Abstract
The majority of clinical studies on moyamoya disease (MMD) have focused on anterior circulation. The disease involvement of posterior circulation in MMD, mainly in the posterior cerebral artery (PCA), has been mentioned since the early 1980s, and it has been repeatedly emphasized as one of the most important factors related to poor prognosis in MMD. However, its clinical features and outcome have only been elucidated during the last few years. In this review, the angiographic definition of PCA stenosis is summarized. The clinical features are elucidated as being either early-onset or delayed-onset, according to the time of PCA stenosis diagnosis in reference to the anterior circulation revascularization surgeries. The surgical strategy and hypothesis on the mechanism of PCA stenosis is also briefly mentioned. It appears that some MMD patients may show PCA stenosis during the early or late course of the disease and that the presenting symptoms may vary. Because the hemodynamic compromise caused by PCA stenosis may respond well to surgical treatment, clinicians should be aware of the condition, especially during follow-up of MMD patients.
Keywords: Moyamoya disease, Posterior cerebral artery, Posterior circulation
INTRODUCTION
Moyamoya disease (MMD) was initially defined as 'steno-occlusive changes of the carotid fork with abnormal vascular network in the base of the brain"21). However, the steno-occlusive changes were also noted in posterior circulation, mainly the posterior cerebral artery (PCA)13,16). MMD has been widely described as a steno-occlusive disease involving the major intracranial arteries since the late 1990s2). Additionally, clinical series on MMD have repeatedly shown PCA stenosis to be one of the most significant factors related to poor outcome8,9,17). However, the majority of clinical studies on MMD have focused on anterior circulation, and clinical features of posterior circulation in MMD have not been elucidated thoroughly. Reports on the symptoms and surgical outcome of PCA stenosis have started to increase during the last few years. Some recent papers on the progressive PCA stenosis found during follow-up of patients after revascularization of the anterior circulation are noteworthy7,10,17).
In this review, the radiologic features and surgical treatment of the PCA territory in MMD will be described. The presentation of PCA involvement in MMD in the initial, preoperative period and during long-term follow-up after anterior circulation revascularization surgery will be addressed separately.
DEFINITION OF PCA STENOSIS
Angiographic definition of the stenosis of the PCA may vary between reports due to the progressive nature of the disease, the role of the PCA as a source of the leptomeningeal collateral to the anterior circulation territory, and the unfamiliarity of the concept. Just as the Suzuki grade describes the progression of stenosis in the internal carotid artery (ICA), anterior cerebral artery (ACA), and middle cerebral artery (MCA), the stenosis of the PCA also varies in degree of severity as follows : 1) normal, 2) slightly involved : small stenotic lesion in the quadrigeminal segment of the PCA with normal filling of most of the cortical branches of the PCA, 3) moderately involved : only few cortical branches of the PCA are visualized, and 4) severely involved : occlusive lesion progresses into the trunk of the basilar artery, or into the vertebral arteries13). Another group later proposed the following criteria, focusing on the stenosis and the presence of PCA moyamoya vessels (similar to the Suzuki grade): 1) Stage 1 : No steno-occlusive changes in the PCA, 2) Stage 2 : Stenosis of the PCA with absent or slightly developed PCA moyamoya, with relatively good visualization of PCA branches, 3) Stage 3 : Severe stenosis or virtually complete occlusion of the PCA with well-developed PCA moyamoya : a few PCA branches are opacified through PCA moyamoya, and 4) Stage 4 : Complete occlusion of the PCA with small amount of PCA moyamoya, without opacification of any apparent PCA branches18). Other reports have used the simplified classification of 1) normal and 2) stenosis11,19). The issue becomes even more complicated when the hemodynamic status of the corresponding territory is considered. Abundant leptomeningeal collaterals may be present even when the PCA is stenotic when the hemodynamic need in the anterior circulation territory is strong enough. Therefore, a combined interpretation of the morphological status of the PCA with the hemodynamic status of the PCA territory is necessary to decide on the clinically relevant, functional stenosis of the PCA.
