Abstract
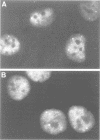
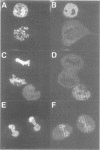
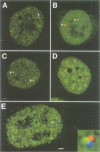
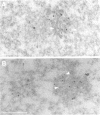
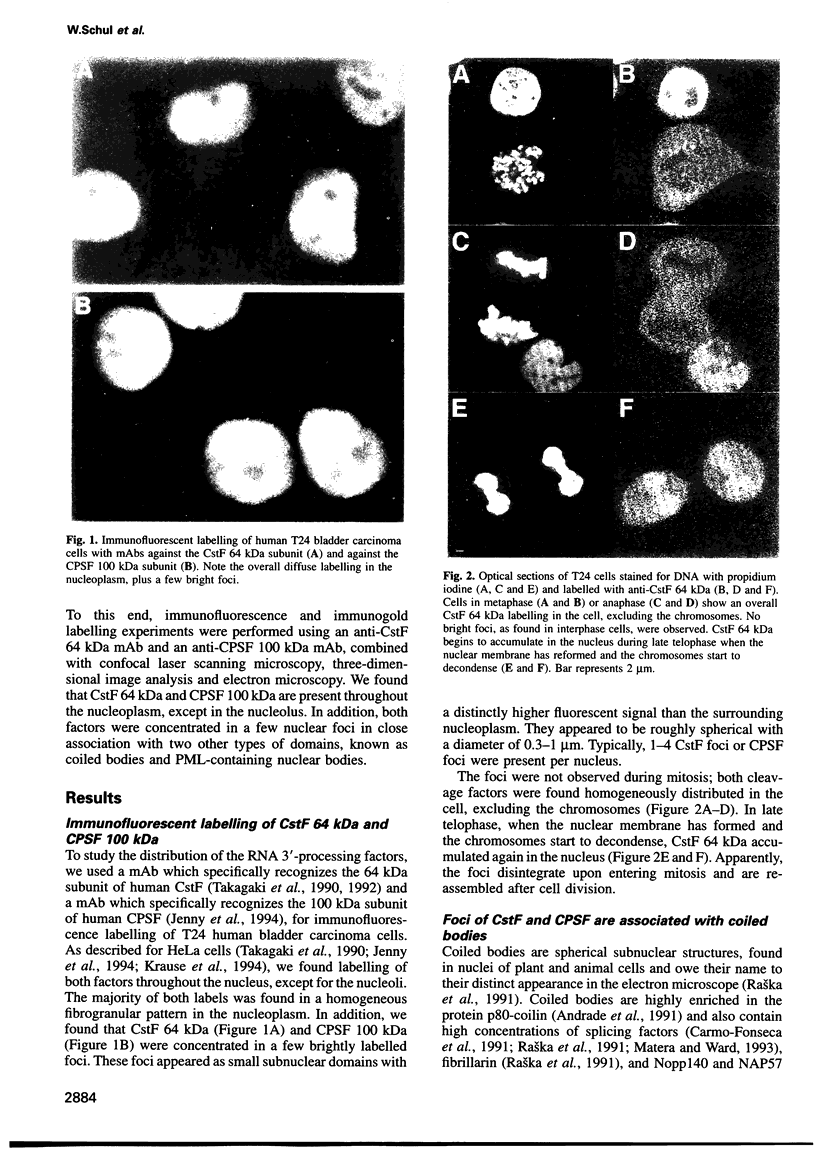
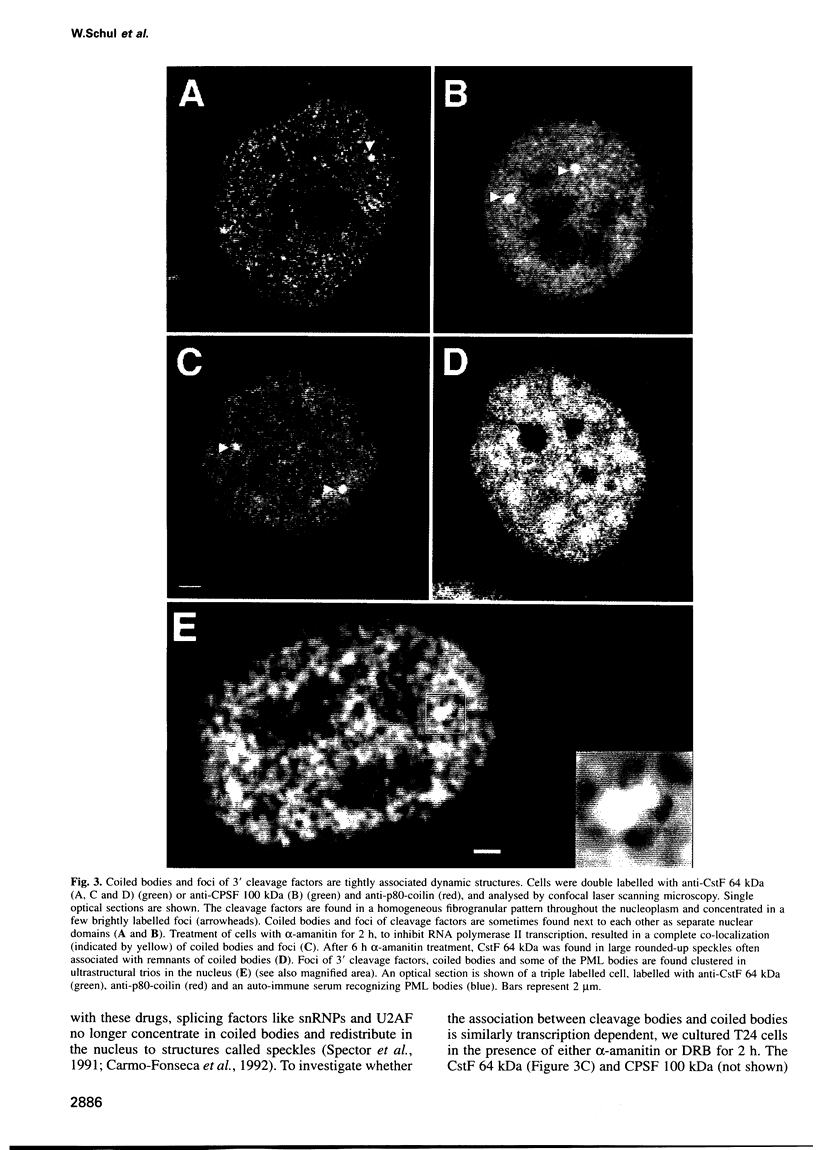
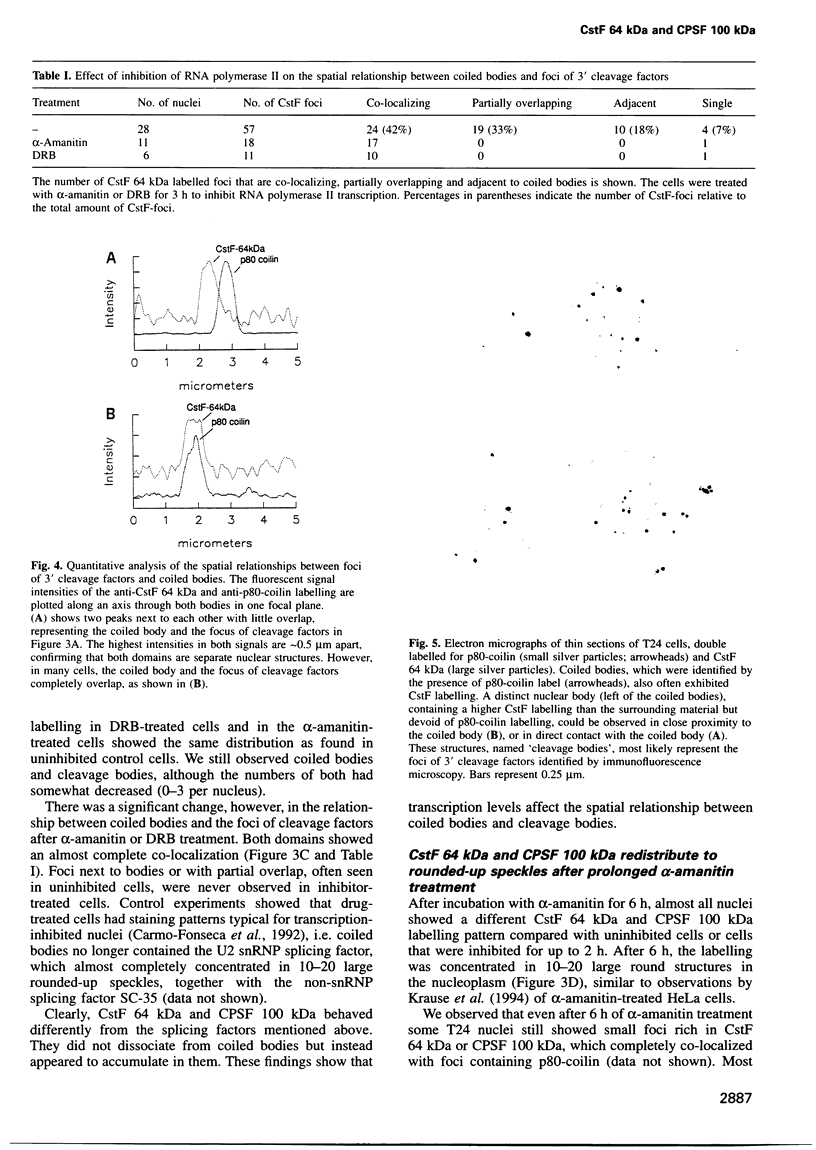
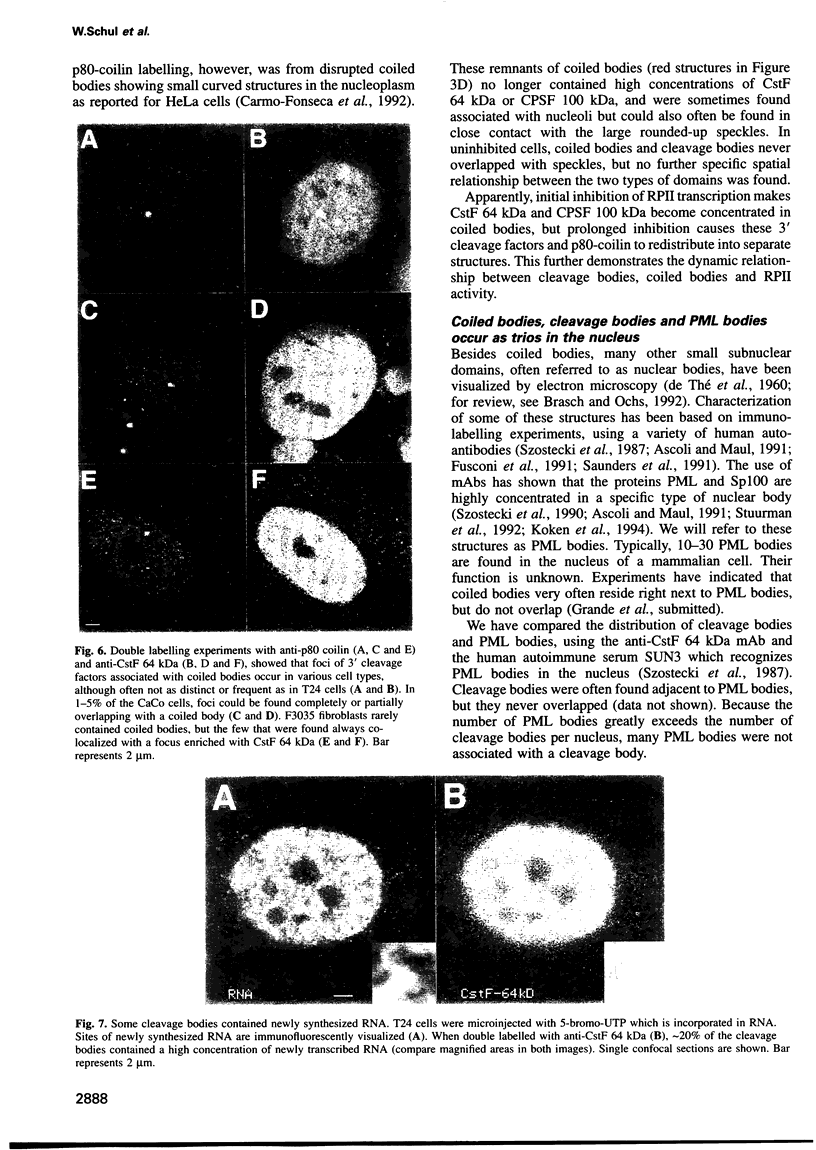
The cleavage stimulation factor (CstF), and the cleavage and polyadenylation specificity factor (CPSF) are necessary for 3'-terminal processing of polyadenylated mRNAs. To study the distribution of 3' cleavage factors in the nuclei of human T24 cells, monoclonal antibodies against the CstF 64 kDa subunit and against the CPSF 100 kDa subunit were used for immunofluorescent labelling. CstF 64 kDa and CPSF 100 kDa were distributed in a fibrogranular pattern in the nucleoplasm and, in addition, were concentrated in 1-4 bright foci. Double immunofluorescence labelling experiments revealed that the foci either overlapped with, or resided next to, a coiled body. Inhibition of transcription with alpha-amanitin or 5,6-dichloro-beta-D-ribofuranosyl-benzimidazole (DRB) resulted in the complete co-localization of coiled bodies and foci containing 3' cleavage factors. Electron microscopy on immunogold double-labelled cells revealed that the foci represent compact spherical fibrous structures, we named 'cleavage bodies', intimately associated with coiled bodies. We found that approximately 20% of the cleavage bodies contained a high concentration of newly synthesized RNA, whereas coiled bodies were devoid of nascent RNA. Our results suggest that the cleavage bodies that contain RNA are those that are adjacent to a coiled body. These findings reveal a dynamic and transcription-dependent interaction between different subnuclear domains, and suggest a relationship between coiled bodies and specific transcripts.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade L. E., Chan E. K., Raska I., Peebles C. L., Roos G., Tan E. M. Human autoantibody to a novel protein of the nuclear coiled body: immunological characterization and cDNA cloning of p80-coilin. J Exp Med. 1991 Jun 1;173(6):1407–1419. doi: 10.1084/jem.173.