Abstract
Atrial fibrillation (AF) associated with fibrosis is characterized by the appearance of interstitial myofibroblasts. These cells are responsible for the uncontrolled deposition of the extracellular matrix, which pathologically separate cardiomyocyte bundles. The enhanced fibrosis is thought to contribute to arrhythmias “indirectly” because a collagenous septum is a passive substrate for propagation, resulting in impulse conduction block and/or zigzag conduction. However, the emerging results demonstrate that myofibroblasts in vitro also promote arrhythmogenesis due to direct implications upon cardiomyocyte electrophysiology. This electrical interference may be considered beneficial as it resolves any conduction blocks; however, the passive properties of myofibroblasts might cause a delay in impulse propagation, thus promoting AF due to discontinuous slow conduction. Moreover, low-polarized myofibroblasts reduce, via cell-density dependence, the fast driving inward current for cardiac impulse conduction, therefore resulting in arrhythmogenic uniformly slow propagation. Critically, the subsequent reduction in cardiomyocytes resting membrane potential in vitro significantly increases the likelihood of ectopic activity. Myofibroblast densities and the degree of coupling at cellular border zones also impact upon this likelihood. By considering future in vivo studies, which identify myofibroblasts “per se” as a novel targets for cardiac arrhythmias, this review aims to describe the implications of noncardiomyocyte view in the context of AF.
1. Introduction
The normal function of the heart is a painstaking cooperation between cardiomyocytes and fibroblasts. It is well known that cardiomyocytes provide the “pumping” function of the organ, whereas fibroblasts are responsible for organizing the cellular scaffold and maintaining the proper 3D-network and thus the normal mechanical function. Moreover, fibroblasts contribute importantly to the uniformity of the excitable substrate and to the continuous and rapid electrical activation of the myocardium. In the healthy normal heart, fibrosis-related arrhythmia is normally absent, which indicates that although fibroblasts outnumber cardiomyocytes roughly three-to-one [1], they do not exert any arrhythmogenic effect. Though there is a general assumption that cardiomyocytes play the crucial role in atrial arrhythmogenesis, little is known regarding an “active” role of the connective tissue in this respect.
A variety of pathological conditions, including pressure overload, volume overload, infarction, and aging [2], induces structural remodelling of the heart leading to heart failure and cardiac arrhythmias. This structural remodelling involves changes in the 3D organization of the heart and is based on complex and diverse responses to injury; as a result, all types of cardiac cells are involved. Histopathologically, cardiac remodelling typically involves changes in myocytes size (hypertrophy), the activation and proliferation of fibroblasts, uncontrolled deposition of the extra cellular matrix (ECM), and cell death [3]. This is in favour of the beginning and perpetuation of supraventricular and ventricular arrhythmias due to the presence of collagenous septa, which physically separate regions of cardiomyocytes, thus inducing structural discontinuities at cellular and tissue levels. This can result in conduction block and zigzag conduction, both of which permit structurally determined reentrant propagation of cardiac impulse.
Functionally, cardiac remodelling leads to mechanical dysfunction which increases the likelihood of life-threatening cardiac arrhythmias [10]. Consequently, arrhythmias arising from structurally remodelled hearts are caused by changes in electrical properties of cardiomyocytes and/or by the remodelling of the ECM.
Electrically, remodelling of cardiomyocytes affects a large number of ion channels, ionic pumps, and proteins [11, 12]. Furthermore, redistribution and regulation of gap junction proteins (connexins) alter the physiological anisotropic ratio, which causes abnormal impulse propagation, thus enabling reentrant electrical activity [13].
2. Role of Myofibroblasts in Perpetual Atrial Fibrillation
Atrial interstitial fibrosis has been shown to increase with age in humans and has been observed in patients with atrial fibrillation (AF) [14, 15], in animal models of aging [16, 17] and in congestive heart failure [18]. Through these studies, it has been shown that atrial fibrosis creates a substrate that promotes AF. Increased collagen deposition has been documented in patients with AF secondary to mitral valve disease versus those in sinus rhythm [19]. Extracellular matrix volume and composition correlate with AF persistence [20]. These findings highlight the association between atrial fibrosis and AF, although determining the causal importance of tissue fibrosis in AF occurrence and persistence remains an important challenge.
AF is also capable of enhancing atrial fibrosis. In human lone AF, it has been shown that long-term assessment of patients diagnosed with AF, which had normal sized atria upon diagnosis, does lead to structural remodeling of the atria causing atrial enlargement and dilatation over a subsequent period of 20 months [21]. The studies suggest that atrial fibrosis acts as both a trigger and a by-product of AF, potentially through a mechanism affecting signaling pathways associated with atrial dilatation [22, 23].
The mechanism of AF that is associated with an increased level of fibrosis is still under debate as both focal and reentrant mechanisms have been observed in patients and animal models of AF. In the dog model of ventricular tachypacing induced congestive heart failure, atrial fibrosis causes localized regions of conduction slowing, increasing conduction heterogeneity and providing an AF substrate [18]. Conduction abnormalities provide a basis for unidirectional conduction block and macroreentry [24].
In contrast, in the study by Stambler et al. on dogs with rapid ventricular pacing-induced congestive heart failure, AF was shown to be focal in origin caused by triggered activity [25]. This triggered activity was shown to be produced by delayed afterdepolarizations initiated by intracellular Ca2+ overload. Drugs that reduced intracellular Ca2+ levels (verapamil, flunarizine, and ryanodine) all terminated AF. Fenelon et al. expanded on this study by performing biatrial mapping in dogs with heart failure and showed that the majority of AF episodes had a focal mechanism [26].
There is evidence that atrial fibrosis is associated with a profound remodelling of the atrial pacemaker complex. It has been shown that the function of the SAN declines in AF [27], heart failure [28–30], and with age [31], conditions associated with an increased level of fibrosis. A strong correlation between these conditions and the incidence of sick sinus syndrome has been observed [31, 32]. It should be noted that, histologically, the healthy SAN is distinguished from the surrounding atrial muscle by a remarkably large amount of interstitium (e.g., up to 75%–90% of SAN volume in cat) [33]. It allows SAN electrical insulation from the surrounding atrial myocardium, except for several critical conduction pathways. Indeed, the SAN as a leading pacemaker requires both anatomical (fibrosis, fat, and blood vessels) and/or functional (paucity of connexins) barriers to protect it from the hyperpolarizing influence of the surrounding myocardium [34–36]. The presence of conduction barriers and pathways explain how a small cluster of pacemaker cells in the SAN pacemaker complex manages to depolarize widely distributed areas of the right atria. An increased level of interstitial fibrosis can further insulate the SAN thereby altering the delicate balance between depolarized cells (source) and the resting tissue ahead (sink) [37].
