Abstract
Objective. The aim of this study was to assess the clinical value of absolute eosinophil count, serum IgE, ESR and CRP as longitudinal biomarkers of disease activity and predictors of relapse in eosinophilic granulomatosis with polyangiitis (Churg-Strauss, EGPA).
Methods. Patients were selected from an observational EGPA cohort. Absolute eosinophil count, IgE, ESR and CRP were measured quarterly. Disease activity was defined by validated assessment tools. The association of tests with disease activity was assessed via regression models, adjusting for repeated measures and treatment status. Survival analysis was used to determine if laboratory tests were predictive of the 3 month future flare risk.
Results. Seventy-four per cent of 892 study visits in 141 patients occurred while patients were on treatment, mostly during remission or mild disease activity, defined as a BVAS for Wegener’s granulomatosis (BVAS/WG) of 1 or 2. Correlations between absolute eosinophil count, IgE, ESR and CRP were mostly low or non-significant (r = −0.08 to 0.44). There were few weak associations with disease activity [absolute eosinophil count: OR) 1.01/100 U (95% CI 1.01, 1.02); ESR: OR 1.15/10 mg/l increase (95% CI 1.04, 1.27)]. When BVAS/WG ≥1 defined active disease, the absolute eosinophil count [hazard ratio (HR) 1.01/100 U (95% CI 1.01, 1.02)] was weakly predictive of flare. When BVAS/WG ≥3 defined active disease, ESR was weakly predictive of flare [HR 1.52/10 mm/h increase (95% CI 1.17, 1.67)].
Conclusion. The absolute eosinophil count, IgE, ESR and CRP have limitations as longitudinal biomarkers of disease activity or predictors of flare in EGPA. These findings suggest that novel biomarkers of disease activity for EGPA are needed.
Keywords: vasculitis, eosinophilic granulomatosis with polyangiitis, Churg–Strauss syndrome, biomarker, eosinophil
Introduction
Eosinophilic granulomatosis with polyangiitis (EGPA, Churg–Strauss syndrome) is a multisystem disease characterized by asthma and other symptoms of allergy, necrotizing vasculitis involving small to medium-sized blood vessels and marked tissue and peripheral blood eosinophilia. The pathogenesis of EGPA is currently unclear, but evidence implicates eosinophils, T lymphocytes and humoral responses as potential mediators of tissue damage [1]. Approximately 40% of subjects with EGPA develop ANCA [2, 3]. The pattern of organ involvement and clinical outcomes of patients with EGPA can differ depending on ANCA status [2–4], which may reflect the different pathogenic mechanisms underlying ANCA-positive vs ANCA-negative EGPA.
Measurement of peripheral eosinophil counts, serum IgE, ESR and CRP is common clinical practice in the longitudinal assessment of patients with EGPA, and each of these laboratory tests has been associated with disease activity in EGPA [4–7]. Although these tests are routinely measured at clinical visits, there are few data evaluating their performance as longitudinal biomarkers of disease activity and predictors of relapse. Studies that have established associations between these common laboratory tests and disease activity in EGPA tend to be cross-sectional and focused on samples obtained at the time of diagnosis, when the degree of disease activity is typically high, limiting the ability to examine within-subject performance of these tests over time and in association with milder disease activity [4, 5]. Furthermore, most of these studies are based on small sample sizes, limiting the ability to study ANCA status as an effect modifier and treatment status as a potential confounder [5–7].
The objective of this study was to examine the performance characteristics of peripheral eosinophil counts, serum IgE, ESR and CRP as markers of disease activity and predictors of relapse in a longitudinal, observational cohort of patients with EGPA.
Methods
Patient selection
Patients enrolled in the Vasculitis Clinical Research Consortium (VCRC) Longitudinal Study of EGPA (Churg–Strauss) between 2006 and 2013 were selected for analysis. The VCRC is a multicentre research infrastructure dedicated to conducting clinical research on different forms of systemic vasculitis. The VCRC Longitudinal Study in EGPA is an observational cohort comprising patients in North America seen quarterly or annually, and additionally during disease flares, at eight centres with clinical expertise in vasculitis. All patients enrolled in the VCRC Longitudinal Study in EGPA fulfilled the 1990 ACR classification criteria for EGPA [8]. Ethical approval was obtained through the institutional review board at each participating centre and all participants provided written informed consent in accordance with the Declaration of Helsinki.