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou M., Carmo-Fonseca M., Ferreira J., Lamond A. I. Nuclear organization of splicing snRNPs during differentiation of murine erythroleukemia cells in vitro. J Cell Biol. 1993 Dec;123(5):1055–1068. doi: 10.1083/jcb.123.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli C. A., Maul G. G. Identification of a novel nuclear domain. J Cell Biol. 1991 Mar;112(5):785–795. doi: 10.1083/jcb.112.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienroth S., Wahle E., Suter-Crazzolara C., Keller W. Purification of the cleavage and polyadenylation factor involved in the 3'-processing of messenger RNA precursors. J Biol Chem. 1991 Oct 15;266(29):19768–19776. [PubMed] [Google Scholar]
- Brasch K., Ochs R. L. Nuclear bodies (NBs): a newly "rediscovered" organelle. Exp Cell Res. 1992 Oct;202(2):211–223. doi: 10.1016/0014-4827(92)90068-j. [DOI] [PubMed] [Google Scholar]
- Callan H. G., Gall J. G., Murphy C. Histone genes are located at the sphere loci of Xenopus lampbrush chromosomes. Chromosoma. 1991 Dec;101(4):245–251. doi: 10.1007/BF00365156. [DOI] [PubMed] [Google Scholar]
- Carlsson K., Aslund N., Mossberg K., Philip J. Simultaneous confocal recording of multiple fluorescent labels with improved channel separation. J Microsc. 1994 Dec;176(Pt 3):287–299. doi: 10.1111/j.1365-2818.1994.tb03527.x. [DOI] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Pepperkok R., Carvalho M. T., Lamond A. I. Transcription-dependent colocalization of the U1, U2, U4/U6, and U5 snRNPs in coiled bodies. J Cell Biol. 1992 Apr;117(1):1–14. doi: 10.1083/jcb.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Tollervey D., Pepperkok R., Barabino S. M., Merdes A., Brunner C., Zamore P. D., Green M. R., Hurt E., Lamond A. I. Mammalian nuclei contain foci which are highly enriched in components of the pre-mRNA splicing machinery. EMBO J. 1991 Jan;10(1):195–206. doi: 10.1002/j.1460-2075.1991.tb07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofori G., Keller W. 3' cleavage and polyadenylation of mRNA precursors in vitro requires a poly(A) polymerase, a cleavage factor, and a snRNP. Cell. 1988 Sep 9;54(6):875–889. doi: 10.1016/s0092-8674(88)91263-9. [DOI] [PubMed] [Google Scholar]