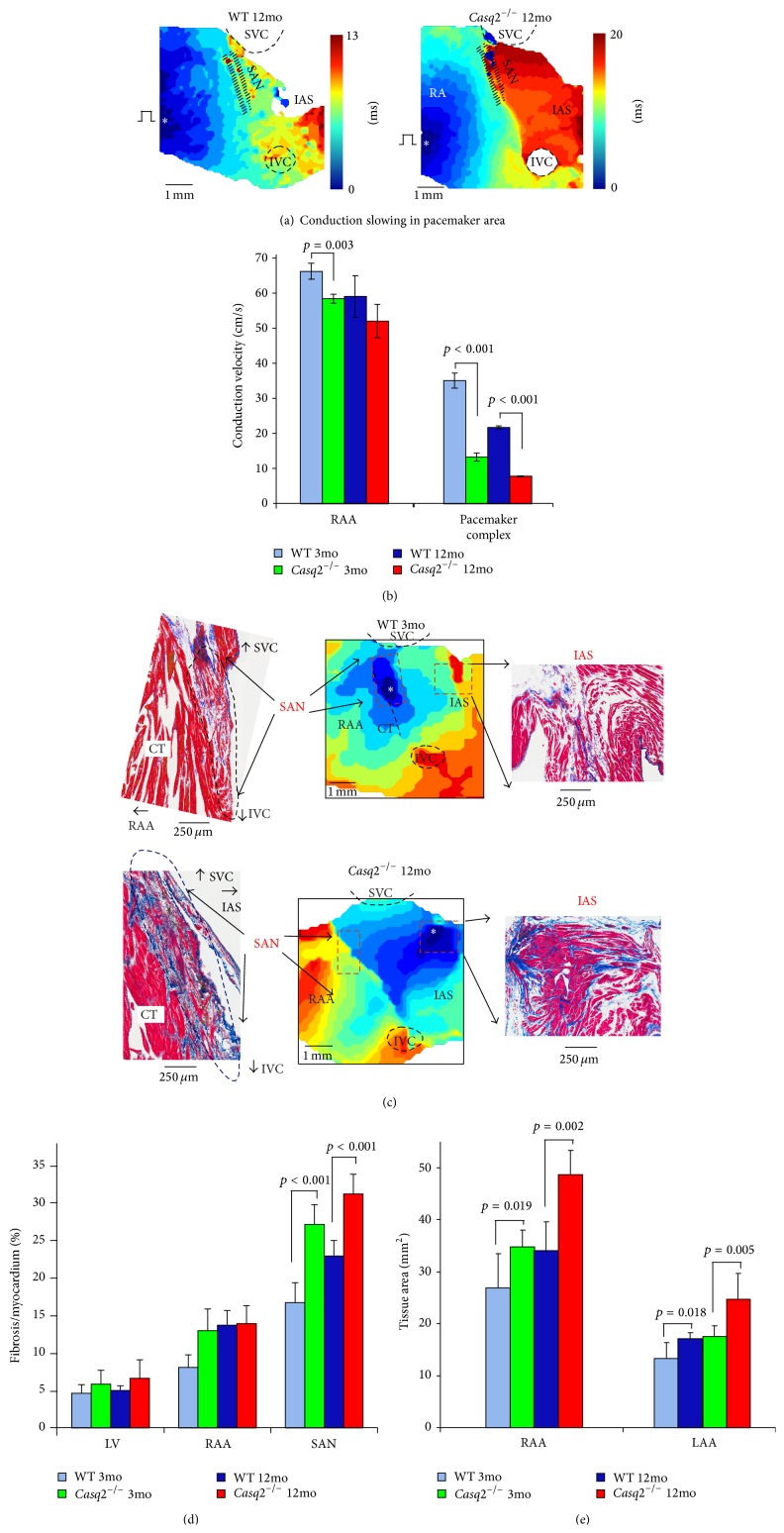
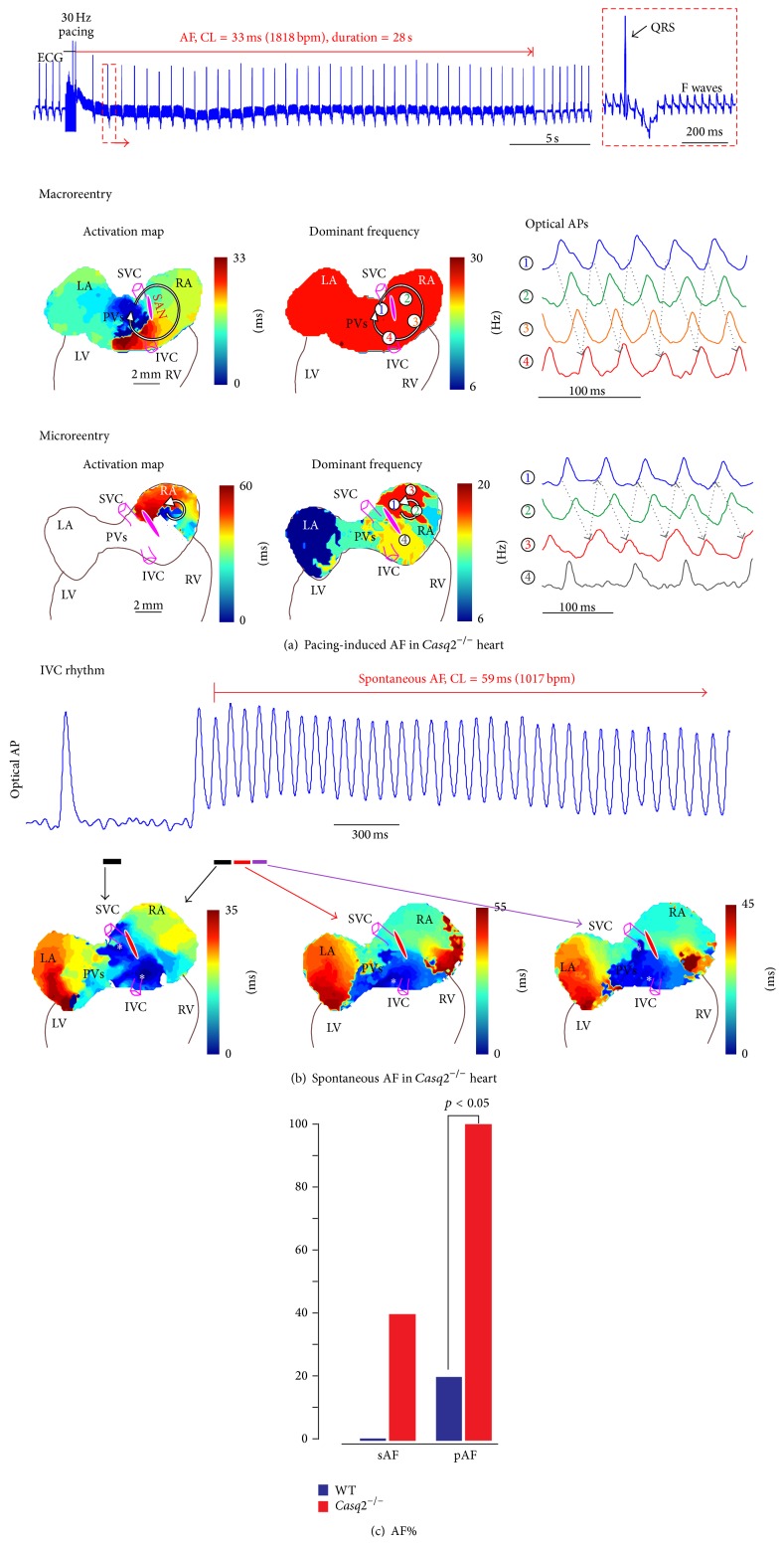
On the other hand, an increased fibrosis can unmask the latent pacemakers by forming specialized, isolated clusters of pacemakers located throughout the atrial pacemaker complex [4, 38]. It has been known for over a century that pacemaker cells are widely distributed throughout the entire region located between the superior and inferior vena cava and between the crista terminalis and intra-atrial septum [33, 39]. Canine and human studies [40–44] have revealed an extensive distributed system of atrial pacemakers, the atrial pacemaker complex, which extends well beyond the anatomically defined SAN and includes primary and subsidiary pacemakers located within the right atrium. Functional anatomy of the atrial pacemaker complex has been extensively studied in mouse models of sick sinus syndrome. Recently, in calsequestrin 2 null mice which were characterized by an increased susceptibility to AF, we have shown, using a high-resolution optical mapping and 3D atrial immunohistology a selective interstitial fibrosis in the atrial pacemaker complex [4], Figure 1. Deletion of calsequestrin 2 depressed primary SAN activity and conduction, but enhanced atrial ectopic activity and AF associated with macro- and micro-reentry during autonomic stimulation (Figure 2). It depressed primary SAN activity and conduction but enhanced atrial ectopic activity and AF associated with macro- and microreentry during autonomic stimulation (Figure 2). Thus, the latent pacemakers will be more stable compared to the primary pacemaker, SAN, probably due to protective electrical insulation role of fibrosis. Such aberrantly isolated clusters of latent pacemakers could become activated and take over the role of the leading pacemaker which can be exaggerated during the abnormal response to autonomic stimulation.
Figure 1.
Enhanced fibrosis, sinoatrial node (SAN) conduction blocks, and atrial enlargement in Casq2−/− hearts. (a) Representative examples of atrial activation during SAN recovery time (SANRT) measurements in 12-month old (mo) wild type (WT, left) and 12 mo Casq2−/− (right) mice at baseline. Activation maps were obtained at continuous pacing (S1S1 = 100 ms) during SANRT measurements at baseline. SVC and IVC: superior and inferior vena cava; RAA and LAA: right and left atrial appendages; RV and LV: right and left ventricles; CT: crista terminalis; IAS: interatrial septum; AVJ: atrioventricular junction. (b) Average data for conduction velocity measured in RAA and within the pacemaker complex at S1S1 = 100 ms pacing in both 3 mo and 12 mo WT and Casq2−/− mice. (c) Histological analysis of the atrial pacemaker complex in WT and Casq2−/− mice is shown. Top: 3 mo WT mouse demonstrates a typical SAN activation at control. Histological staining of the same SAN preparation shown in activation maps confirms location of the SAN. Sections were cut through the SAN preparation parallel to the surface. An enlarged part of the stained preparation (marked by a red dotted rectangle on the activation map) demonstrates the compact part of the SAN (blue rectangle) separated from the atrial muscle (green rectangle) on the other side by connective tissue. Bottom: 12 mo Casq2−/− heart demonstrates structural remodeling of the atrial pacemaker complex. (d) Average ratio of fibrotic tissue content to cardiac tissue measured in different areas in both 3 mo and 12 mo WT and Casq2−/− mouse hearts. (e) Atrial tissue area calculated for both right and left atria in 3 mo and 12 mo WT and Casq2−/− hearts (reprinted with permission from [4]).
Figure 2.
Increased susceptibility to atrial flutter/fibrillation (AF) in Casq2−/− hearts. (a) Rapid-pacing-induced AF in Casq2−/− hearts under isoproterenol and acetylcholine treatment. The pseudo-ECG showed that rapid pacing at 30 Hz induced AF which lasted for 28 seconds. The regular fibrillatory (F) waves indicating atrial flutter is zoomed in on the right. Both macro- and microreentry were drivers for the AF in Casq2−/− hearts. The reentrant circuits are shown in the activation maps on the left; maps in the middle show the dominant frequency (or the reciprocal of the averaged cycle length) at various locations. During macroreentry, dominant frequency was uniform. In contrast, during microreentry, dominant frequency was locally higher in the microreentry circuit area. On the right are the sample optical action potentials (OAPs), whose locations were marked by numbers in the frequency maps. Abbreviations are the same as those in Figure 1. (b) Spontaneous AF occurred under Isoproterenol (Iso) and acetylcholine (ACh) treatment. As shown in OAPs and activation maps, the heart was under slow stable IVC rhythm, and then one of the IVC beats triggered a rapid burst of atrial activity. Representative OAP trace from the IVC region is shown. (c) Percentage of animals with AF inducibility in the control and Casq2−/− groups (sAF: spontaneous AF; pAF: pacing-induced AF) (reprinted with permission from [4]).