Clinical and serological assessments
VCRC clinician investigators performed comprehensive clinical assessments of patients with EGPA at each study visit and recorded clinical and laboratory data using standardized data collection forms. A patient was classified as having ANCA-positive disease if either immunofluorescence or antigen-specific ELISA testing (anti-PR3 or anti-MPO) was ever positive at any point during the disease. Treatment status was recorded as a categorical variable (on treatment or off treatment) for each study visit. Patients were considered on treatment if they were taking oral glucocorticoids and/or other immunosuppressive medication at the time of the study visit. Asthma-specific treatments were not included in the definition of on treatment.
Complete blood count with differential, serum IgE and CRP levels and ESR were measured at each study visit per standard clinical laboratory practice and techniques at the respective study centres. All laboratory data were entered into a centralized data repository and the units of measurement were standardized across the centres for each laboratory test. Absolute eosinophil count was calculated by multiplying the total white blood cell count by the percentage of eosinophils. For categorical-based analyses, the following thresholds were used to define abnormal laboratory values: absolute eosinophil count >350 cells/µl, serum IgE >432 mg/l, ESR >20 mm/h and CRP >5.0 mg/l.
Since there is no standardized disease-specific activity assessment tool for EGPA, different definitions of disease activity were studied in parallel. Both the BVAS [9] and the BVAS for Wegener’s granulomatosis (BVAS/WG) [10] were used to assess disease activity; these tools typically produce highly correlated results [11]. Different threshold scores (≥1, ≥3) were used to define active disease. A score ≥3 on the BVAS or BVAS/WG corresponds to at least one severe disease manifestation or more than one minor disease manifestation. Dyspnoea or wheeze is scored as a 2 point item on the BVAS when attributable to active vasculitis. The presence or absence of asthma symptoms as determined by the examining clinician investigator was recorded at each visit. Per investigator consensus, asthma symptoms alone were not considered sufficient evidence of disease activity in EGPA, so symptoms of wheeze without other evidence of disease activity were not scored on the BVAS. There are no disease activity items related to asthma scored on the BVAS/WG.
Disease remission was defined as a BVAS or BVAS/WG score of 0. Disease relapse was defined as disease activity at the time of the study visit where remission was noted at the 3 month antecedent visit. Mild disease was defined as a BVAS/WG score of 1 or 2. In addition to the BVAS and BVAS/WG, the examining physician provided a global assessment of disease activity (active disease versus remission) at each study visit.
Statistical methods
Fisher’s exact test and the Wilcoxon rank sum test were used to compare prevalence and distribution, respectively, between groups. Correlations among absolute eosinophil count, IgE and CRP levels and ESR were performed using Spearman’s rank sum. Performance characteristics (sensitivity, specificity) of categorically defined laboratory tests, alone and in combination, were calculated in association with disease activity. Logistic regression, without adjustment for repeated measures, was performed to calculate the area under the curve (AUC) for each laboratory test in association with disease activity. The AUC corresponds to the area under a receiver operating characteristic curve and can range from 0.5 (zero discrimination) to 1.0 (perfect test). Multivariable models, adjusting for repeated measures (i.e. multiple study visits for the same patient) and treatment status, were run to examine the association of laboratory tests with disease activity using generalized estimating equation logistic regression.
In patients evaluated quarterly, survival analysis was used to determine whether laboratory test values were predictive of 3 month future relapse risk. The counting process approach was used to account for repeated events in the survival analysis models [12]. In the counting process approach, each disease relapse was assumed to be an independent event, and a patient could contribute to the risk set for an event as long as the patient was under observation at the time the event occurred. In both logistic regression and survival analysis models, laboratory tests analysed as continuous, independent variables and alternative definitions of disease activity were used to define disease activity as the dependent variable. All statistical analyses were done using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
There were 892 total study visits made by 141 patients with EGPA. Clinical and laboratory characteristics of the cohort are provided in Table 1. There was significantly more renal involvement in ANCA-positive compared with ANCA-negative disease (27% vs 4%, P < 0.01). There was a trend towards more cardiac, pulmonary and gastrointestinal involvement in ANCA-negative patients and more neurological manifestations in ANCA-positive patients. Quarterly, as opposed to annual, study visits occurred in 81 patients, accounting for 753 study visits. The majority of study visits (74%) occurred while patients were on treatment for EGPA and 65% of all study visits occurred while patients were taking prednisone.
Table 1.