EARLY-ONSET PCA STENOSIS (BEFORE ANTERIOR CIRCULATION REVASCULARIZATION SURGERY)
Approximately 20-30% of pediatric MMD patients present with PCA stenosis at the time of the initial diagnosis5,14,17). At that time, the symptoms of the patient are dominated by the anterior circulation territory and mainly consist of motor-type transient ischemic attacks (TIAs). PCA-specific symptoms are related to the visual function and include visual field defects, decreased visual acuity, transient blindness, scotomas, etc.14). However, not all patients with hemodynamic insufficiency in the PCA territory (evident on image studies) may present with the "PCA-specific" symptoms.
Similar to the ACA territory, the choice to manage the PCA territory insufficiency surgically seems to vary between centers. Previous reports have mentioned that 'PCA-specific' symptoms may be resolved by surgical revascularization of the MCA territories5,13). Others have supported the feasibility and good results of additional PCA territory revascularization1). It is noteworthy that one of the first reports on the PCA in MMD mentioned that the indirect redistribution effect may be accounted for when the PCA territory insufficiency is mainly due to the 'vascular steal' through the collaterals supplying the anterior circulation. However, because the anterior circulation revascularization surgery does not lead to direct revascularization of the visual cortex, direct revascularization surgery of the visual cortex is needed in cases of persistent progression of PCA stenosis to prevent permanent infarction.
Several features on the early-onset PCA stenosis have been elucidated. First, a strong correlation between the presence of infarction (regardless of the vascular territory) and PCA stenosis have been repeatedly shown17,19,22,23). Interestingly, no such correlation has been noted between the severity of ICA stenosis and the occurrence of parenchymal ischemic lesions. This may be explained by the role of the PCA as the supplier of leptomeningeal feeders to the anterior circulation. In other words, as PCA stenosis progresses, the compensatory blood flow to the ischemic brain through the leptomeningeal collateral decreases and results in infarction. In addition, similar association between the severity of ICA steno-occlusion and the frequency of PCA stenosis has been suggested5,22). Those with PCA stenosis tended to be in a more advanced stage of ICA stenosis. However, steno-occlusion of the PCA was observed even in those with less advanced ICA in younger pediatric patients, suggesting a weaker correlation between the ICA and PCA disease progression in the younger group5). Finally, PCA involvement is one of the most important factors related to poor prognosis in MMD9). In fact, correlation between homozygous c.14576G>A variant of the ring finger protein 213 and early onset and aggressive PCA involvement has recently been shown, providing evidence for the clinical significance of PCA stenosis as a prognostic factor15).
DELAYED-ONSET PCA STENOSIS (AFTER ANTERIOR CIRCULATION REVASCULARIZATION SURGERY)
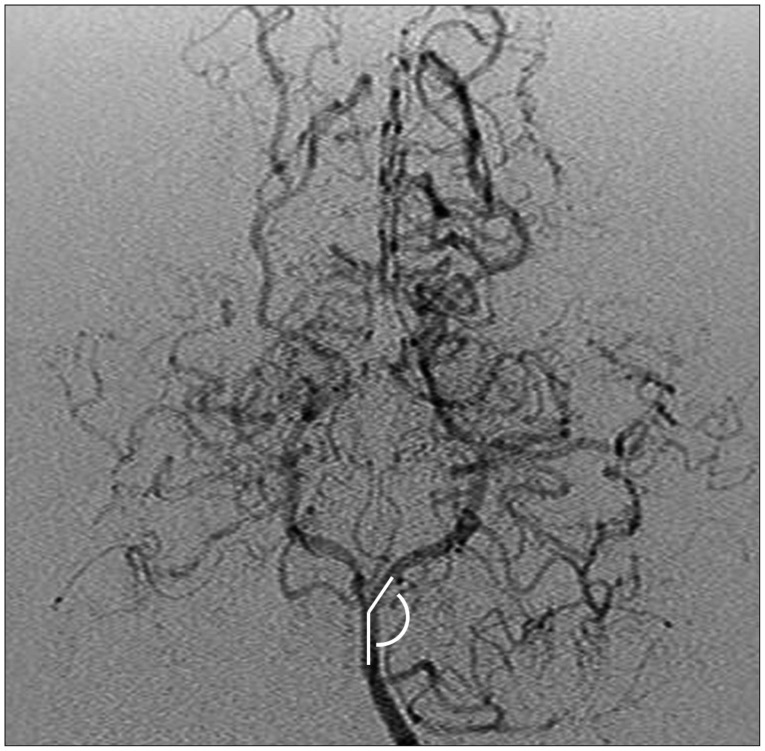
As the data on long-term follow-up of MMD patients accumulates, studies on progressive changes of the PCA have recently increased1,3,4,5,6,7,12). The possibility of progressive stenosis of the PCA was mentioned in one of the earliest reports focusing on the posterior circulation in MMD14). The report specifically elaborated on a patient that initially underwent anterior circulation revascularization surgery and subsequently underwent additional surgery of the PCA 5 years later. However, the incidence and clinical features of delayed-onset PCA stenosis have not been addressed until recently. One study involving 23 patients reported progression of angiographic PCA staging in 47.7% and symptomatic PCA progression in 21.0% of patients during a minimum follow-up of 3 years after anterior