- Dyck J. A., Maul G. G., Miller W. H., Jr, Chen J. D., Kakizuka A., Evans R. M. A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell. 1994 Jan 28;76(2):333–343. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- Frey M. R., Matera A. G. Coiled bodies contain U7 small nuclear RNA and associate with specific DNA sequences in interphase human cells. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):5915–5919. doi: 10.1073/pnas.92.13.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusconi M., Cassani F., Govoni M., Caselli A., Farabegoli F., Lenzi M., Ballardini G., Zauli D., Bianchi F. B. Anti-nuclear antibodies of primary biliary cirrhosis recognize 78-92-kD and 96-100-kD proteins of nuclear bodies. Clin Exp Immunol. 1991 Feb;83(2):291–297. doi: 10.1111/j.1365-2249.1991.tb05630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J. G., Stephenson E. C., Erba H. P., Diaz M. O., Barsacchi-Pilone G. Histone genes are located at the sphere loci of newt lampbrush chromosomes. Chromosoma. 1981;84(2):159–171. doi: 10.1007/BF00399128. [DOI] [PubMed] [Google Scholar]
- Gall J. G., Tsvetkov A., Wu Z., Murphy C. Is the sphere organelle/coiled body a universal nuclear component? Dev Genet. 1995;16(1):25–35. doi: 10.1002/dvg.1020160107. [DOI] [PubMed] [Google Scholar]
- Gilmartin G. M., Nevins J. R. An ordered pathway of assembly of components required for polyadenylation site recognition and processing. Genes Dev. 1989 Dec;3(12B):2180–2190. doi: 10.1101/gad.3.12b.2180. [DOI] [PubMed] [Google Scholar]
- Gilmartin G. M., Nevins J. R. Molecular analyses of two poly(A) site-processing factors that determine the recognition and efficiency of cleavage of the pre-mRNA. Mol Cell Biol. 1991 May;11(5):2432–2438. doi: 10.1128/mcb.11.5.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Verdun D. The nucleolus today. J Cell Sci. 1991 Jul;99(Pt 3):465–471. doi: 10.1242/jcs.99.3.465. [DOI] [PubMed] [Google Scholar]