Clinical and demographic differences in the EGPA study cohort by ANCA status
| Variable | Total | ANCA negative | ANCA positive |
|---|---|---|---|
| Patients, n (%) | 141 | 76 (54) | 65 (46) |
| Study visits, n (%) | 892 | 490 (55) | 402 (45) |
| Sex, female*, n (%) | 82 (58) | 53 (70) | 29 (45) |
| Age at first observation, median (range), years | 55 (21–82) | 51 (21–82) | 57 (23–82) |
| Race, Caucasian, n (%) | 131 (93) | 72 (95) | 59 (91) |
| Disease duration at first study visit, median (range), years | 1.5 (0–29.8) | 1.6 (0–21.7) | 1.3 (0–29.8) |
| Clinical manifestations (prevalence), % | |||
| Asthma | 94 | 97 | 91 |
| Cardiac | 23 | 27 | 19 |
| Dermatological | 58 | 65 | 51 |
| ENT | 89 | 90 | 87 |
| Gastrointestinal | 18 | 23 | 13 |
| Musculoskeletal | 55 | 56 | 54 |
| Neurological | 69 | 63 | 76 |
| Pulmonary (non-asthma) | 66 | 72 | 60 |
| Renal* | 15 | 4 | 27 |
| On prednisone* | |||
| Study visits, n (%) | 579 (65) | 348 (71) | 231 (57) |
| On other immunosuppressants, n (%) | |||
| Study visits | 433 (49) | 248 (51) | 185 (46) |
| AZA | 205 (23) | 114 (23) | 91 (23) |
| CYC | 10 (1) | 3 (1) | 7 (2) |
| MTX | 179 (21) | 90 (18) | 89 (22) |
| On prednisone or other immunosuppressants, n (%) | 659 (74) | 376 (77) | 283 (70) |
| BVAS/WG score, n (%) | 0: 766 (86) | 0: 414 (85) | 0: 352 (88) |
| ≥1: 99 (11) | ≥1: 56 (11) | ≥1: 43 (11) | |
| ≥3: 33 (4) | ≥3: 17 (3) | ≥3: 16 (4) | |
| Eosinophil count, median (range), 103/mm | 262.0 (0–18 096) | 276.0 (0–7083) | 229.4 (0–18 096) |
| IgE, median (range), mg/dl* | 60.0 (0–21 925) | 38.4 (0–21 925) | 85.0 (2–7298) |
| ESR, median (range), mm/h | 8.0 (1–94) | 8.0 (1–72) | 8.0 (1–94) |
| CRP, median (range), mg/l | 2.2 (0–203) | 2.0 (0–98) | 2.6 (0–203) |
*Indicates statistically significant differences (P < 0.05) between ANCA-positive and ANCA-negative subjects with EGPA. Categorical variables were assessed by Fisher’s exact test and continuous variables were assessed by the Wilcoxon rank sum test. BVAS/WG: BVAS for Wegener’s granulomatosis; EGPA: eosinophilic granulomatosis with polyangiitis.
The majority of study visits were made during disease remission. Different definitions of remission yielded similar overall frequencies of remission visits (BVAS 90%, BVAS/WG 86%, physician’s assessment 89%). Disease activity was mild (BVAS/WG 1 or 2) in 66 of the 99 study visits made during periods of active disease, and 45 study visits during active disease occurred in patients who were not taking treatments for EGPA. BVAS/WG scores did not significantly differ between patients with active untreated vs treated disease (median BVAS/WG 2 in both groups). Asthma symptoms were noted during 58 (6.5%) study visits and the majority of these visits (69%) occurred at times of disease remission.
Of 892 study visits, the number of recorded laboratory measurements was as follows: absolute eosinophil count, 829 observations; IgE, 525 observations; ESR, 823 observations; CRP, 812 observations. Missing data in IgE occurred because measurement of serum IgE was not routine practice for several of the study centres. Absolute eosinophil count, ESR and CRP did not differ significantly by ANCA status; however, ANCA-positive patients had significantly higher levels of serum IgE (P < 0.01).