circulation revascularization surgery7). Another study retrospectively reviewed the postoperative follow-up course (mean follow-up : 8.3 years) of 88 patients, and 29 of the 166 PCAs showed radiologic progression while 15 of them were symptomatic and needed surgical treatment11). Although the incidence rate is somewhat different between studies, progressive PCA stenosis in MMD after anterior circulation revascularization surgery is not a rare event but an expected course in a fair number of patients. The study by Lee et al.11) investigated the preoperative factors related to progressive stenosis of the PCA through evaluation of the clinical course of 88 MMD patients. Multivariate analysis revealed that presence of infarction at the time of diagnosis and a smaller angle between the P1 segment of the PCA and the basilar artery (Fig. 1) were statistically significant factors. Younger age at the time of diagnosis, the other most important prognostic factor in MMD besides PCA stenosis, was marginally significant by univariate analysis (p=0.054) and not significant by multivariate evaluation. A smaller angle between the PCA and basilar artery was suspected to create more shear stress on the vessel wall according to a previous computational study20), possibly resulting in stenosis of the PCA in MMD patients.
Fig. 1. The angle between basilar artery and P1 segment of PCA. PCA : posterior cerebral artery.
The presenting symptoms, time between the anterior circulation revascularization surgeries and detection of symptomatic PCA stenosis in this postoperative group of patients, differed by the type of initial operation. When anterior circulation was not addressed in the initial surgery, the patients presented with a motor-type transient ischemic attack, similar to symptoms of anterior circulation ischemia7). When the anterior circulation was revascularized, initially the most common symptom was headache, followed by visual symptoms12). Additionally, when the initial surgery did not include the ACA territory, patients developed new symptoms due to PCA stenosis by 18 months on average after initial diagnosis7). In the series where ACA territory was revascularized, the average time interval was 4.9 years11). However, in the latter study, it was emphasized that the 'time event' of PCA stenosis was not condensed to some specific time window but was rather dispersed throughout the follow-up (earliest : 1.8 years, latest : 10.8 years).
A recent study focused specifically on patients who developed progressive, symptomatic PCA stenosis during the follow-up after revascularization of the ACA and MCA territories showed that the diagnosis of the condition may be rather challenging10). At the initial time of diagnosis of the disease, the majority of the patients will present with the typical motor-type TIA, and their images will represent hemodynamic insufficiency and ICA stenosis. However, the symptom and clinical course of the patients with delayed PCA stenosis may vary widely between patients, thereby making the diagnosis difficult. The symptoms are not 'typical' (headache may be caused by various reasons in MMD patients), and the results of the noninvasive imaging are not as uniform as at the initial diagnosis. Whereas 84% of patients showed decreased reserve on diamox single photon emission computed tomography (d-SPECT) at the initial diagnosis of MMD9), only 41% of patients showed 'diagnostic' findings on d-SPECT at the time of delayed PCA stenosis10). Although perfusion MRI was as diagnostic for the delayed PCA stenosis (79%)10) as for the initial diagnosis (89%)9), the inconsistency of the work-up results and atypical symptoms halts the use of gold standard, invasive angiography during the follow-up. Therefore, the authors suggested additional evaluation using MRA and EEG to find more supportive evidence for progressive PCA stenosis before performing angiography to confirm the diagnosis10).
