Abstract
In humans, defects in peroxisome assembly result in the peroxisome biogenesis disorders (PBDs), a group of genetically heterogeneous, lethal recessive diseases. We have identified the human gene PXAAA1 based upon its similarity to PpPAS5, a gene required for peroxisome assembly in the yeast Pichia pastoris. Expression of PXAAA1 restored peroxisomal protein import in fibroblasts from 16 unrelated members of complementation group 4 (CG4) of the PBD. Consistent with this observation, CG4 patients carry mutations in PXAAA1. The product of this gene, Pxaaa1p, belongs to the AAA family of ATPases and appears to be a predominantly cytoplasmic protein. Substitution of an arginine for the conserved lysine residue in the ATPase domain of Pxaaa1p abolished its biological activity, suggesting that Pxaaa1p is an ATPase. Furthermore, Pxaaa1p is required for stability of the predominantly cytoplasmic PTS1 receptor, Pxr1p. We conclude that Pxaaa1p plays a direct role in peroxisomal protein import and is required for PTS1 receptor activity.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. D., Soares M. B., Kerlavage A. R., Fields C., Venter J. C. Rapid cDNA sequencing (expressed sequence tags) from a directionally cloned human infant brain cDNA library. Nat Genet. 1993 Aug;4(4):373–380. doi: 10.1038/ng0893-373. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bellion E., Goodman J. M. Proton ionophores prevent assembly of a peroxisomal protein. Cell. 1987 Jan 16;48(1):165–173. doi: 10.1016/0092-8674(87)90367-9. [DOI] [PubMed] [Google Scholar]
- Brocard C., Kragler F., Simon M. M., Schuster T., Hartig A. The tetratricopeptide repeat-domain of the PAS10 protein of Saccharomyces cerevisiae is essential for binding the peroxisomal targeting signal-SKL. Biochem Biophys Res Commun. 1994 Nov 15;204(3):1016–1022. doi: 10.1006/bbrc.1994.2564. [DOI] [PubMed] [Google Scholar]
- Confalonieri F., Duguet M. A 200-amino acid ATPase module in search of a basic function. Bioessays. 1995 Jul;17(7):639–650. doi: 10.1002/bies.950170710. [DOI] [PubMed] [Google Scholar]
- Crane D. I., Kalish J. E., Gould S. J. The Pichia pastoris PAS4 gene encodes a ubiquitin-conjugating enzyme required for peroxisome assembly. J Biol Chem. 1994 Aug 26;269(34):21835–21844. [PubMed] [Google Scholar]
- Dodt G., Braverman N., Wong C., Moser A., Moser H. W., Watkins P., Valle D., Gould S. J. Mutations in the PTS1 receptor gene, PXR1, define complementation group 2 of the peroxisome biogenesis disorders. Nat Genet. 1995 Feb;9(2):115–125. doi: 10.1038/ng0295-115. [DOI] [PubMed] [Google Scholar]
- Drwinga H. L., Toji L. H., Kim C. H., Greene A. E., Mulivor R. A. NIGMS human/rodent somatic cell hybrid mapping panels 1 and 2. Genomics. 1993 May;16(2):311–314. doi: 10.1006/geno.1993.1190. [DOI] [PubMed] [Google Scholar]
- Erdmann R., Veenhuis M., Mertens D., Kunau W. H. Isolation of peroxisome-deficient mutants of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5419–5423. doi: 10.1073/pnas.86.14.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann R., Wiebel F. F., Flessau A., Rytka J., Beyer A., Fröhlich K. U., Kunau W. H. PAS1, a yeast gene required for peroxisome biogenesis, encodes a member of a novel family of putative ATPases. Cell. 1991 Feb 8;64(3):499–510. doi: 10.1016/0092-8674(91)90234-p. [DOI] [PubMed] [Google Scholar]