Correlation of laboratory tests
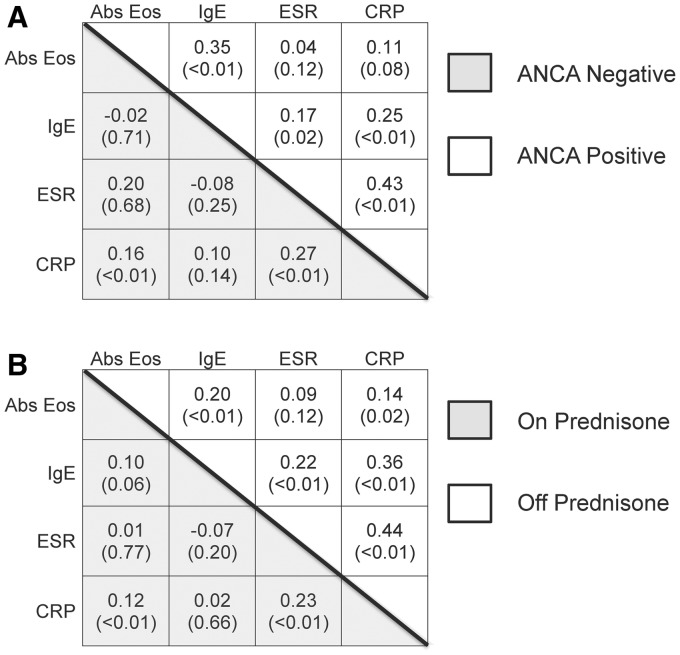
Correlations among absolute eosinophil count, serum IgE and CRP values and ESR were mostly low or non-significant. Correlation matrices stratified by ANCA status (Fig. 1A) were strikingly similar to the correlation matrices stratified by prednisone use (Fig. 1B). There was moderate, significant correlation among laboratory tests in patients who were not taking prednisone, and correlation was dramatically reduced by prednisone use.
Fig. 1.
Correlation matrices of common laboratory tests in EGPA
Correlation between absolute eosinophil count, serum IgE, ESR and CRP differed among subjects with eosinophilic granulomatosis with polyangiitis (EGPA, Churg–Strauss) according to (A) ANCA status and (B) prednisone status. Spearman’s correlation coefficients are presented with P-values in parenthesis. Abs Eos: absolute eosinophil counts; EGPA: eosinophilic granulomatosis with polyangiitis.
Association of laboratory tests and disease activity
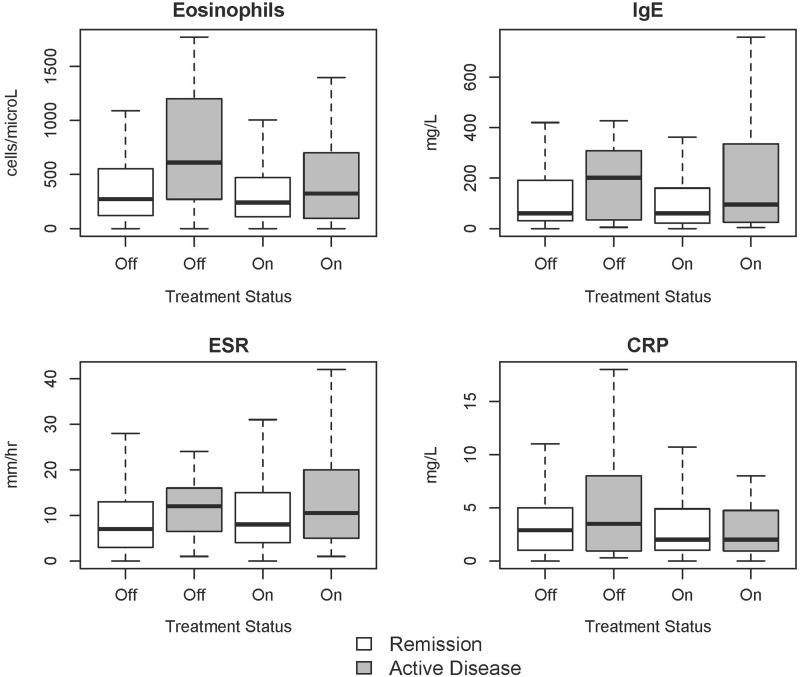
Median values of the laboratory tests during active disease vs remission were as follows: absolute eosinophil count, 379.6 vs 245.7 cells/µl (P < 0.01); IgE, 105.0 vs 59.5 mg/l (P = 0.06); CRP, 2.7 vs 2.1 mg/l (P = 0.61); ESR, 11.0 vs 8.0 mm/h (P = 0.01). Relationships between different laboratory tests and disease activity stratified by treatment status (prednisone and/or other immunosuppressants) are shown in boxplots in Fig. 2. Although none of the comparative analyses stratified by treatment status met statistical significance, the median values of each laboratory test were highest in patients with untreated, active disease. In patients on treatment, median laboratory values tended to be higher during active disease vs remission for all laboratory tests except CRP, and differences in laboratory values between active disease and remission were less pronounced in patients who were on treatment compared with patients off treatment at the time of sampling.