SURGICAL PROCEDURES
Indirect revascularization using omental flap transplantation has been described for PCA territory operation14). Additionally, the feasibility and efficacy of encephalo-duro-arterio synangiosis using a galeoperiosteal flap containing the occipital artery has been reported1). Direct occipital artery and posterior cerebral artery bypass surgery combined with an indirect procedure was performed successfully by other groups19). The surgical outcome of delayed PCA stenosis was excellent, regardless of the surgical method used. Almost all of the patients reported symptomatic and radiologic improvement4,7,10).
THEORIES ON THE MECHANISM OF STENOSIS
PCA stenosis in MMD may be explained by the main disease process of MMD, which consists of progressive steno-occlusion of major intracranial arteries18,19). In addition, secondary stenosis may also occur resulting from involution of the PCA due to development of sufficient collaterals to the anterior circulation after revascularization surgeries or due to a decrease in blood flow demand in the infarcted areas7).
CONCLUSION
PCA stenosis is an important clinical factor related to poor outcome in MMD disease. It may be detected initially or after anterior circulation revascularization surgery. Clinicians should be aware of the possibility of progressive PCA stenosis during the postoperative follow-up period and its various clinical presentations. Appropriate clinical suspicion and integrative interpretation of multiple work-up results may lead to the diagnosis and treatment of progressive PCA stenosis.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HI12C0066).
References
- 1.Choi IJ, Hong SH, Cho BK, Wang KC, Kim SK. Encephalo-duro-arterio-synangiosis (EDAS) using occipital artery in children with Moyamoya disease. J Korean Neurosurg Soc. 2005;38:413–418. [Google Scholar]
- 2.Fukui M Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis ('moyamoya' disease) Clin Neurol Neurosurg. 1997;99(Suppl 2):S238–S240. [PubMed] [Google Scholar]
- 3.Funaki T, Takahashi JC, Takagi Y, Yoshida K, Araki Y, Kikuchi T, et al. Impact of posterior cerebral artery involvement on long-term clinical and social outcome of pediatric moyamoya disease. J Neurosurg Pediatr. 2013;12:626–632. doi: 10.3171/2013.9.PEDS13111. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi T, Shirane R, Tominaga T. Additional surgery for postoperative ischemic symptoms in patients with moyamoya disease : the effectiveness of occipital artery-posterior cerebral artery bypass with an indirect procedure : technical case report. Neurosurgery. 2009;64:E195–E196. discussion E196. doi: 10.1227/01.NEU.0000336311.60660.26. [DOI] [PubMed] [Google Scholar]
- 5.Hishikawa T, Tokunaga K, Sugiu K, Date I. Assessment of the difference in posterior circulation involvement between pediatric and adult patients with moyamoya disease. J Neurosurg. 2013;119:961–965. doi: 10.3171/2013.6.JNS122099. [DOI] [PubMed] [Google Scholar]
- 6.Hishikawa T, Tokunaga K, Sugiu K, Date I. Long-term outcomes in adult patients with ischemic-type moyamoya disease involving posterior circulation. Acta Neurochir (Wien) 2014;156:1745–1751. doi: 10.1007/s00701-014-2136-3. [DOI] [PubMed] [Google Scholar]
- 7.Huang AP, Liu HM, Lai DM, Yang CC, Tsai YH, Wang KC, et al. Clinical significance of posterior circulation changes after revascularization in patients with moyamoya disease. Cerebrovasc Dis. 2009;28:247–257. doi: 10.1159/000228254. [DOI] [PubMed] [Google Scholar]
- 8.Imaizumi T, Hayashi K, Saito K, Osawa M, Fukuyama Y. Long-term outcomes of pediatric moyamoya disease monitored to adulthood. Pediatr Neurol. 1998;18:321–325. doi: 10.1016/s0887-8994(97)00209-9. [DOI] [PubMed] [Google Scholar]
