSW). The relative ability of V. fischeri strains to colonize was determined by creating mixed inocula of wild-type V. fischeri (carrying green fluorescent protein [GFP] and Erm resistance genes at the Tn7 site; TIM302) and the appropriate mutant strain carrying only the Erm resistance gene at the Tn7 site. A separate cocolonization of TIM302 and wild-type V. fischeri carrying only the gene for Ermr (TIM313) was included to control for carriage of GFP. To control for the possibility of a secondary site mutation, TIM302 was also competed against a complemented strain carrying the gene and its native promoter region in cis at the Tn7 site of the mutant strain. All animals were transferred to fresh, uninoculated FSW at 16 h and again at approximately 40 h after inoculation. To compete strains in the presence of chitin sugars, the FSW was supplemented with 20 mM GlcNAc following the 16-h initial inoculation, and the water was subsequently exchanged for fresh 20 mM GlcNAc–FSW at least every 12 h. At 48 h postinoculation, individual animal luminescence was determined using a TD 20/20 luminometer (Turner Design, Sunnyvale, CA), and the animals were frozen for surface sterilization. Animals were homogenized, and the homogenate was plated to determine the CFU per light organ. Uninoculated animals had no detectible luminescence or CFU in all experiments.
Growth competition in culture.
Wild-type V. fischeri, containing a chromosomal copy of the GFP gene in the Tn7 site (52, 53), was coinoculated with one of several mutant strains or a wild-type marker control carrying only the Erm resistance gene at the Tn7 site. For each pairing of strains, growth competition was determined in 0.1× SWTO (1/10 the concentration of yeast extract, tryptone, and glycerol with the normal concentration of salts and buffer; containing 50 mM Tris-HCl, pH 7.5) either with or without the addition of 20 mM GlcNAc. The resulting cultures were incubated in a 24-well polystyrene plate at 28°C with continuous shaking in a GENioPro plate reader (Tecan, Research Triangle Park, NC). At 0 and 3 h of incubation, 50-µl aliquots of cultures were removed from the test plate, serially diluted, and plated to determine the CFU of both strains in culture. The relative competitive index of the two strains was calculated by dividing the ratio of the two strains at 3 h by the ratio at 0 h.
SUPPLEMENTAL MATERIAL
Diagram of central metabolic pathways described in this study that are affected by chitin sugar catabolism. Download
Relative promoter activity for the glpFK locus during growth in a tryptone-based medium in the absence or presence of 20 mM of either GlcN or chitobiose. Download
Characterization of cytochrome oxidase-deficient V. fischeri and the contribution of GlcNAc to oxygen consumption. Download
Cocolonization competition in the squid light organ during the first 48 h after inoculation. Download
Strains and plasmids used in this study.
Supplemental methods. Download
ACKNOWLEDGMENTS
We thank E. Stabb and D. Colton for contributive discussions. We also thank H. Blackwell and her research group for the gift of synthetic octanoyl homoserine lactone (C8-HSL).
This work was funded by NIH grants RR12294/OD11024 to E.G.R. and M. McFall-Ngai and AI050661 to M. McFall-Ngai and E.G.R., as well as NSF grant MCB1050687 to A.K.D. M.P. was funded by a China Scholarship Council State Scholarship Fund Award. J.A.S. was funded by an NSF Graduate Research Fellowship, the Chemical Biology Training Program (UW Madison, NIH-NIGMS T32 GM008505), and the Microbes in Health and Disease Training Program (UW Madison NIH-NIAID T32 AI055397).