- Faber K. N., Keizer-Gunnink I., Pluim D., Harder W., Ab G., Veenhuis M. The N-terminus of amine oxidase of Hansenula polymorpha contains a peroxisomal targeting signal. FEBS Lett. 1995 Jan 3;357(2):115–120. doi: 10.1016/0014-5793(94)01317-t. [DOI] [PubMed] [Google Scholar]
- Fransen M., Brees C., Baumgart E., Vanhooren J. C., Baes M., Mannaerts G. P., Van Veldhoven P. P. Identification and characterization of the putative human peroxisomal C-terminal targeting signal import receptor. J Biol Chem. 1995 Mar 31;270(13):7731–7736. doi: 10.1074/jbc.270.13.7731. [DOI] [PubMed] [Google Scholar]
- Gietl C., Faber K. N., van der Klei I. J., Veenhuis M. Mutational analysis of the N-terminal topogenic signal of watermelon glyoxysomal malate dehydrogenase using the heterologous host Hansenula polymorpha. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3151–3155. doi: 10.1073/pnas.91.8.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover J. R., Andrews D. W., Rachubinski R. A. Saccharomyces cerevisiae peroxisomal thiolase is imported as a dimer. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10541–10545. doi: 10.1073/pnas.91.22.10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Hosken N., Wilkinson J., Subramani S. A conserved tripeptide sorts proteins to peroxisomes. J Cell Biol. 1989 May;108(5):1657–1664. doi: 10.1083/jcb.108.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Krisans S., Keller G. A., Subramani S. Antibodies directed against the peroxisomal targeting signal of firefly luciferase recognize multiple mammalian peroxisomal proteins. J Cell Biol. 1990 Jan;110(1):27–34. doi: 10.1083/jcb.110.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
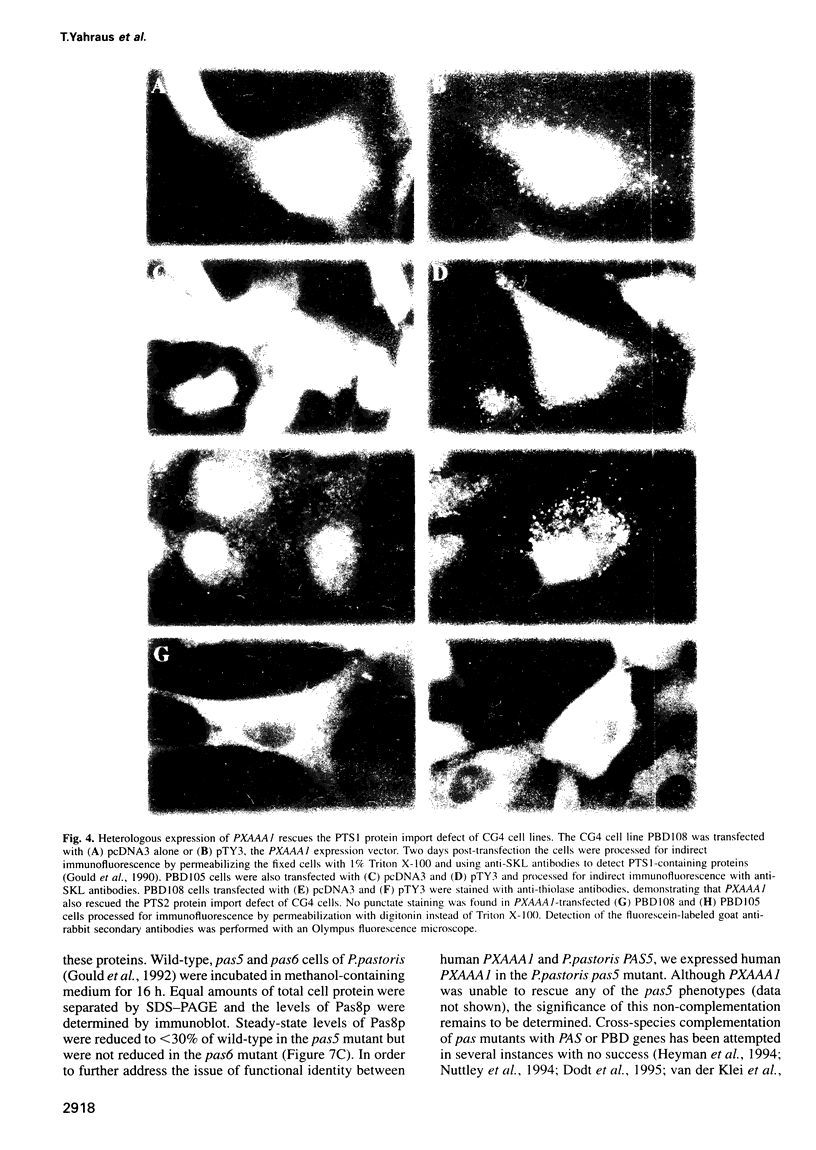
- Gould S. J., McCollum D., Spong A. P., Heyman J. A., Subramani S. Development of the yeast Pichia pastoris as a model organism for a genetic and molecular analysis of peroxisome assembly. Yeast. 1992 Aug;8(8):613–628. doi: 10.1002/yea.320080805. [DOI] [PubMed] [Google Scholar]
- Grant S. G., Jessee J., Bloom F. R., Hanahan D. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4645–4649. doi: 10.1073/pnas.87.12.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D., Kostka S., Kraft R., Dingwall C., Laskey R. A., Hartmann E., Prehn S. Two different subunits of importin cooperate to recognize nuclear localization signals and bind them to the nuclear envelope. Curr Biol. 1995 Apr 1;5(4):383–392. doi: 10.1016/s0960-9822(95)00079-0. [DOI] [PubMed] [Google Scholar]
- Hachiya N., Komiya T., Alam R., Iwahashi J., Sakaguchi M., Omura T., Mihara K. MSF, a novel cytoplasmic chaperone which functions in precursor targeting to mitochondria. EMBO J. 1994 Nov 1;13(21):5146–5154. doi: 10.1002/j.1460-2075.1994.tb06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman J. A., Monosov E., Subramani S. Role of the PAS1 gene of Pichia pastoris in peroxisome biogenesis. J Cell Biol. 1994 Dec;127(5):1259–1273. doi: 10.1083/jcb.127.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaka T., Small G. M., Lazarow P. B. Translocation of acyl-CoA oxidase into peroxisomes requires ATP hydrolysis but not a membrane potential. J Cell Biol. 1987 Dec;105(6 Pt 2):2915–2922. doi: 10.1083/jcb.105.6.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James G. L., Goldstein J. L., Pathak R. K., Anderson R. G., Brown M. S. PxF, a prenylated protein of peroxisomes. J Biol Chem. 1994 May 13;269(19):14182–14190. [PubMed] [Google Scholar]