- 9.Kim SK, Cho BK, Phi JH, Lee JY, Chae JH, Kim KJ, et al. Pediatric moyamoya disease : an analysis of 410 consecutive cases. Ann Neurol. 2010;68:92–101. doi: 10.1002/ana.21981. [DOI] [PubMed] [Google Scholar]
- 10.Lee JY, Choi YH, Cheon JE, Paeng JC, Ryu HW, Kim KJ, et al. Delayed posterior circulation insufficiency in pediatric moyamoya disease. J Neurol. 2014;261:2305–2313. doi: 10.1007/s00415-014-7484-7. [DOI] [PubMed] [Google Scholar]
- 11.Lee JY, Kim SK, Cheon JE, Choi JW, Phi JH, Kim IO, et al. Posterior cerebral artery involvement in moyamoya disease : initial infarction and angle between PCA and basilar artery. Childs Nerv Syst. 2013;29:2263–2269. doi: 10.1007/s00381-013-2123-7. [DOI] [PubMed] [Google Scholar]
- 12.Lee JY, Phi JH, Wang KC, Cho BK, Shin MS, Kim SK. Neurocognitive profiles of children with moyamoya disease before and after surgical intervention. Cerebrovasc Dis. 2011;31:230–237. doi: 10.1159/000321901. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto S, Kikuchi H, Karasawa J, Nagata I, Ikota T, Takeuchi S. Study of the posterior circulation in moyamoya disease. Clinical and neuroradiological evaluation. J Neurosurg. 1984;61:1032–1037. doi: 10.3171/jns.1984.61.6.1032. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto S, Kikuchi H, Karasawa J, Nagata I, Ihara I, Yamagata S. Study of the posterior circulation in moyamoya disease. Part 2 : Visual disturbances and surgical treatment. J Neurosurg. 1986;65:454–460. doi: 10.3171/jns.1986.65.4.0454. [DOI] [PubMed] [Google Scholar]
- 15.Miyatake S, Miyake N, Touho H, Nishimura-Tadaki A, Kondo Y, Okada I, et al. Homozygous c.14576G>A variant of RNF213 predicts early-onset and severe form of moyamoya disease. Neurology. 2012;78:803–810. doi: 10.1212/WNL.0b013e318249f71f. [DOI] [PubMed] [Google Scholar]
- 16.Moen M, Levine SR, Newman DS, Dull-Baird A, Brown GG, Welch KM. Bilateral posterior cerebral artery strokes in a young migraine sufferer. Stroke. 1988;19:525–528. doi: 10.1161/01.str.19.4.525. [DOI] [PubMed] [Google Scholar]
- 17.Mugikura S, Higano S, Shirane R, Fujimura M, Shimanuki Y, Takahashi S. Posterior circulation and high prevalence of ischemic stroke among young pediatric patients with Moyamoya disease : evidence of angiography-based differences by age at diagnosis. AJNR Am J Neuroradiol. 2011;32:192–198. doi: 10.3174/ajnr.A2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mugikura S, Takahashi S, Higano S, Shirane R, Kurihara N, Furuta S, et al. The relationship between cerebral infarction and angiographic characteristics in childhood moyamoya disease. AJNR Am J Neuroradiol. 1999;20:336–343. [PMC free article] [PubMed] [Google Scholar]
- 19.Mugikura S, Takahashi S, Higano S, Shirane R, Sakurai Y, Yamada S. Predominant involvement of ipsilateral anterior and posterior circulations in moyamoya disease. Stroke. 2002;33:1497–1500. doi: 10.1161/01.str.0000016828.62708.21. [DOI] [PubMed] [Google Scholar]
- 20.Seol HJ, Shin DC, Kim YS, Shim EB, Kim SK, Cho BK, et al. Computational analysis of hemodynamics using a two-dimensional model in moyamoya disease. J Neurosurg Pediatr. 2010;5:297–301. doi: 10.3171/2009.10.PEDS09452. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki J, Kodama N. Moyamoya disease--a review. Stroke. 1983;14:104–109. doi: 10.1161/01.str.14.1.104. [DOI] [PubMed] [Google Scholar]
- 22.Yamada I, Himeno Y, Suzuki S, Matsushima Y. Posterior circulation in moyamoya disease : angiographic study. Radiology. 1995;197:239–246. doi: 10.1148/radiology.197.1.7568830. [DOI] [PubMed] [Google Scholar]
- 23.Yamada I, Murata Y, Umehara I, Suzuki S, Matsushima Y. SPECT and MRI evaluations of the posterior circulation in moyamoya disease. J Nucl Med. 1996;37:1613–1617. [PubMed] [Google Scholar]