- Jenny A., Hauri H. P., Keller W. Characterization of cleavage and polyadenylation specificity factor and cloning of its 100-kilodalton subunit. Mol Cell Biol. 1994 Dec;14(12):8183–8190. doi: 10.1128/mcb.14.12.8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W., Bienroth S., Lang K. M., Christofori G. Cleavage and polyadenylation factor CPF specifically interacts with the pre-mRNA 3' processing signal AAUAAA. EMBO J. 1991 Dec;10(13):4241–4249. doi: 10.1002/j.1460-2075.1991.tb05002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koken M. H., Puvion-Dutilleul F., Guillemin M. C., Viron A., Linares-Cruz G., Stuurman N., de Jong L., Szostecki C., Calvo F., Chomienne C. The t(15;17) translocation alters a nuclear body in a retinoic acid-reversible fashion. EMBO J. 1994 Mar 1;13(5):1073–1083. doi: 10.1002/j.1460-2075.1994.tb06356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause S., Fakan S., Weis K., Wahle E. Immunodetection of poly(A) binding protein II in the cell nucleus. Exp Cell Res. 1994 Sep;214(1):75–82. doi: 10.1006/excr.1994.1235. [DOI] [PubMed] [Google Scholar]
- Lamond A. I., Carmo-Fonseca M. The coiled body. Trends Cell Biol. 1993 Jun;3(6):198–204. doi: 10.1016/0962-8924(93)90214-l. [DOI] [PubMed] [Google Scholar]
- Malatesta M., Zancanaro C., Martin T. E., Chan E. K., Amalric F., Lührmann R., Vogel P., Fakan S. Cytochemical and immunocytochemical characterization of nuclear bodies during hibernation. Eur J Cell Biol. 1994 Oct;65(1):82–93. [PubMed] [Google Scholar]
- Manders E. M., Stap J., Brakenhoff G. J., van Driel R., Aten J. A. Dynamics of three-dimensional replication patterns during the S-phase, analysed by double labelling of DNA and confocal microscopy. J Cell Sci. 1992 Nov;103(Pt 3):857–862. doi: 10.1242/jcs.103.3.857. [DOI] [PubMed] [Google Scholar]
- Marzluff W. F. Histone 3' ends: essential and regulatory functions. Gene Expr. 1992;2(2):93–97. [PMC free article] [PubMed] [Google Scholar]