- Kalish J. E., Theda C., Morrell J. C., Berg J. M., Gould S. J. Formation of the peroxisome lumen is abolished by loss of Pichia pastoris Pas7p, a zinc-binding integral membrane protein of the peroxisome. Mol Cell Biol. 1995 Nov;15(11):6406–6419. doi: 10.1128/mcb.15.11.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Regulation of translation in eukaryotic systems. Annu Rev Cell Biol. 1992;8:197–225. doi: 10.1146/annurev.cb.08.110192.001213. [DOI] [PubMed] [Google Scholar]
- Kragler F., Langeder A., Raupachova J., Binder M., Hartig A. Two independent peroxisomal targeting signals in catalase A of Saccharomyces cerevisiae. J Cell Biol. 1993 Feb;120(3):665–673. doi: 10.1083/jcb.120.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause T., Kunau W. H., Erdmann R. Effect of site-directed mutagenesis of conserved lysine residues upon Pas1 protein function in peroxisome biogenesis. Yeast. 1994 Dec;10(12):1613–1620. doi: 10.1002/yea.320101210. [DOI] [PubMed] [Google Scholar]
- Kunau W. H., Beyer A., Franken T., Götte K., Marzioch M., Saidowsky J., Skaletz-Rorowski A., Wiebel F. F. Two complementary approaches to study peroxisome biogenesis in Saccharomyces cerevisiae: forward and reversed genetics. Biochimie. 1993;75(3-4):209–224. doi: 10.1016/0300-9084(93)90079-8. [DOI] [PubMed] [Google Scholar]
- Latterich M., Fröhlich K. U., Schekman R. Membrane fusion and the cell cycle: Cdc48p participates in the fusion of ER membranes. Cell. 1995 Sep 22;82(6):885–893. doi: 10.1016/0092-8674(95)90268-6. [DOI] [PubMed] [Google Scholar]
- Maquat L. E. When cells stop making sense: effects of nonsense codons on RNA metabolism in vertebrate cells. RNA. 1995 Jul;1(5):453–465. [PMC free article] [PubMed] [Google Scholar]
- Marzioch M., Erdmann R., Veenhuis M., Kunau W. H. PAS7 encodes a novel yeast member of the WD-40 protein family essential for import of 3-oxoacyl-CoA thiolase, a PTS2-containing protein, into peroxisomes. EMBO J. 1994 Oct 17;13(20):4908–4918. doi: 10.1002/j.1460-2075.1994.tb06818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum D., Monosov E., Subramani S. The pas8 mutant of Pichia pastoris exhibits the peroxisomal protein import deficiencies of Zellweger syndrome cells--the PAS8 protein binds to the COOH-terminal tripeptide peroxisomal targeting signal, and is a member of the TPR protein family. J Cell Biol. 1993 May;121(4):761–774. doi: 10.1083/jcb.121.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNew J. A., Goodman J. M. An oligomeric protein is imported into peroxisomes in vivo. J Cell Biol. 1994 Dec;127(5):1245–1257. doi: 10.1083/jcb.127.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud J., Brody L. C., Steel G., Fontaine G., Martin L. S., Valle D., Mitchell G. Strand-separating conformational polymorphism analysis: efficacy of detection of point mutations in the human ornithine delta-aminotransferase gene. Genomics. 1992 Jun;13(2):389–394. doi: 10.1016/0888-7543(92)90258-t. [DOI] [PubMed] [Google Scholar]
- Moser A. B., Rasmussen M., Naidu S., Watkins P. A., McGuinness M., Hajra A. K., Chen G., Raymond G., Liu A., Gordon D. Phenotype of patients with peroxisomal disorders subdivided into sixteen complementation groups. J Pediatr. 1995 Jul;127(1):13–22. doi: 10.1016/s0022-3476(95)70250-4. [DOI] [PubMed] [Google Scholar]