Similar results have been observed in other genetically engineered mouse models. Deletion of some structural proteins (such as Cx40 [45, 46], ankyrin-B [38], liver kinase B1 (LKB1) [47], natriuretic peptide receptor C [48], or overexpression of tumor necrosis factor- (TNF-) α [49]) has been linked to enhanced fibrosis, depression of the SAN function, and increased atrial arrhythmogenesis. Shift of the leading pacemaker outside of the SAN structure and a beat-to-beat competition between different pacemakers have been revealed in these mouse models and resulted in heart rate irregularities, tachy-brady arrhythmias, and AF. Interestingly, autonomic stimulation [4] or consecutive thermal ablation of such ectopic sites [45] resulted in leading pacemaker shift back to the SAN but at a prolonged intrinsic cycle length.
In addition to the concept that enhanced interstitial fibrosis contributes to cardiac arrhythmias “indirectly” by affecting passive properties of impulse conduction, recent studies demonstrate that at least a paracrine interaction, or likely a direct electrical coupling, exists between the cardiomyocytes and (myo)fibroblasts (MFBs, see paragraphs below). It has been suggested that structural remodelling including fibrosis of the SAN complex could be attributable to abnormal Ca2+ handling in the pacemaker cells [28]. Enhanced diastolic Ca2+ could directly lead to increased fibrosis within the SAN complex as well as in the latent pacemaker areas by favouring downstream activation of apoptosis due to cytosolic Ca2+ overload. In fact, it has been suggested that chronic Ca2+ leak from the sarcoplasmic reticulum can directly stimulate cell damage and fibrogenesis [50]. Increased intracellular [Ca2+] could also stimulate activation of the multifunctional Ca2+/calmodulin-dependent protein kinase II (CaMKII) which in turn promotes myocardial dysfunction [51] and heart failure [52], SAN cell apoptosis, increased fibrosis and alternating atrial arrhythmogenesis [28].
Finally, the emerging results demonstrate that MFBs in vitro also promote cardiac arrhythmogenesis due to direct implications upon cardiomyocyte electrophysiology [9, 53]. When coupled to cardiomyocytes, MFBs have a depolarizing effect on cardiomyocyte resting membrane potential, which can lead to partial or total sodium channel inactivation. Recent studies have indicated that depolarization in the resting membrane potential of fibroblasts is the most critical factor promoting cardiomyocyte early after depolarizations ectopic activity [8, 54].
3. Role of Myofibroblasts in the Heart
Under the pathological conditions like hypertension, fibrosis, and infarction, MFBs appear in the myocardium. These cells have an important role in reparative fibrosis; they share a phenotype with fibroblasts and smooth-muscle cells and were first identified years ago in skin wound tissue [55] and granulation tissue [56]. However, it is not yet known if these cells are resident changed-phenotype-fibroblasts, endothelial-mesenchymal derived cells or from fibrocytes [57, 58]. Their role is merely reparative and they disappear following programmed cell death. Currently, the most reliable marker for MFBs is alpha-smooth-muscle actin (αSMA), which is expressed in smooth-muscle cells but not in fibroblasts. It has also been shown that MFBs participate in the process of reparative fibrosis in the lung [59], liver [60], and pancreas [61], where they produce excessive ECM, a process similar to that of fibrotic heart remodelling. The trigger for recruitments of MFBs to the diseased heart is not fully understood. The local upregulation of cytokines including foremost TGF-B1 seems to play a prominent role. However it has been demonstrated that tissue stiffening following excessive ECM deposition drives transdifferentiation of precursor cells into forming fibrogenic MFBs [62]. Myofibroblasts themselves produce uncontrolled ECM; hence, a vicious circle ensues. It has also been shown that variation in oxygen (O2) concentration plays a key role in the proliferation of cardiac MFBs. Adult mouse cardiac fibroblasts cultured at 21% O2 express de novoαSMA [63]; in contrast the same was observed when human fetal cardiac fibroblasts were exposed to low percentage O2 [64].
4. Electrical Communications between MFBs and Adjacent Parenchymal Cells
MFBs form gap junctions with the resident parenchymal cells and can exist in different organs like skin [65], intestines [66], and bladder walls [67]. In the healthy heart, MFBs are present only in the valve leaflets; postinfarct MFBs appear in large numbers a few days after injury at the site of infarction. These MFBs differ from those in skin wounds as they can persist in the infarct area for 20 years [68, 69] whilst maintaining intimate contact with the surviving cardiomyocytes.
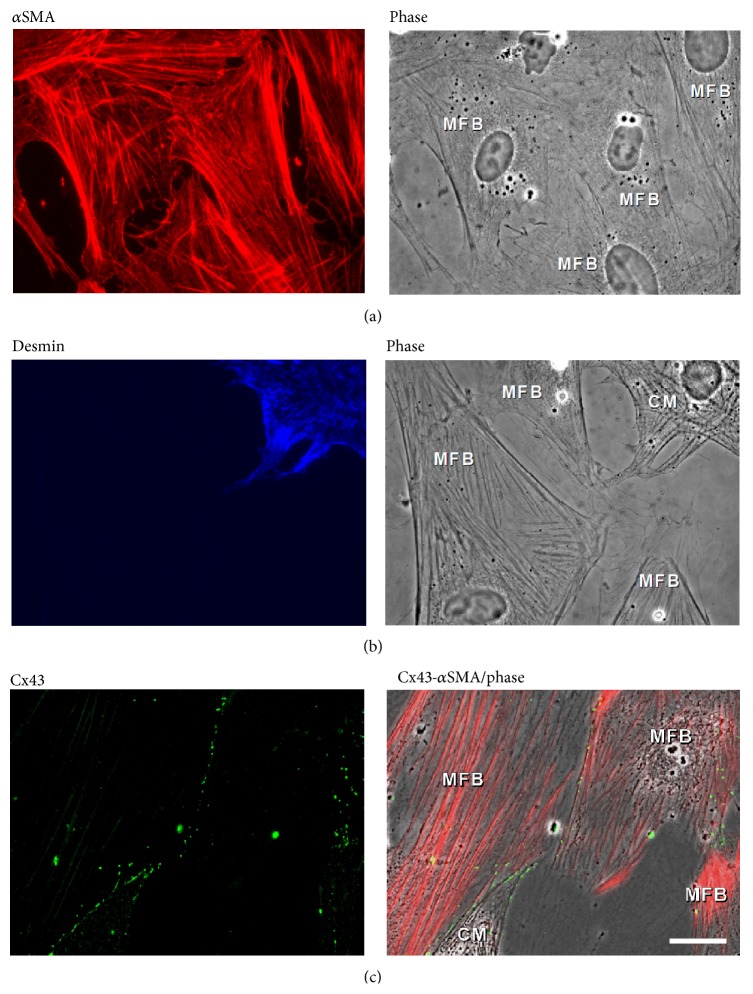
It is assumed that MFBs primarily differentiate from resident fibroblasts. This process is initiated by transforming growth factor β (TGFβ), followed by an activation of several “canonical” cellular pathways (Smad, ERK, P38 kinase, AP-1 but not JNK) [59]. In culture, neonatal rat cardiac fibroblasts undergo transdifferentiation into MFBs. De novo expression of αSMA increases in parallel with the expression of connexin43 (Cx43) [70]; thus, cutback in expression of Cx43 by small interfering RNAs technique significantly inhibits αSMA expression. There is evidence that fibroblasts in the infarct scar tissue express Cx43 and Cx45 [71]. Other investigations have demonstrated that these fibroblasts are in fact (myo) fibroblasts (Figure 4) [72]. However, questions about electrical coupling between MFBs and cardiomyocytes remain unanswered. It is essential to note that it is not yet reported whether MFBs in vivo establish a heterocellular electronic coupling with cardiomyocytes. We have successfully engineered the heterocellular contact in vitro by coculturing neonatal rat cardiomyocytes and MFBs from cardiac origin (Figure 3). As expected [73], these fibroblasts became MFBs when cultured on rigid substrates (glass coverslips). This was confirmed by observed de novo expression of αSMA (Figure 3(a)). We have also demonstrated that in vitro MFBs express gap junction proteins Cx43 and 45 (not Cx40) at MFB-MFB cell-cell contacts and importantly also at MFB-CM cell-cell contacts (Figure 3(c)) [7]. The successful establishment of this heterocellular contact in vitro together with our previous investigations into a variety of histoarchitectures in vivo now allows for the study of two different situations normally encountered in the infarcted heart.