- Matera A. G., Ward D. C. Nucleoplasmic organization of small nuclear ribonucleoproteins in cultured human cells. J Cell Biol. 1993 May;121(4):715–727. doi: 10.1083/jcb.121.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier U. T., Blobel G. NAP57, a mammalian nucleolar protein with a putative homolog in yeast and bacteria. J Cell Biol. 1994 Dec;127(6 Pt 1):1505–1514. doi: 10.1083/jcb.127.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raska I., Andrade L. E., Ochs R. L., Chan E. K., Chang C. M., Roos G., Tan E. M. Immunological and ultrastructural studies of the nuclear coiled body with autoimmune antibodies. Exp Cell Res. 1991 Jul;195(1):27–37. doi: 10.1016/0014-4827(91)90496-h. [DOI] [PubMed] [Google Scholar]
- Raska I., Dundr M., Koberna K., Melcák I., Risueño M. C., Török I. Does the synthesis of ribosomal RNA take place within nucleolar fibrillar centers or dense fibrillar components? A critical appraisal. J Struct Biol. 1995 Jan-Feb;114(1):1–22. doi: 10.1006/jsbi.1995.1001. [DOI] [PubMed] [Google Scholar]
- Raska I. Nuclear ultrastructures associated with the RNA synthesis and processing. J Cell Biochem. 1995 Sep;59(1):11–26. doi: 10.1002/jcb.240590103. [DOI] [PubMed] [Google Scholar]
- Raska I., Ochs R. L., Salamin-Michel L. Immunocytochemistry of the cell nucleus. Electron Microsc Rev. 1990;3(2):301–353. doi: 10.1016/0892-0354(90)90006-e. [DOI] [PubMed] [Google Scholar]
- Roth M. B. Spheres, coiled bodies and nuclear bodies. Curr Opin Cell Biol. 1995 Jun;7(3):325–328. doi: 10.1016/0955-0674(95)80086-7. [DOI] [PubMed] [Google Scholar]
- Sachs A., Wahle E. Poly(A) tail metabolism and function in eucaryotes. J Biol Chem. 1993 Nov 5;268(31):22955–22958. [PubMed] [Google Scholar]
- Saunders W. S., Cooke C. A., Earnshaw W. C. Compartmentalization within the nucleus: discovery of a novel subnuclear region. J Cell Biol. 1991 Nov;115(4):919–931. doi: 10.1083/jcb.115.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer U., Thiry M., Goessens G. Structure, function and assembly of the nucleolus. Trends Cell Biol. 1993 Jul;3(7):236–241. doi: 10.1016/0962-8924(93)90123-i. [DOI] [PubMed] [Google Scholar]
- Smith K. P., Carter K. C., Johnson C. V., Lawrence J. B. U2 and U1 snRNA gene loci associate with coiled bodies. J Cell Biochem. 1995 Dec;59(4):473–485. doi: 10.1002/jcb.240590408. [DOI] [PubMed] [Google Scholar]