- Motley A., Hettema E., Distel B., Tabak H. Differential protein import deficiencies in human peroxisome assembly disorders. J Cell Biol. 1994 May;125(4):755–767. doi: 10.1083/jcb.125.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttley W. M., Brade A. M., Eitzen G. A., Veenhuis M., Aitchison J. D., Szilard R. K., Glover J. R., Rachubinski R. A. PAY4, a gene required for peroxisome assembly in the yeast Yarrowia lipolytica, encodes a novel member of a family of putative ATPases. J Biol Chem. 1994 Jan 7;269(1):556–566. [PubMed] [Google Scholar]
- Osumi T., Tsukamoto T., Hata S., Yokota S., Miura S., Fujiki Y., Hijikata M., Miyazawa S., Hashimoto T. Amino-terminal presequence of the precursor of peroxisomal 3-ketoacyl-CoA thiolase is a cleavable signal peptide for peroxisomal targeting. Biochem Biophys Res Commun. 1991 Dec 31;181(3):947–954. doi: 10.1016/0006-291x(91)92028-i. [DOI] [PubMed] [Google Scholar]
- Rowe L. B., Nadeau J. H., Turner R., Frankel W. N., Letts V. A., Eppig J. T., Ko M. S., Thurston S. J., Birkenmeier E. H. Maps from two interspecific backcross DNA panels available as a community genetic mapping resource. Mamm Genome. 1994 May;5(5):253–274. doi: 10.1007/BF00389540. [DOI] [PubMed] [Google Scholar]
- Santos M. J., Imanaka T., Shio H., Small G. M., Lazarow P. B. Peroxisomal membrane ghosts in Zellweger syndrome--aberrant organelle assembly. Science. 1988 Mar 25;239(4847):1536–1538. doi: 10.1126/science.3281254. [DOI] [PubMed] [Google Scholar]
- Shimozawa N., Suzuki Y., Orii T., Moser A., Moser H. W., Wanders R. J. Standardization of complementation grouping of peroxisome-deficient disorders and the second Zellweger patient with peroxisomal assembly factor-1 (PAF-1) defect. Am J Hum Genet. 1993 Apr;52(4):843–844. [PMC free article] [PubMed] [Google Scholar]
- Slawecki M. L., Dodt G., Steinberg S., Moser A. B., Moser H. W., Gould S. J. Identification of three distinct peroxisomal protein import defects in patients with peroxisome biogenesis disorders. J Cell Sci. 1995 May;108(Pt 5):1817–1829. doi: 10.1242/jcs.108.5.1817. [DOI] [PubMed] [Google Scholar]
- Small G. M., Szabo L. J., Lazarow P. B. Acyl-CoA oxidase contains two targeting sequences each of which can mediate protein import into peroxisomes. EMBO J. 1988 Apr;7(4):1167–1173. doi: 10.1002/j.1460-2075.1988.tb02927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto U., Pepperkok R., Ansorge W., Just W. W. Import of firefly luciferase into mammalian peroxisomes in vivo requires nucleoside triphosphates. Exp Cell Res. 1993 Mar;205(1):66–75. doi: 10.1006/excr.1993.1059. [DOI] [PubMed] [Google Scholar]
- Spong A. P., Subramani S. Cloning and characterization of PAS5: a gene required for peroxisome biogenesis in the methylotrophic yeast Pichia pastoris. J Cell Biol. 1993 Nov;123(3):535–548. doi: 10.1083/jcb.123.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinkels B. W., Gould S. J., Bodnar A. G., Rachubinski R. A., Subramani S. A novel, cleavable peroxisomal targeting signal at the amino-terminus of the rat 3-ketoacyl-CoA thiolase. EMBO J. 1991 Nov;10(11):3255–3262. doi: 10.1002/j.1460-2075.1991.tb04889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X., Waterham H. R., Veenhuis M., Cregg J. M. The Hansenula polymorpha PER8 gene encodes a novel peroxisomal integral membrane protein involved in proliferation. J Cell Biol. 1995 Feb;128(3):307–319. doi: 10.1083/jcb.128.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. J., Garboczi D. N., Pedersen P. L. Mutational analysis of the consensus nucleotide binding sequences in the rat liver mitochondrial ATP synthase beta-subunit. J Biol Chem. 1992 Oct 5;267(28):20331–20338. [PubMed] [Google Scholar]