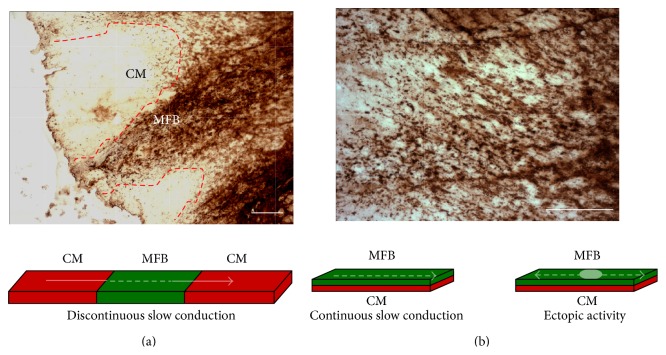
Figure 4.
Characteristics of heterocellular interaction between myofibroblasts and cardiomyocytes in regionally infarcted rat heart. (a) Top. Immunohistochemistry picture of rat heart slices after 37 weeks of coronary occlusion. A region of myofibroblasts (brown) stained for αSMA physically separates two bundles of cardiac myocytes. Bottom. Schematic representation of heterocellular culture mimics the in vivo situation. (b) Same as A with a MFBs stratum infiltrated into cardiac tissue (top) with schematic represented situation (bottom) (with courtesy: Dr. Alex Lyon, NHLI, Imperial College, London. Unpublished).
Figure 3.
Phenotype characteristics and electrical coupling between myofibroblasts and cardiomyocytes. (a) Immunocytochemistry and phase contrast picture shows expression of αSMA (red) in cardiac fibroblasts, which have differentiated to myofibroblasts after 3 days in culture. (b) Parenchymal cardiomyocytes and not stromal myofibroblasts express desmin (blue). (c) Myofibroblast αSMA positive cells (red) express Cx43 (green) at cell-cell contacts and at contacts with cardiomyocytes. The corresponding phase contrast picture shows spatially the contact between coculture of myofibroblasts and cardiomyocytes (reprinted with permission from [5]).
Immunohistochemical images of chronic infarct in rat cardiac tissue (37 weeks) shown in Figure 4 demonstrate that there is an intimate contact between the areas heavy populated with MFBs (αSMA, brown) and the areas of surviving cardiomyocytes (white). We hypothesised that an area of MFBs might (i) interrupt or affect impulse propagation and (ii) induce ectopic activity, due to electrical coupling.
5. Areas of Myofibroblasts Linking Up Separate Bundles of Cardiomyocytes
The general assumption is that cardiac impulse conduction is blocked at site where cardiomyocyte areas are in contact with collagenous septa or with fibrotic tissue (like infarct regions or sutures site follow heart transplantation). This latter case sporadically reports an unexpected synchronization between donor and recipient heart [74]. Because MFBs are present in the fibrotic tissue, we tested this hypothesis, by engineering the situation represented in Figure 4(a) top, in vitro, by seeding cardiomyocytes in a geometrical defined pattern, and interrupted them with an insert of MFBs (Figure 4(a), bottom).
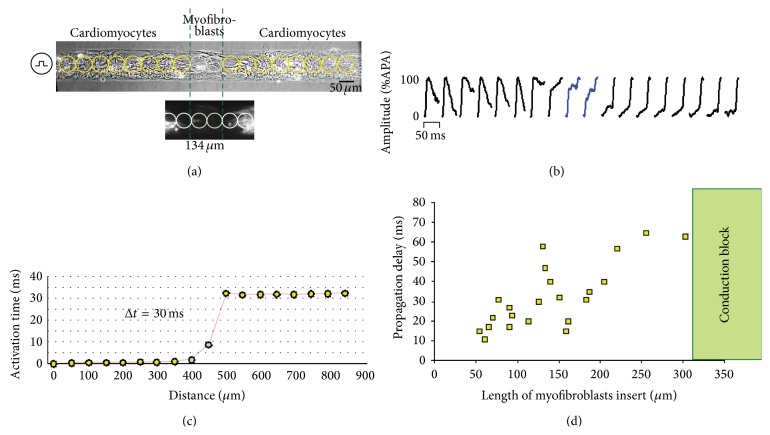
Details of the experiments are represented in Figure 5(a) [6]. Strands were stimulated from the left hand side and the characteristics of impulse propagation were assessed optically after being exposed to a voltage sensitive dye [75]. For the final analysis, we took into consideration only the insert of MFBs without “cardiomyocytes contamination” (Figure 5(a), lower panel). Each photodiode recorded an optical action potential upstroke, which was correlated with the activation time. Whereas activation time was rapid in the cardiomyocyte area, a passive local electrotonic transmission induced a delay of 30 ms across the MFB insert (Figure 5(c)). As shown in Figure 5(d), the delay is strictly related to the length of the insert. Under these experimental conditions MFBs can support impulse propagation up to ~320 μm; at lengths greater than this, propagation invariably failed. These experiments demonstrate that the heterocellular electrical coupling between the two cell populations can reinstate conduction across an interrupted network of cardiomyocytes resulting in a discontinuity of propagation. In consequence, one has to take into consideration that patchy fibrosis as encountered in the remodelled atria (i.e., ageing) [76] may alter the normal pattern of propagation by inducing discontinuous slow conduction, playing a key role in the context of reentrant circuits.
Figure 5.
Myofibroblasts act as passive electrical paths for impulse conduction. (a) Heterocellular construct of a left-stimulated strand of cardiomyocytes (top) interrupt by a pure region (length = 134 μm) of myomesin-deficient myofibroblasts (bottom). Circles indicate the optical mapped area detected from each photodetector. (b) Optical action potential upstrokes recorded in a detected length of ~50 μm. (c) Activation times reconstruct from each action potential upstrokes; in both cardiomyocytes areas, the activation is almost immediate whereas the propagation into myofibroblast area exhibits a delay of 30 ms. (d) Summary of propagated delays related to the inserts' length, therefore indicating a passive electronic transmission of up to ~320 μm (modified with permission from [6]).
6. Myofibroblasts Overlaid as to Completely Cover an Area of Cardiomyocytes
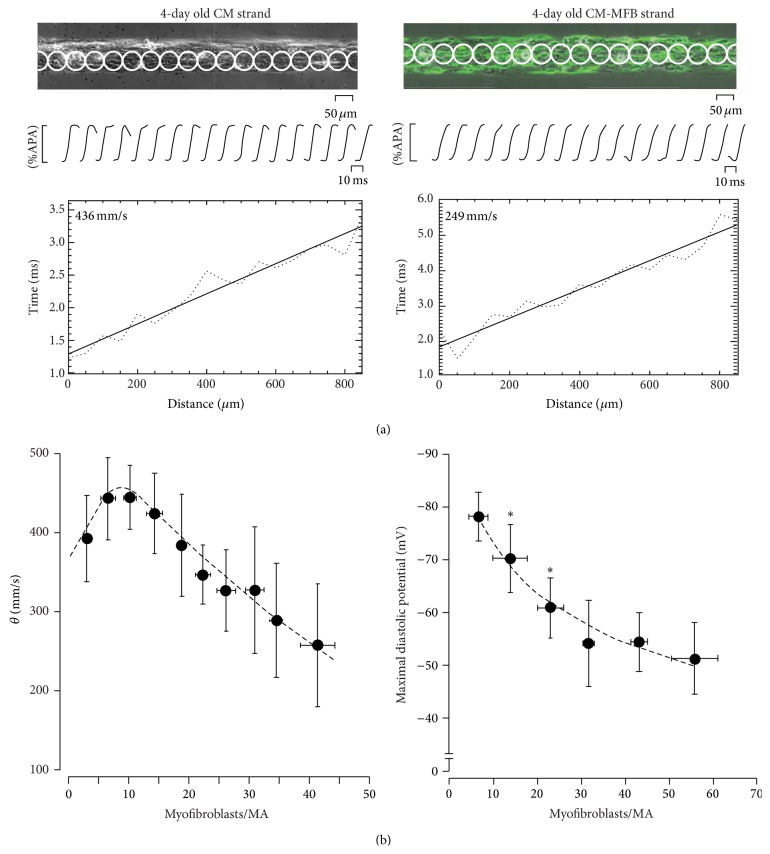
To further investigate any direct electrical coupling consequences, MFBs were also cultured in order to overlay the cardiomyocytes. In Figure 4(b), the situation is such that the fibrotic area is heavily populated with MFBs, which infiltrate and diffuse throughout the area of cardiomyocytes, thus increasing the heterotissue interaction (as compared with Figure 4(a)). Due to the depolarized resting membrane potential of MFBs [7], we hypothesized that these circumstances, together with the increased electrical cell-to-cell interaction area, might produce a large depolarized region which can affect local impulse propagation. This, in turn, might reduce the conduction velocity for the cardiac tissue, which is in contact with MFBs. Utilising the same pattern growth technique we engineered 80 μm wide strands of neonatal rat ventricular cardiomyocytes, to which a layer of MFBs is seeded on top of [7] (Figure 4(b), bottom). The conduction velocity (θ) in control preparations, which are virtually devoid of MFBs, is high (~43 cm/s, Figure 6(a), left). However, the presence of MFBs drastically reduces θ by up to 25 cm/s (Figure 6(a), right). An overall analysis of θ dependence on MFB coverage area is represented in Figure 6(b) left; θ denotes biphasic behaviour towards the number of MFBs per measured area. This behaviour is highly reminiscent of the phenomena of supernormal conduction in cardiac tissue investigated both in vivo [77] and in vitro [78]. Both demonstrated that θ is biphasically dependent on the gradual increment of extracellular potassium concentration. Similar, but in a MFBs density-dependent manner, our results show a similar behaviour, suggesting that MFBs may directly depolarize cardiomyocytes resembling the well-known depolarizing effect of [K+]out. This hypothesis was proved by conventional intracellular microelectrode techniques for measuring diastolic resting membrane potential (V m). We found that MFBs gradually depolarized cardiomyocytes in a density-dependent manner (Figure 6(b), right) where recorded V m dropped from ~−80 mV at a MFB density less than 5% to ~−55 mV when more than 40% of the cardiomyocytes area is covered by MFBs. This data propose that, assuming the same effect in vivo at the epicardial border zone where the minimal wall thickness is comparable to a two-dimensional layer, infiltrated laminae of MFBs might induce epicardium slow conduction velocity. In contrast, in a 3D architecture (ventricular wall) the coupling of cardiomyocytes bordering infarct area might counterbalance this depolarizing effect. The consequences in the context of AF are clear: MFBs can directly depolarize the surrounding cardiomyocytes tissue and thus lead to local conduction slowing and enhance the likelihood of arrhythmia (cf. paragraph below).