- Spector D. L., Fu X. D., Maniatis T. Associations between distinct pre-mRNA splicing components and the cell nucleus. EMBO J. 1991 Nov;10(11):3467–3481. doi: 10.1002/j.1460-2075.1991.tb04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. L. Macromolecular domains within the cell nucleus. Annu Rev Cell Biol. 1993;9:265–315. doi: 10.1146/annurev.cb.09.110193.001405. [DOI] [PubMed] [Google Scholar]
- Stuurman N., de Graaf A., Floore A., Josso A., Humbel B., de Jong L., van Driel R. A monoclonal antibody recognizing nuclear matrix-associated nuclear bodies. J Cell Sci. 1992 Apr;101(Pt 4):773–784. doi: 10.1242/jcs.101.4.773. [DOI] [PubMed] [Google Scholar]
- Szostecki C., Guldner H. H., Netter H. J., Will H. Isolation and characterization of cDNA encoding a human nuclear antigen predominantly recognized by autoantibodies from patients with primary biliary cirrhosis. J Immunol. 1990 Dec 15;145(12):4338–4347. [PubMed] [Google Scholar]
- Szostecki C., Krippner H., Penner E., Bautz F. A. Autoimmune sera recognize a 100 kD nuclear protein antigen (sp-100). Clin Exp Immunol. 1987 Apr;68(1):108–116. [PMC free article] [PubMed] [Google Scholar]
- Takagaki Y., MacDonald C. C., Shenk T., Manley J. L. The human 64-kDa polyadenylylation factor contains a ribonucleoprotein-type RNA binding domain and unusual auxiliary motifs. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1403–1407. doi: 10.1073/pnas.89.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagaki Y., Manley J. L., MacDonald C. C., Wilusz J., Shenk T. A multisubunit factor, CstF, is required for polyadenylation of mammalian pre-mRNAs. Genes Dev. 1990 Dec;4(12A):2112–2120. doi: 10.1101/gad.4.12a.2112. [DOI] [PubMed] [Google Scholar]
- Takagaki Y., Ryner L. C., Manley J. L. Four factors are required for 3'-end cleavage of pre-mRNAs. Genes Dev. 1989 Nov;3(11):1711–1724. doi: 10.1101/gad.3.11.1711. [DOI] [PubMed] [Google Scholar]
- Thiry M. Cytochemical and immunocytochemical study of coiled bodies in different cultured cell lines. Chromosoma. 1994 Jul;103(4):268–276. doi: 10.1007/BF00352251. [DOI] [PubMed] [Google Scholar]