Fig. 2.
Boxplots of laboratory tests in association with disease and treatment status
Boxplots are provided for values of absolute eosinophil count, serum IgE, ESR and CRP in association with disease activity (BVAS/WG 0 vs ≥1) and treatment status (prednisone and/or other immunosuppressants). The boxes signify the middle 50% of scores (interquartile range) and the median values are represented by a black line within each box. The lines extending above and below the boxes represent the range of the upper and lower quartiles. Outliers are not shown. BVAS/WG: BVAS for Wegener’s granulomatosis.
The performance characteristics of the laboratory tests are listed in Table 2. A pattern of low sensitivity and high specificity was observed for IgE, CRP and ESR in association with disease activity. Sensitivity of these three laboratory tests improved minimally when restricting analyses to patients off treatment, and sensitivity improved modestly, particularly for ESR and CRP, when increasing the BVAS/WG threshold to define disease activity from 1 to 3. Absolute eosinophil count displayed moderate sensitivity and specificity as a marker of disease activity. If the threshold to define elevated absolute eosinophil count was raised from 500 to 1500 cells/µl, there was low sensitivity (11%) and high specificity (97%) in association with disease activity, as 83 of 776 subjects with a BVAS/WG score >0 had an absolute eosinophil count of <1500 cells/µl and 20 of 30 subjects had a BVAS/WG score of 0 despite an absolute eosinophil count of >1500 cells/µl. For all laboratory tests, the AUC was poor, indicating that varying thresholds to define abnormal laboratory values had minimal impact on improving the overall performance characteristics of the tests. When tests were considered in combination, allowing for up to two missing values per subject, sensitivity improved but remained <80% if a positive result was defined by any one abnormal laboratory test result. If abnormalities in all measured tests defined a positive result, specificity was excellent (>95%), but sensitivity was poor (<10%).
Table 2.
Performance characteristics in association with disease activity for selected biomarkers for eosinophilic granulomatosus with polyangiitis
| TP | FP | TN | FN | Sens, % | Spec, % | AUC | TP | FP | TN | FN | Sens, % | Spec, % | AUC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total cohort—BVAS/WG ≥1 |
Subjects off treatment—BVAS/WG ≥1 |
|||||||||||||
| Eos | 49 | 269 | 444 | 44 | 53 | 62 | 0.60 | 17 | 65 | 89 | 22 | 44 | 58 | 0.52 |
| IgE | 10 | 56 | 400 | 44 | 19 | 88 | 0.61 | 5 | 12 | 76 | 17 | 23 | 86 | 0.63 |
| ESR | 19 | 103 | 607 | 71 | 21 | 85 | 0.58 | 8 | 31 | 124 | 30 | 21 | 80 | 0.60 |
| CRP | 21 | 156 | 546 | 66 | 24 | 78 | 0.51 | 10 | 48 | 109 | 25 | 29 | 69 | 0.50 |
| Any | 69 | 396 | 370 | 30 | 70 | 48 | 0.59 | 28 | 95 | 75 | 17 | 62 | 44 | 0.53 |
| All | 1 | 9 | 757 | 98 | 1 | 99 | 0.50 | 1 | 4 | 166 | 44 | 2 | 98 | 0.50 |
| Total cohort—BVAS/WG ≥3 | Subjects off treatment—BVAS/WG ≥3 | |||||||||||||
| Eos | 14 | 304 | 470 | 18 | 44 | 61 | 0.64 | 6 | 76 | 100 | 11 | 35 | 57 | 0.50 |
| IgE | 5 | 61 | 425 | 19 | 21 | 88 | 0.56 | 4 | 13 | 83 | 10 | 29 | 87 | 0.76 |
| ESR | 11 | 111 | 658 | 20 | 36 | 86 | 0.53 | 4 | 35 | 142 | 12 | 25 | 80 | 0.60 |
| CRP | 10 | 167 | 591 | 21 | 33 | 78 | 0.58 | 7 | 51 | 125 | 9 | 44 | 71 | 0.53 |
| Any | 26 | 439 | 393 | 7 | 79 | 47 | 0.63 | 13 | 110 | 87 | 5 | 72 | 44 | 0.58 |
| All | 1 | 9 | 823 | 32 | 3 | 99 | 0.51 | 1 | 4 | 193 | 17 | 6 | 98 | 0.52 |
All: all measured labs abnormal; Any: abnormal Eos, IgE, ESR or CRP; AUC: area under the curve; BVAS/WG: BVAS for Wegener’s granulomatosis; Eos: absolute eosinophil count; FN: false negative (abnormal lab and BVAS/WG >0); FP: false positive (abnormal lab and BVAS/WG = 0); Sens: sensitivity; Spec: specificity; TN: true negative (normal lab and BVAS/WG = 0); TP: true positive (abnormal lab and BVAS/WG >0).