Figure 6.
Impulse conduction in myofibroblasts coated cardiomyocyte strands. (a) Same as Figure 3(a) for optical action potential recording in a pure (left) and αSMA positive myofibroblasts (green) coated cardiomyocyte strand (right). Heterocellular construct shows a reduced conduction velocity (249 mm/s) compared to control (436 mm/s, p < 0.001). (b) Overall analysis of conduction velocity and maximal diastolic potential related to MFBs density calculated as number of cells per measurement area. Left: conduction velocity denotes biphasic behaviour due to the supernormal sodium based conduction follow gradual depolarization that occurs from the increment of myofibroblasts density. Right: myofibroblasts reduce maximal diastolic potential in a cell density-dependent manner (modified with permission from [7]).
7. Myofibroblasts Induce Ectopic Activity in Cardiac Tissue
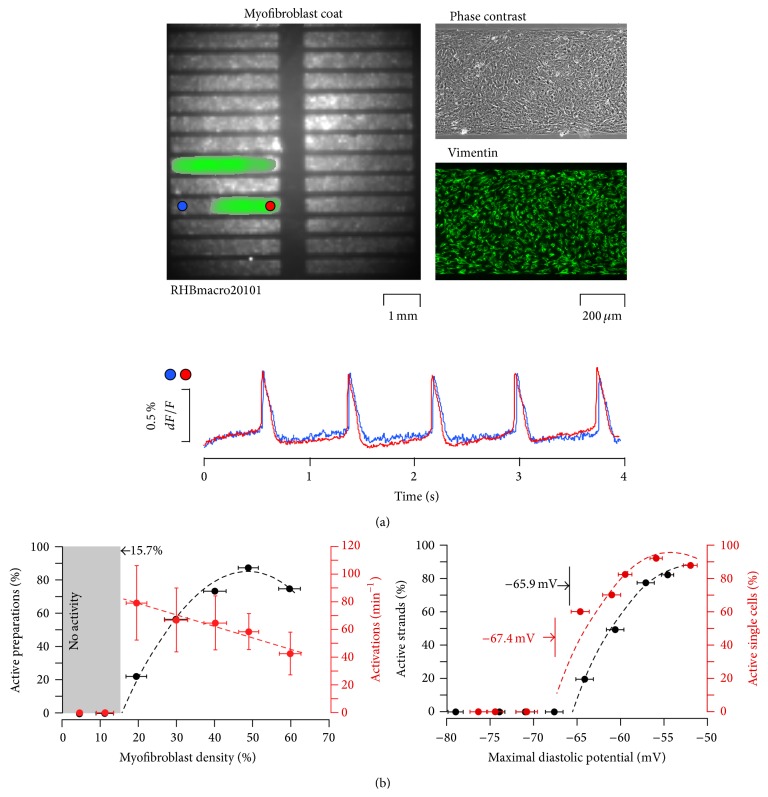
The last part of the study sought to investigate the intimate contact between cardiomyocytes and MFBs (Figure 4(b), right) and how ectopic electrical activity was elicited following heterocellular coupling [8]. An in vitro fibrotic situation was created by coating strands of neonatal rat ventricular myocytes with increasing densities of MFBs (Figure 7). Spontaneous electrical activities were recorded for 4 seconds. The overview of preparation in Figure 7(a) corresponds to a single frame taken from such recording. In this point in time the quasicompletely coverage of MFB elicits spontaneous activity in two strands; the recording shows action potentials occurred regularly with a frequency of 75 bpm. Crucially, the presence of spontaneous activity is strictly proportional to MFBs densities (Figure 7, top). When MFB coverage was below 16% all the preparations were invariably quiescent; however, when coverage was above ~80%, all preparations exhibited spontaneous activity. In contrast, we found beat frequency reduced from ~80 bpm (20% MFBs density) to ~40 bpm (60% MFBs density) due to progressive reduction in the diastolic membrane potential (Figure 6(b)). A comparison of cardiac membrane potential between isolated cardiomyocytes and heterocellular strands is demonstrated in Figure 7(b), right. Gradual reduction of resting V m in single cardiomyocytes was examined using a patch clamp technique. Stepwise depolarization during injection of 30 sec long current pulses exhibits electrical spontaneous activity elicited at membrane potential less negative than −67 mV. These results were similar to those found in the heterocellular strands where spontaneous impulse initiation was induced with a minimal density of MFBs corresponding to ~−66 mV. These findings indicate that the heterocellular coupling between MFBs and cardiomyocytes might structurally form an ectopic focus. The firing area is preferentially generated from MFBs and not from the injured cardiomyocytes. In our experiments, the cardiac network appears healthy but ectopic activity could be as well induced.
Figure 7.
Myofibroblasts induce ectopic activity. (a) Upper row: overview of a preparation consisted of 24 myofibroblasts coated with cardiomyocyte strands (0.6 × 4.5 mm). Right: details of the microarchitecture (a phase contrast photo) and of the MFB cover layer (vimentin immunostaining) of an individual strand. Lower row: propagated action potential recorded optically at specific sites (blue and red circles in the overview). (b) Left: ectopic spontaneously active strands and spontaneous frequency correlate with myofibroblast density. Spontaneous activity is invariably absent when myofibroblast density is less than 15.7% of the total area examined. Right: electrical spontaneous activity correlated with maximal diastolic potential. Similar to active strands where membrane potential threshold for elicit automaticity is −65.9 mV, single cardiomyocytes elicit spontaneous action potentials at −67.4 mV (modified with permission from [8]).
8. Is the Myofibroblast a Possible Target for Atrial Fibrillation?
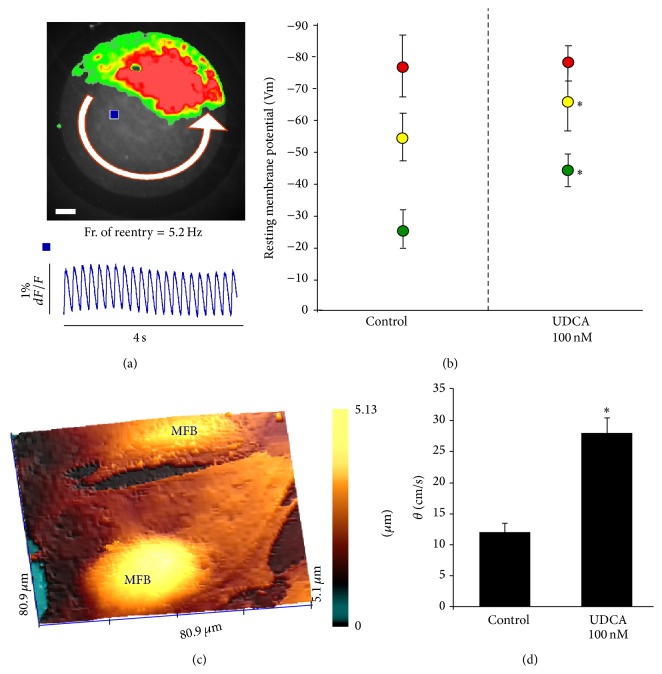
Recently we have investigated the possibility to target MFB in order to suppress electrical disorders in the heart. Thanks to the collaboration with Professor Gorelik and Professor Williamson at Imperial College London, we have discovered that MFBs transiently appear during heart development and they can be responsible for fetal arrhythmias [79]. Clinically, it has been associated with a pregnant disease called intrahepatic cholestasis and the prognosis ameliorates after administration of ursodeoxycholic acid (UDCA). Because MFBs tend to depolarize the coupled cardiomyocytes, we sought to investigate whether UDCA may directly target MFBs. We found that UDCA hyperpolarizes MFB membrane potential by targeting the sulphonylurea receptor of IK1 channel [9], reestablishing the normal conduction velocity and terminating reentrant arrhythmia (Figure 8). A double-blind randomized placebo-controlled crossover trial is under investigation by administering UDCA in patient with chronic heart failure [80].