Footnotes
Citation Pan M, Schwartzman JA, Dunn AK, Lu Z, Ruby EG. 2015. A single host-derived glycan impacts key regulatory nodes of symbiont metabolism in a coevolved mutualism. mBio 6(4):e00811-15. doi:10.1128/mBio.00811-15
REFERENCES
- 1.McFall-Ngai MJ. 2014. The importance of microbes in animal development: lessons from the squid-vibrio symbiosis. Annu Rev Microbiol 68:177–194. doi: 10.1146/annurev-micro-091313-103654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maynard CL, Elson CO, Hatton RD, Weaver CT. 2012. Reciprocal interactions of the intestinal microbiota and immune system. Nature 489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert SF. 2014. A holobiont birth narrative: the epigenetic transmission of the human microbiome. Front Genet 5:282. doi: 10.3389/fgene.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douglas AE. 2014. Molecular dissection of nutrient exchange at the insect-microbial interface. Curr Opin J Insect Sci 4:23–28. doi: 10.1016/j.cois.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Pacheco AR, Barile D, Underwood MA, Mills DA. 2015. The impact of the milk glycobiome on the neonate gut microbiota. Annu Rev Animal Biosci 3:419–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comstock LE. 2009. Importance of glycans to the host-Bacteroides mutualism in the mammalian intestine. Cell Host Microbe 5:522–526. doi: 10.1016/j.chom.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Engel P, Moran NA. 2013. Functional and evolutionary insights into the simple yet specific gut microbiota of the honey bee from metagenomic analysis. Gut Microbes 4:60–65. doi: 10.4161/gmic.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson MC, Graf J. 2012. Bacterial symbioses of the medicinal leech Hirudo verbana. Gut Microbes 3:322–331. doi: 10.4161/gmic.20227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McFall-Ngai M. 2014. Divining the essence of symbiosis: insights from the squid-vibrio model. PLoS Biol 12:e1001783. doi: 10.1371/journal.pbio.1001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KH, Ruby EG. 1994. Effect of the squid host on the abundance and distribution of symbiotic Vibrio fischeri in nature. Appl Environ Microbiol 60:1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stabb EV, Visick KL. 2013. Vibrio fischeri:squid symbiosis, p 497–532. In Rosenberg E, Delong EF, Lory S, Stackebrandt E, Thompson F (ed), The prokaryotes. Springer, Berlin, Germany. [Google Scholar]
- 12.Verma SC, Miyashiro T. 2013. Quorum sensing in the squid-vibrio symbiosis. Int J Mol Sci 14:16386–16401. doi: 10.3390/ijms140816386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch EJ, Miyashiro T, McFall-Ngai MJ, Ruby EG. 2014. Features governing symbiont persistence in the squid-vibrio association. Mol Ecol 23:1624–1634. doi: 10.1111/mec.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visick Kl FJ, Doino J, McFall-Ngai M, Ruby EG. 2000. Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J Bacteriol 182:4578–4586. doi: 10.1128/JB.182.16.4578-4586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Studer SV, Schwartzman JA, Ho JS, Geske GD, Blackwell HE, Ruby EG. 2014. Non-native acylated homoserine lactones reveal that LuxIR quorum sensing promotes symbiont stability. Environ Microbiol 16:2623–2634. doi: 10.1111/1462-2920.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunn AK. 2012. Vibrio fischeri metabolism: symbiosis and beyond. Adv Microb Physiol 61:37–68. doi: 10.1016/B978-0-12-394423-8.00002-0. [DOI] [PubMed] [Google Scholar]
- 17.Mandel MJ, Schaefer AL, Brennan CA, Heath-Heckman EA, DeLoney-Marino CR, McFall-Ngai MJ, Ruby EG. 2012. Squid-derived chitin oligosaccharides are a chemotactic signal during colonization by Vibrio fischeri. Appl Environ Microbiol 78:4620–4626. doi: 10.1128/AEM.00377-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oldroyd GE. 2013. Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nat Rev Microbiol 11:252–263. doi: 10.1038/nrmicro2990. [DOI] [PubMed] [Google Scholar]
- 19.Killiny N, Prado SS, Almeida RP. 2010. Chitin utilization by the insect-transmitted bacterium Xylella fastidiosa. Appl Environ Microbiol 76:6134–6140. doi: 10.1128/AEM.01036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaston J, Goodrich-Blair H. 2010. Common trends in mutualism revealed by model associations between invertebrates and bacteria. FEMS Microbiol Rev 34:41–58. doi: 10.1111/j.1574-6976.2009.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aluwihare LI, Repeta DJ, Pantoja S, Johnson CG. 2005. Two chemically distinct pools of organic nitrogen accumulate in the ocean. Science 308:1007–1010. doi: 10.1126/science.1108925. [DOI] [PubMed] [Google Scholar]