- Tuma R. S., Stolk J. A., Roth M. B. Identification and characterization of a sphere organelle protein. J Cell Biol. 1993 Aug;122(4):767–773. doi: 10.1083/jcb.122.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle E., Keller W. The biochemistry of 3'-end cleavage and polyadenylation of messenger RNA precursors. Annu Rev Biochem. 1992;61:419–440. doi: 10.1146/annurev.bi.61.070192.002223. [DOI] [PubMed] [Google Scholar]
- Wahle E. The end of the message: 3'-end processing leading to polyadenylated messenger RNA. Bioessays. 1992 Feb;14(2):113–118. doi: 10.1002/bies.950140208. [DOI] [PubMed] [Google Scholar]
- Wansink D. G., Schul W., van der Kraan I., van Steensel B., van Driel R., de Jong L. Fluorescent labeling of nascent RNA reveals transcription by RNA polymerase II in domains scattered throughout the nucleus. J Cell Biol. 1993 Jul;122(2):283–293. doi: 10.1083/jcb.122.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis K., Rambaud S., Lavau C., Jansen J., Carvalho T., Carmo-Fonseca M., Lamond A., Dejean A. Retinoic acid regulates aberrant nuclear localization of PML-RAR alpha in acute promyelocytic leukemia cells. Cell. 1994 Jan 28;76(2):345–356. doi: 10.1016/0092-8674(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Wu C. H., Gall J. G. U7 small nuclear RNA in C snurposomes of the Xenopus germinal vesicle. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6257–6259. doi: 10.1073/pnas.90.13.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z. A., Murphy C., Callan H. G., Gall J. G. Small nuclear ribonucleoproteins and heterogeneous nuclear ribonucleoproteins in the amphibian germinal vesicle: loops, spheres, and snurposomes. J Cell Biol. 1991 May;113(3):465–483. doi: 10.1083/jcb.113.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de THE, RIVIERE M., BERNHARD W. [Examination by electron microscope of the VX2 tumor of the domestic rabbit derived from the Shope papilloma]. Bull Assoc Fr Etud Cancer. 1960 Oct-Dec;47:570–584. [PubMed] [Google Scholar]
- van Driel R., Wansink D. G., van Steensel B., Grande M. A., Schul W., de Jong L. Nuclear domains and the nuclear matrix. Int Rev Cytol. 1995;162A:151–189. doi: 10.1016/s0074-7696(08)61231-0. [DOI] [PubMed] [Google Scholar]