Figure 8.
Myofibroblast as a possible cell target for antiarrhythmic therapy. (a) Reentrant excitation on myofibroblasts coated cardiomyocytes monolayer. Top: colour coded reentrant propagation. Bottom optical action potential traces. (b) UDCA hyperpolarizes MFBs membrane potential only. Red circles: cardiomyocytes monolayers. Yellow circles: heterocellular monolayers. Green circles: MFB monolayers. p < 0.05. (c) Topographical images obtained by scanning ion conductance microscopy of a MFB embedded in a monolayer. (d) Effect of UDCA on impulse propagation velocity in myofibroblast coated with cardiomyocytes strands (modified with permission from [9]).
9. Outlook
Electrical communication between the stromal and parenchyma tissue has been the focus of much research over the last 50 years. Certain “myths,” like that we are using a total of 10% of our brain, have been dispelled, including the discovery that indeed only 10% of the human brain is made of neurons, with the rest comprised of “Glia,” identified as stromal “glue” or nerve “putty,” which merely fills the spaces within the parenchyma tissue. In the last two decades researchers have shown that Glia cells express gap junctions and interact directly with neurons [81, 82]. In vitro, stromal cardiac MFBs are electrically and mechanically coupled with cardiomyocytes and this pairing disturbs the electrical homeostasis of the parenchymal cardiac tissue [83, 84]. If the same situation in pathological cardiac tissue will be observed in vivo, MFBs could be considered as a new cellular target for cardiac arrhythmia (Figure 9). Strategies might focus on (i) inducing the MFBs “inactive” (overturn the phenotype back to fibroblast and thus circumvent the electrical coupling) or (ii) targeting these cells, pharmacologically or genetically, for radically hyperpolarization [9, 85]. Evidence obtained so far requires further characterization in order to fully understand the impact(s) of heterocellular interactions upon the complex 3D remodelling of the cytoarchitecture, which occurs during heart failure.
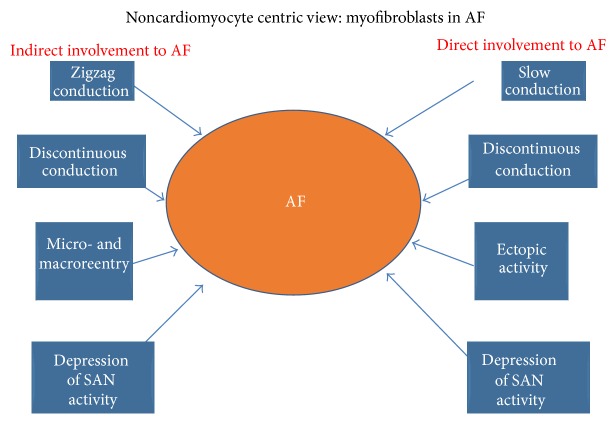
Figure 9.
Schematic representation of a “well-known” (indirect) and “proposed” (direct) involvement of myofibroblasts in atrial fibrillation (AF).
Interestingly, there is speculation that MFBs might appear transiently during heart development and follow the partial state of fetal hypoxia [64] and in the aged heart [76, 86]. Additional studies are necessary to understand if MFBs are not only proarrhythmogenic in heart failure but also during heart development and aging; these are frequently subjected to other pathological “MFB-triggering” situations (diabetes, autoimmune disorder, and metabolic diseases).
Problems regarding engraftment for tissue regeneration have also been investigated and reveal that if other cells, less polarised than cardiomyocytes, were to form gap junctions with cardiomyocytes, spontaneous activity could be induced [87]. This was highlighted by our study where coating cardiomyocytes with Cx43-transfected-HeLa cells ([8], data not shown) give rise to spontaneous activity. Further studies are necessary in order to understand and predict an accurate arrhythmogenic mechanism following cell engraftment in heart failure models using possibly or conductive patches [88], embryonic cardiomyocytes [89], and progenitor stem cells [90]. Regarding the cell therapy, it is also unclear whether engraftment will perturb the electrical homeostasis of cardiac tissue due to an intrinsic resting membrane potential, and there exists the possibility that the engrafted cells might electrically couple with MFBs. Both of these may cause the regenerated cardiac tissue engraftment to become an unexpected source of arrhythmia.
Acknowledgments
This work is funded by Young Research Grant GR-2009.1530528, Italian Ministry of Health, to Michele Miragoli and Flagship Project Nanomax, (miRNano), Italian National Research Council, to Michele Miragoli.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Banerjee I., Fuseler J. W., Price R. L., Borg T. K., Baudino T. A. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. The American Journal of Physiology—Heart and Circulatory Physiology. 2007;293(3):H1883–H1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 2.Rohr S. Myofibroblasts in diseased hearts: new players in cardiac arrhythmias? Heart Rhythm. 2009;6(6):848–856. doi: 10.1016/j.hrthm.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 3.Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiological Reviews. 1999;79(1):215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 4.Glukhov A. V., Kalyanasundaram A., Lou Q., et al. Calsequestrin 2 deletion causes sinoatrial node dysfunction and atrial arrhythmias associated with altered sarcoplasmic reticulum calcium cycling and degenerative fibrosis within the mouse atrial pacemaker complex. European Heart Journal. 2015;36(11):686–697. doi: 10.1093/eurheartj/eht452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zipes D. P., Jalife J. Cardiac Electrophysiology: From Cell to Bedside. 5th. Philadelphia, Pa, USA: Saunders/Elsevier; 2009. [Google Scholar]
- 6.Gaudesius G., Miragoli M., Thomas S. P., Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circulation Research. 2003;93(5):421–428. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 7.Miragoli M., Gaudesius G., Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circulation Research. 2006;98(6):801–810. doi: 10.1161/01.RES.0000214537.44195.a3. [DOI] [PubMed] [Google Scholar]
- 8.Miragoli M., Salvarani N., Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circulation Research. 2007;101(8):755–758. doi: 10.1161/CIRCRESAHA.107.160549. [DOI] [PubMed] [Google Scholar]
- 9.Miragoli M., Sheikh Abdul Kadir S. H., Sheppard M. N., et al. A protective antiarrhythmic role of ursodeoxycholic acid in an in vitro rat model of the cholestatic fetal heart. Hepatology. 2011;54(4):1282–1292. doi: 10.1002/hep.24492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adamson P. B., Barr R. C., Callans D. J., et al. The perplexing complexity of cardiac arrhythmias: beyond electrical remodeling. Heart Rhythm. 2005;2(6):650–659. doi: 10.1016/j.hrthm.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Heijman J., Voigt N., Nattel S., Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circulation Research. 2014;114(9):1483–1499. doi: 10.1161/circresaha.114.302226. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki Y. K., Nishida K., Kato T., Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124(20):2264–2274. doi: 10.1161/circulationaha.111.019893. [DOI] [PubMed] [Google Scholar]
- 13.Kontogeorgis A., Li X., Kang E. Y., et al. Decreased connexin43 expression in the mouse heart potentiates pacing-induced remodeling of repolarizing currents. The American Journal of Physiology—Heart and Circulatory Physiology. 2008;295(5):H1905–H1916. doi: 10.1152/ajpheart.590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frustaci A., Chimenti C., Bellocci F., Morgante E., Russo M. A., Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96(4):1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 15.Kostin S., Klein G., Szalay Z., Hein S., Bauer E. P., Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovascular Research. 2002;54(2):361–379. doi: 10.1016/S0008-6363(02)00273-0. [DOI] [PubMed] [Google Scholar]
- 16.Anyukhovsky E. P., Sosunov E. A., Plotnikov A., et al. Cellular electrophysiologic properties of old canine atria provide a substrate for arrhythmogenesis. Cardiovascular Research. 2002;54(2):462–469. doi: 10.1016/s0008-6363(02)00271-7. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi H., Wang C., Miyauchi Y., et al. Aging-related increase to inducible atrial fibrillation in the rat model. Journal of Cardiovascular Electrophysiology. 2002;13(8):801–808. doi: 10.1046/j.1540-8167.2002.00801.x. [DOI] [PubMed] [Google Scholar]
- 18.Li D., Fareh S., Leung T. K., Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100(1):87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 19.Boldt A., Wetzel U., Lauschke J., et al. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart. 2004;90(4):400–405. doi: 10.1136/hrt.2003.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J., Cui G., Esmailian F., et al. Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation. 2004;109(3):363–368. doi: 10.1161/01.cir.0000109495.02213.52. [DOI] [PubMed] [Google Scholar]
- 21.Sanfilippo A. J., Abascal V. M., Sheehan M., et al. Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation. 1990;82(3):792–797. doi: 10.1161/01.cir.82.3.792. [DOI] [PubMed] [Google Scholar]