- Tsukamoto T., Miura S., Fujiki Y. Restoration by a 35K membrane protein of peroxisome assembly in a peroxisome-deficient mammalian cell mutant. Nature. 1991 Mar 7;350(6313):77–81. doi: 10.1038/350077a0. [DOI] [PubMed] [Google Scholar]
- Van der Leij I., Franse M. M., Elgersma Y., Distel B., Tabak H. F. PAS10 is a tetratricopeptide-repeat protein that is essential for the import of most matrix proteins into peroxisomes of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11782–11786. doi: 10.1073/pnas.90.24.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn-Brouwer T., van der Leij I., Hemrika W., Distel B., Tabak H. F. Sequence of the PAS8 gene, the product of which is essential for biogenesis of peroxisomes in Saccharomyces cerevisiae. Biochim Biophys Acta. 1993 Nov 16;1216(2):325–328. doi: 10.1016/0167-4781(93)90166-b. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Lingappa V. R. Mechanism of protein translocation across the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1986;2:499–516. doi: 10.1146/annurev.cb.02.110186.002435. [DOI] [PubMed] [Google Scholar]
- Walton P. A., Hill P. E., Subramani S. Import of stably folded proteins into peroxisomes. Mol Biol Cell. 1995 Jun;6(6):675–683. doi: 10.1091/mbc.6.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton P. A., Wendland M., Subramani S., Rachubinski R. A., Welch W. J. Involvement of 70-kD heat-shock proteins in peroxisomal import. J Cell Biol. 1994 Jun;125(5):1037–1046. doi: 10.1083/jcb.125.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland M., Subramani S. Cytosol-dependent peroxisomal protein import in a permeabilized cell system. J Cell Biol. 1993 Feb;120(3):675–685. doi: 10.1083/jcb.120.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland M., Subramani S. Presence of cytoplasmic factors functional in peroxisomal protein import implicates organelle-associated defects in several human peroxisomal disorders. J Clin Invest. 1993 Nov;92(5):2462–2468. doi: 10.1172/JCI116854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebel F. F., Kunau W. H. The Pas2 protein essential for peroxisome biogenesis is related to ubiquitin-conjugating enzymes. Nature. 1992 Sep 3;359(6390):73–76. doi: 10.1038/359073a0. [DOI] [PubMed] [Google Scholar]
- Wiemer E. A., Nuttley W. M., Bertolaet B. L., Li X., Francke U., Wheelock M. J., Anné U. K., Johnson K. R., Subramani S. Human peroxisomal targeting signal-1 receptor restores peroxisomal protein import in cells from patients with fatal peroxisomal disorders. J Cell Biol. 1995 Jul;130(1):51–65. doi: 10.1083/jcb.130.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. W., Lazarow P. B. PEB1 (PAS7) in Saccharomyces cerevisiae encodes a hydrophilic, intra-peroxisomal protein that is a member of the WD repeat family and is essential for the import of thiolase into peroxisomes. J Cell Biol. 1995 Apr;129(1):65–80. doi: 10.1083/jcb.129.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoop M. J., Ab G. Import of proteins into peroxisomes and other microbodies. Biochem J. 1992 Sep 15;286(Pt 3):657–669. doi: 10.1042/bj2860657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Klei I. J., Hilbrands R. E., Swaving G. J., Waterham H. R., Vrieling E. G., Titorenko V. I., Cregg J. M., Harder W., Veenhuis M. The Hansenula polymorpha PER3 gene is essential for the import of PTS1 proteins into the peroxisomal matrix. J Biol Chem. 1995 Jul 21;270(29):17229–17236. doi: 10.1074/jbc.270.29.17229. [DOI] [PubMed] [Google Scholar]