- 22.Hunt DE, Gevers D, Vahora NM, Polz MF. 2008. Conservation of the chitin utilization pathway in the Vibrionaceae. Appl Environ Microbiol 74:44–51. doi: 10.1128/AEM.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meibom KL, Li XB, Nielsen AT, Wu C-Y, Roseman S, Schoolnik GK. 2004. The Vibrio cholerae chitin utilization program. Proc Natl Acad Sci U S A 101:2524–2529. doi: 10.1073/pnas.0308707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bassler BL, Yu C, Lee YC, Roseman S. 1991. Chitin utilization by marine bacteria. Degradation and catabolism of chitin oligosaccharides by Vibrio furnissii. J Biol Chem 266:24276–24286. [PubMed] [Google Scholar]
- 25.Blokesch M. 2012. Chitin colonization, chitin degradation and chitin-induced natural competence of Vibrio cholerae are subject to catabolite repression. Environ Microbiol 14:1898–1912. doi: 10.1111/j.1462-2920.2011.02689.x. [DOI] [PubMed] [Google Scholar]
- 26.Houot L, Chang S, Absalon C, Watnick PI. 2010. Vibrio cholerae phosphoenolpyruvate phosphotransferase system control of carbohydrate transport, biofilm formation, and colonization of the germfree mouse intestine. Infect Immun 78:1482–1494. doi: 10.1128/IAI.01356-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kremer N, Philipp EE, Carpentier M-C, Brennan CA, Kraemer L, Altura MA, Augustin R, Häsler R, Heath-Heckman EA, Peyer SM, Schwartzman J, Rader BA, Ruby EG, Rosenstiel P, McFall-Ngai MJ. 2013. Initial symbiont contact orchestrates host-organ-wide transcriptional changes that prime tissue colonization. Cell Host Microbe 14:183–194. doi: 10.1016/j.chom.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyashiro T, Klein W, Oehlert D, Cao X, Schwartzman J, Ruby EG. 2011. The N-acetyl-d-glucosamine repressor NagC of Vibrio fischeri facilitates colonization of Euprymna scolopes. Mol Microbiol 82:894–903. doi: 10.1111/j.1365-2958.2011.07858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartzman JA, Koch E, Heath-Heckman EA, Zhou L, Kremer N, McFall-Ngai MJ, Ruby EG. 2015. The chemistry of negotiation: rhythmic, glycan-driven acidification in a symbiotic conversation. Proc Natl Acad Sci U S A 112:566–571. doi: 10.1073/pnas.1418580112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kremer N, Schwartzman J, Augustin R, Zhou L, Ruby EG, Hourdez S, McFall-Ngai MJ. 2014. The dual nature of haemocyanin in the establishment and persistence of the squid–vibrio symbiosis. Proc Biol Sci 281:20140504. doi: 10.1098/rspb.2014.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Studer SV, Mandel MJ, Ruby EG. 2008. AinS quorum sensing regulates the Vibrio fischeri acetate switch. J Bacteriol 190:5915–5923. doi: 10.1128/JB.00148-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfe AJ. 2005. The acetate switch. Microbiol Mol Biol Rev 69:12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deutscher J, Francke C, Postma PW. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev 70:939–1031. doi: 10.1128/MMBR.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keyhani NO, Roseman S. 1999. Physiological aspects of chitin catabolism in marine bacteria. Biochim Biophys Acta 1473:108–122. doi: 10.1016/S0304-4165(99)00172-5. [DOI] [PubMed] [Google Scholar]
- 35.Dunn AK, Karr EA, Wang Y, Batton AR, Ruby EG, Stabb EV. 2010. The alternative oxidase (AOX) gene in Vibrio fischeri is controlled by NsrR and upregulated in response to nitric oxide. Mol Microbiol 77:44–55. doi: 10.1111/j.1365-2958.2010.07194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Visick KL, O’Shea TM, Klein AH, Geszvain K, Wolfe AJ. 2007. The sugar phosphotransferase system of Vibrio fischeri inhibits both motility and bioluminescence. J Bacteriol 189:2571–2574. doi: 10.1128/JB.01761-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyell NL, Colton DM, Bose JL, Tumen-Velasquez MP, Kimbrough JH, Stabb EV. 2013. Cyclic AMP receptor protein regulates pheromone-mediated bioluminescence at multiple levels in Vibrio fischeri ES114. J Bacteriol 195:5051–5063. doi: 10.1128/JB.00751-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wier AM, Nyholm SV, Mandel MJ, Massengo-Tiassé RP, Schaefer AL, Koroleva I, Splinter-BonDurant S, Brown B, Manzella L, Snir E, Almabrazi H, Scheetz TE, Bonaldo Mde F, Casavant TL, Soares MB, Cronan JE, Reed JL, Ruby EG, McFall-Ngai MJ. 2010. Transcriptional patterns in both host and bacterium underlie a daily rhythm of anatomical and metabolic change in a beneficial symbiosis. Proc Natl Acad Sci U S A 107:2259–2264. doi: 10.1073/pnas.0909712107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lupp C, Ruby EG. 2005. Vibrio fischeri uses two quorum-sensing systems for the regulation of early and late colonization factors. J Bacteriol 187:3620–3629. doi: 10.1128/JB.187.11.3620-3629.