- 22.Verheule S., Wilson E., Everett T., IV, Shanbhag S., Golden C., Olgin J. Alterations in atrial electrophysiology and tissue structure in a canine model of chronic atrial dilatation due to Mitral Regurgitation. Circulation. 2003;107(20):2615–2622. doi: 10.1161/01.CIR.0000066915.15187.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knackstedt C., Gramley F., Schimpf T., et al. Association of echocardiographic atrial size and atrial fibrosis in a sequential model of congestive heart failure and atrial fibrillation. Cardiovascular Pathology. 2008;17(5):318–324. doi: 10.1016/j.carpath.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Derakhchan K., Li D., Courtemanche M., et al. Method for simultaneous epicardial and endocardial mapping of in vivo canine heart: application to atrial conduction properties and arrhythmia mechanisms. Journal of Cardiovascular Electrophysiology. 2001;12(5):548–555. doi: 10.1046/j.1540-8167.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- 25.Stambler B. S., Fenelon G., Shepard R. K., Clemo H. F., Guiraudon C. M. Characterization of sustained atrial tachycardia in dogs with rapid ventricular pacing-induced heart failure. Journal of Cardiovascular Electrophysiology. 2003;14(5):499–507. doi: 10.1046/j.1540-8167.2003.02519.x. [DOI] [PubMed] [Google Scholar]
- 26.Fenelon G., Shepard R. K., Stambler B. S. Focal origin of atrial tachycardia in dogs with rapid ventricular pacing-induced heart failure. Journal of Cardiovascular Electrophysiology. 2003;14(10):1093–1102. doi: 10.1046/j.1540-8167.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- 27.Elvan A., Wylie K., Zipes D. P. Pacing-induced chronic atrial fibrillation impairs sinus node function in dogs: electrophysiological remodeling. Circulation. 1996;94(11):2953–2960. doi: 10.1161/01.cir.94.11.2953. [DOI] [PubMed] [Google Scholar]
- 28.Swaminathan P. D., Purohit A., Soni S., et al. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. The Journal of Clinical Investigation. 2011;121(8):3277–3288. doi: 10.1172/jci57833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Opthof T., Coronel R., Rademaker H. M. E., Vermeulen J. T., Wilms-Schopman F. J. G., Janse M. J. Changes in sinus node function in a rabbit model of heart failure with ventricular arrhythmias and sudden death. Circulation. 2000;101(25):2975–2980. doi: 10.1161/01.CIR.101.25.2975. [DOI] [PubMed] [Google Scholar]
- 30.Sanders P., Kistler P. M., Morton J. B., Spence S. J., Kalman J. M. Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation. 2004;110(8):897–903. doi: 10.1161/01.cir.0000139336.69955.ab. [DOI] [PubMed] [Google Scholar]
- 31.Yanni J., Tellez J. O., Sutyagin P. V., Boyett M. R., Dobrzynski H. Structural remodelling of the sinoatrial node in obese old rats. Journal of Molecular and Cellular Cardiology. 2010;48(4):653–662. doi: 10.1016/j.yjmcc.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dobrzynski H., Boyett M. R., Anderson R. H. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007;115(14):1921–1932. doi: 10.1161/circulationaha.106.616011. [DOI] [PubMed] [Google Scholar]
- 33.Boyett M. R., Honjo H., Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovascular Research. 2000;47(4):658–687. doi: 10.1016/s0008-6363(00)00135-8. [DOI] [PubMed] [Google Scholar]
- 34.Fedorov V. V., Glukhov A. V., Chang R. Conduction barriers and pathways of the sinoatrial pacemaker complex: their role in normal rhythm and atrial arrhythmias. American Journal of Physiology: Heart and Circulatory Physiology. 2012;302(9):H1773–H1783. doi: 10.1152/ajpheart.00892.2011. [DOI] [PubMed] [Google Scholar]
- 35.Liu J., Dobrzynski H., Yanni J., Boyett M. R., Lei M. Organisation of the mouse sinoatrial node: structure and expression of HCN channels. Cardiovascular Research. 2007;73(4):729–738. doi: 10.1016/j.cardiores.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 36.Glukhov A. V., Hage L. T., Hansen B. J., et al. Sinoatrial node reentry in a canine chronic left ventricular infarct model role of intranodal fibrosis and heterogeneity of refractoriness. Circulation: Arrhythmia and Electrophysiology. 2013;6(5):984–994. doi: 10.1161/circep.113.000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joyner R. W., van Capelle F. J. Propagation through electrically coupled cells. How a smalll SA node drives a large atrium. Biophysical Journal. 1986;50(6):1157–1164. doi: 10.1016/s0006-3495(86)83559-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glukhov A. V., Fedorov V. V., Anderson M. E., Mohler P. J., Efimov I. R. Functional anatomy of the murine sinus node: high-resolution optical mapping of ankyrin-B heterozygous mice. American Journal of Physiology—Heart and Circulatory Physiology. 2010;299(2):H482–H491. doi: 10.1152/ajpheart.00756.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lange G. Action of driving stimuli from intrinsic and extrinsic sources on in situ cardiac pacemaker tissues. Circulation Research. 1965;17(5):449–459. doi: 10.1161/01.res.17.5.449. [DOI] [PubMed] [Google Scholar]
- 40.Boineau J. P., Schuessler R. B., Mooney C. R., et al. Multicentric origin of the atrial depolarization wave: the pacemaker complex. Relation to dynamics of atrial conduction, P-wave changes and heart rate control. Circulation. 1978;58(6):1036–1048. doi: 10.1161/01.cir.58.6.1036. [DOI] [PubMed] [Google Scholar]
- 41.Fedorov V. V., Glukhov A. V., Chang R., et al. Optical mapping of the isolated coronary-perfused human sinus node. Journal of the American College of Cardiology. 2010;56(17):1386–1394. doi: 10.1016/j.jacc.2010.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaskell W. H. On the innervation of the heart, with especial reference to the heart of the tortoise. Annals of Noninvasive Electrocardiology. 2000;5(3):292–311. [Google Scholar]
- 43.Fedorov V. V., Trifonova O. P., Glukhov A. V., Rosen M. R., Rosenshtraukh L. V. The role of mechano-electrical feedback in the cholinergic atrial fibrillation initiation. In: Kamkin A., Kiseleva I., editors. Mechanosensitivity in Cells and Tissues. Moscow, Russia: Academia; 2005. [PubMed] [Google Scholar]
- 44.Sharifov O. F., Fedorov V. V., Beloshapko G. G., Glukhov A. V., Yushmanova A. V., Rosenshtraukh L. V. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. Journal of the American College of Cardiology. 2004;43(3):483–490. doi: 10.1016/j.jacc.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 45.Bagwe S., Berenfeld O., Vaidya D., Morley G. E., Jalife J. Altered right atrial excitation and propagation in connexin40 knockout mice. Circulation. 2005;112(15):2245–2253. doi: 10.1161/CIRCULATIONAHA.104.527325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leaf D. E., Feig J. E., Vasquez C., et al. Connexin40 imparts conduction heterogeneity to atrial tissue. Circulation Research. 2008;103(9):1001–1008. doi: 10.1161/circresaha.107.168997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ozcan C., Battaglia E., Young R., Suzuki G. LKB1 knockout mouse develops spontaneous atrial fibrillation and provides mechanistic insights into human disease process. Journal of the American Heart Association. 2015;4(3) doi: 10.1161/jaha.114.001733.e001733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Egom E. E., Vella K., Hua R., et al. Impaired sinoatrial node function and increased susceptibility to atrial fibrillation in mice lacking natriuretic peptide receptor C. The Journal of Physiology. 2015;593(5):1127–1146. doi: 10.1113/jphysiol.2014.283135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saba S., Janczewski A. M., Baker L. C., et al. Atrial contractile dysfunction, fibrosis, and arrhythmias in a mouse model of cardiomyopathy secondary to cardiac-specific overexpression of tumor necrosis factor-α . American Journal of Physiology—Heart and Circulatory Physiology. 2005;289(4):H1456–H1467. doi: 10.1152/ajpheart.00733.2004. [DOI] [PubMed] [Google Scholar]
- 50.Fauconnier J., Meli A. C., Thireau J., et al. Ryanodine receptor leak mediated by caspase-8 activation leads to left ventricular injury after myocardial ischemia-reperfusion. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(32):13258–13263. doi: 10.1073/pnas.1100286108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Pasquale E., Lodola F., Miragoli M., et al. CaMKII inhibition rectifies arrhythmic phenotype in a patient-specific model of catecholaminergic polymorphic ventricular tachycardia. Cell Death and Disease. 2013;4(10):p. e843. doi: 10.1038/cddis.2013.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Erickson J. R., Joiner M.-L. A., Guan X., et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133(3):462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benamer N., Vasquez C., Mahoney V. M., Steinhardt M. J., Coetzee W. A., Morley G. E. Fibroblast KATP currents modulate myocyte electrophysiology in infarcted hearts. American Journal of Physiology: Heart and Circulatory Physiology. 2013;304(9):H1231–H1239. doi: 10.1152/ajpheart.00878.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen T. P., Xie Y., Garfinkel A., Qu Z., Weiss J. N. Arrhythmogenic consequences of myofibroblastmyocyte coupling. Cardiovascular Research. 2012;93(2):242–251. doi: 10.1093/cvr/cvr292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. The Journal of Pathology. 2003;200(4):500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 56.Hinz B., Gabbiani G. Cell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodeling. Thrombosis and Haemostasis. 2003;90(6):993–1002. doi: 10.1160/TH03-05-0328. [DOI] [PubMed] [Google Scholar]
- 57.van Amerongen M. J., Bou-Gharios G., Popa E. R., et al. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. The Journal of Pathology. 2008;214(3):377–386. doi: 10.1002/path.2281. [DOI] [PubMed] [Google Scholar]
- 58.Bellini A., Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Laboratory Investigation. 2007;87(9):858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 59.Hu Y., Peng J., Feng D., et al. Role of extracellular signal-regulated kinase, p38 kinase, and activator protein-1 in transforming growth factor-β1-induced alpha smooth muscle actin expression in human fetal lung fibroblasts in vitro. Lung. 2006;184(1):33–42. doi: 10.1007/s00408-005-2560-5. [DOI] [PubMed] [Google Scholar]
- 60.Jiao J., Friedman S. L., Aloman C. Hepatic fibrosis. Current Opinion in Gastroenterology. 2009;25(3):223–229. doi: 10.1097/mog.0b013e3283279668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jaster R., Emmrich J. Crucial role of fibrogenesis in pancreatic diseases. Best Practice and Research in Clinical Gastroenterology. 2008;22(1):17–29. doi: 10.1016/j.bpg.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Hinz B., Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Current Opinion in Biotechnology. 2003;14(5):538–546. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 63.Sen C. K., Khanna S., Roy S. Perceived hyperoxia: oxygen-induced remodeling of the reoxygenated heart. Cardiovascular Research. 2006;71(2):280–288. doi: 10.1016/j.cardiores.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 64.Clancy R. M., Zheng P., O'Mahony M., et al. Role of hypoxia and cAMP in the transdifferentiation of human fetal cardiac fibroblasts: implications for progression to scarring in autoimmune-associated congenital heart block. Arthritis & Rheumatism. 2007;56(12):4120–4131. doi: 10.1002/art.23061. [DOI] [PubMed] [Google Scholar]
- 65.Gabbiani G., Chaponnier C., Huttner I. Cytoplasmic filaments and gap junctions in epithelial cells and myofibroblasts during wound healing. The Journal of Cell Biology. 1978;76(3):561–568. doi: 10.1083/jcb.76.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Powell D. W., Mifflin R. C., Valentich J. D., Crowe S. E., Saada J. I., West A. B. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. American Journal of Physiology—Cell Physiology. 1999;277(2):C183–C201. doi: 10.1152/ajpcell.1999.277.2.C183. [DOI] [PubMed] [Google Scholar]
- 67.Fry C. H., Sui G.-P., Kanai A. J., Wu C. The function of suburothelial myofibroblasts in the bladder. Neurourology and Urodynamics. 2007;26(6, supplement):914–919. doi: 10.1002/nau.20483. [DOI] [PubMed] [Google Scholar]
- 68.Sun Y., Kiani M. F., Postlethwaite A. E., Weber K. T. Infarct scar as living tissue. Basic Research in Cardiology. 2002;97(5):343–347. doi: 10.1007/s00395-002-0365-8. [DOI] [PubMed] [Google Scholar]
- 69.Van Den Borne S. W. M., Diez J., Blankesteijn W. M., Verjans J., Hofstra L., Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nature Reviews Cardiology. 2010;7(1):30–37. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 70.Asazuma-Nakamura Y., Dai P., Harada Y., Jiang Y., Hamaoka K., Takamatsu T. Cx43 contributes to TGF-beta signaling to regulate differentiation of cardiac fibroblasts into myofibroblasts. Experimental Cell Research. 2009;315(7):1190–1199. doi: 10.1016/j.yexcr.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 71.Camelliti P., Devlin G. P., Matthews K. G., Kohl P., Green C. R. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovascular Research. 2004;62(2):415–425. doi: 10.1016/j.cardiores.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 72.Willems I. E. M. G., Havenith M. G., De Mey J. G. R., Daemen M. J. A. P. The α-smooth muscle actin-positive cells in healing human myocardial scars. American Journal of Pathology. 1994;145(4):868–875. [PMC free article] [PubMed] [Google Scholar]
- 73.Wang J., Seth A., Mcculloch C. A. Force regulates smooth muscle actin in cardiac fibroblasts. The American Journal of Physiology—Heart and Circulatory Physiology. 2000;279(6):H2776–H2785. doi: 10.1152/ajpheart.2000.279.6.H2776. [DOI] [PubMed] [Google Scholar]
- 74.Bexton R. S., Hellestrand K. J., Cory-Pearce R., et al. Unusual atrial potentials in a cardiac transplant recipient. Possible synchronization between donor and recipient atria. Journal of Electrocardiology. 1983;16(3):313–321. doi: 10.1016/s0022-0736(83)80012-0. [DOI] [PubMed] [Google Scholar]
- 75.Rohr S. Determination of impulse conduction characteristics at a microscopic scale in patterned growth heart cell cultures using multiple site optical recording of transmembrane voltage. Journal of Cardiovascular Electrophysiology. 1995;6(7):551–568. doi: 10.1111/j.1540-8167.1995.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 76.Rossi S., Fortunati I., Carnevali L., et al. The effect of aging on the specialized conducting system: a telemetry ECG study in rats over a 6 month period. PLoS ONE. 2014;9(11) doi: 10.1371/journal.pone.0112697.e112697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kagiyama Y., Hill J. L., Gettes L. S. Interaction of acidosis and increased extracellular potassium on action potential characteristics and conduction in guinea pig ventricular muscle. Circulation Research. 1982;51(5):614–623. doi: 10.1161/01.RES.51.5.614. [DOI] [PubMed] [Google Scholar]
- 78.Rohr S., Kucera J. P., Kléber A. G. Slow conduction in cardiac tissue, I: effects of a reduction of excitability versus a reduction of electrical coupling on microconduction. Circulation Research. 1998;83(8):781–794. doi: 10.1161/01.res.83.8.781. [DOI] [PubMed] [Google Scholar]
- 79.Williamson C., Miragoli M., Sheikh Abdul Kadir S., et al. Bile acid signaling in fetal tissues: implications for intrahepatic cholestasis of pregnancy. Digestive Diseases. 2011;29(1):58–61. doi: 10.1159/000324130. [DOI] [PubMed] [Google Scholar]
- 80.von Haehling S., Schefold J. C., Jankowska E. A., et al. Ursodeoxycholic acid in patients with chronic heart failure: a double-blind, randomized, placebo-controlled, crossover trial. Journal of the American College of Cardiology. 2012;59(6):585–592. doi: 10.1016/j.jacc.2011.10.880. [DOI] [PubMed] [Google Scholar]
- 81.Nagy J. I., Dudek F. E., Rash J. E. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Research Reviews. 2004;47(1–3):191–215. doi: 10.1016/j.brainresrev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 82.Rouach N., Koulakoff A., Giaume C. Neurons set the tone of gap junctional communication in astrocytic networks. Neurochemistry International. 2004;45(2-3):265–272. doi: 10.1016/j.neuint.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 83.Rosker C., Salvarani N., Schmutz S., Grand T., Rohr S. Abolishing myofibroblast arrhythmogeneicity by pharmacological ablation of α-smooth muscle actin containing stress fibers. Circulation Research. 2011;109(10):1120–1131. doi: 10.1161/circresaha.111.244798. [DOI] [PubMed] [Google Scholar]
- 84.Grand T., Salvarani N., Jousset F., Rohr S. Aggravation of cardiac myofibroblast arrhythmogeneicity by mechanical stress. Cardiovascular Research. 2014;104(3):489–500. doi: 10.1093/cvr/cvu227. [DOI] [PubMed] [Google Scholar]
- 85.Yankelson L., Feld Y., Bressler-Stramer T., et al. Cell therapy for modification of the myocardial electrophysiological substrate. Circulation. 2008;117(6):720–731. doi: 10.1161/CIRCULATIONAHA.106.671776. [DOI] [PubMed] [Google Scholar]
- 86.Rossi S., Baruffi S., Bertuzzi A., et al. Ventricular activation is impaired in aged rat hearts. American Journal of Physiology—Heart and Circulatory Physiology. 2008;295(6):H2336–H2347. doi: 10.1152/ajpheart.00517.2008. [DOI] [PubMed] [Google Scholar]
- 87.Pijnappels D. A., Schalij M. J., van Tuyn J., et al. Progressive increase in conduction velocity across human mesenchymal stem cells is mediated by enhanced electrical coupling. Cardiovascular Research. 2006;72(2):282–291. doi: 10.1016/j.cardiores.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 88.Cingolani E., Ionta V., Cheng K., Giacomello A., Cho H. C., Marban E. Engineered electrical conduction tract restores conduction in complete heart block: from in vitro to in vivo proof of concept. Journal of the American College of Cardiology. 2014;64(24):2575–2585. doi: 10.1016/j.jacc.2014.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roell W., Lewalter T., Sasse P., et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450(7171):819–824. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 90.Kajstura J., Urbanek K., Rota M., et al. Cardiac stem cells and myocardial disease. Journal of Molecular and Cellular Cardiology. 2008;45(4):505–513. doi: 10.1016/j.yjmcc.2008.05.025. [DOI] [PubMed] [Google Scholar]