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zoetendal EG, Raes J, van den Bogert B, Arumugam M, Booijink CC, Troost FJ, Bork P, Wels M, de Vos WM, Kleerebezem M. 2012. The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J 6:1415–1426. doi: 10.1038/ismej.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonnenburg JL, Xu J, Leip DD, Chen C-H, Westover BP, Weatherford J, Buhler JD, Gordon JI. 2005. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307:1955–1959. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 42.Chang PV, Hao L, Offermanns S, Medzhitov R. 2014. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A 111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Görke B, Stülke J. 2008. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol 6:613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- 44.Hang S, Purdy AE, Robins WP, Wang Z, Mandal M, Chang S, Mekalanos JJ, Watnick PI. 2014. The acetate switch of an intestinal pathogen disrupts host insulin signaling and lipid metabolism. Cell Host Microbe 16:592–604. doi: 10.1016/j.chom.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goto Y, Obata T, Kunisawa J, Sato S, Ivanov II, Lamichhane A, Takeyama N, Kamioka M, Sakamoto M, Matsuki T, Setoyama H, Imaoka A, Uematsu S, Akira S, Domino SE, Kulig P, Becher B, Renauld JC, Sasakawa C, Umesaki Y. 2014. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 345:1254009. doi: 10.1126/science.1254009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boettcher KJ, Ruby EG. 1990. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J Bacteriol 172:3701–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandel MJ, Stabb EV, Ruby EG. 2008. Comparative genomics-based investigation of resequencing targets in Vibrio fischeri: focus on point miscalls and artefactual expansions. BMC Genomics 9:138. doi: 10.1186/1471-2164-9-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stabb EV, Butler MS, Adin DM. 2004. Correlation between osmolarity and luminescence of symbiotic Vibrio fischeri strain ES114. J Bacteriol 186:2906–2908. doi: 10.1128/JB.186.9.2906-2908.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graf J, Dunlap PV, Ruby EG. 1994. Effect of transposon-induced motility mutations on colonization of the host light organ by Vibrio fischeri. J Bacteriol 176:6986–6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sambrook JF, Fritsch J, Maniantis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Press, Cold Spring Harbor, NY. [Google Scholar]
- 51.Slauch JM, Silhavy TJ. 1991. cis-acting ompF mutations that result in OmpR-dependent constitutive expression. J Bacteriol 173:4039–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCann J, Stabb EV, Millikan DS, Ruby EG. 2003. Population dynamics of Vibrio fischeri during infection of Euprymna scolopes. Appl Environ Microbiol 69:5928–5934. doi: 10.1128/AEM.69.10.5928-5934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyashiro T, Wollenberg MS, Cao X, Oehlert D, Ruby EG. 2010. A single qrr gene is necessary and sufficient for LuxO-mediated regulation in Vibrio fischeri. Mol Microbiol 77:1556–1567. doi: 10.1111/j.1365-2958.2010.07309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diagram of central metabolic pathways described in this study that are affected by chitin sugar catabolism. Download
Relative promoter activity for the glpFK locus during growth in a tryptone-based medium in the absence or presence of 20 mM of either GlcN or chitobiose. Download
Characterization of cytochrome oxidase-deficient V. fischeri and the contribution of GlcNAc to oxygen consumption. Download
Cocolonization competition in the squid light organ during the first 48 h after inoculation. Download
Strains and plasmids used in this study.
Supplemental methods. Download