Multivariable models, which accounted for repeated measures and treatment status, were used to determine the association between the laboratory tests and disease activity over time. Absolute eosinophil count and ESR were significantly associated with disease activity after adjustment for repeated measures and treatment status, but the associations were weak (Table 3). For every 100 U increase in absolute eosinophil count, the prevalence risk ratio (RR) for active disease increased by only 1% [RR 1.01 (95% CI 1.01, 1.02), P < 0.01]. For every 10 U increase in ESR, the prevalence RR for active disease increased by 15% [RR 1.15 (95% CI 1.04, 1.27), P < 0.01]. The results did not significantly differ by definition of disease activity or ANCA status.
Table 3.
Association of common laboratory tests with disease activity in EGPA in multivariable regression models after adjusting for repeated measures and treatment status
| Predictor variable | Prevalence risk ratio (95% CI) | P-value |
|---|---|---|
| Absolute eosinophil count | 1.01 (1.01, 1.02) | <0.01 |
| IgE | 1.02 (0.99, 1.02) | 0.31 |
| ESR | 1.15 (1.04, 1.27) | <0.01 |
| CRP | 0.98 (0.73, 1.03) | 0.90 |
Risk ratios are reported per 100 U increase for absolute eosinophil count and IgE and per 10 U increase for ESR and CRP. EGPA: eosinophilic granulomatosis with polyangiitis.
Prediction of disease relapse
There were few predictors of disease relapse in EGPA and predictors differed depending on the threshold used to define disease activity (Table 4). When BVAS/WG ≥1 defined a disease flare, only absolute eosinophil count was associated with a significant increase in 3 month future relapse risk. When BVAS/WG ≥3 defined a disease flare, only ESR was associated with a significant increase in 3 month relapse risk. Results did not significantly differ by definition of disease activity or ANCA status.
Table 4.
Risk factors to predict future disease relapse in EGPA
| Univariable analysis |
Adjusted for prednisone and other immune medications |
|||
|---|---|---|---|---|
| Predictor variable | Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value |
| BVAS/WG ≥1 defines disease relapse | ||||
| Eos | 1.01 (1.01, 1.02) | <0.01 | 1.01 (1.01, 1.02) | <0.01 |
| IgE | 0.98 (0.97, 1.03) | 0.64 | 0.99 (0.97, 1.04) | 0.53 |
| ESR | 1.23 (1.01, 1.42) | 0.04 | 1.15 (0.99, 1.35) | 0.12 |
| CRP | 1.04 (0.99, 1.17) | 0.60 | 1.01 (0.98, 1.14) | 0.58 |
| Prednisone | 1.16 (0.60, 2.34) | 0.68 | ||
| Other immune medications | 0.45 (0.26, 0.79) | <0.01 | ||
| BVAS/WG ≥3 defines disease relapse | ||||
| Absolute eosinophil countEos | 1.01 (1.01, 1.02) | <0.01 | 1.00 (1.00, 1.01) | 0.25 |
| IgE | 0.99 (0.99, 1.00) | 0.15 | 0.99 (0.99, 1.00) | 0.49 |
| ESR | 1.64 (1.27, 1.75) | <0.01 | 1.52 (1.17, 1.67) | <0.01 |
| CRP | 1.01 (0.99, 1.03) | 0.06 | 1.09 (0.99, 1.26) | 0.28 |
| Prednisone | 1.37 (0.45, 3.22) | 0.72 | ||
| Other immune medications | 0.76 (0.18, 0.92) | 0.03 | ||
Risk ratios for Eos and IgE reported per 100 U increments. Risk ratios for ESR and CRP reported per 10 U increments.
BVAS/WG: BVAS for Wegener’s granulomatosis; EGPA: eosinophilic granulomatosis with polyangiitis; Eos: absolute eosinophil count.
Discussion
This analysis of data from a large longitudinal cohort demonstrates that absolute eosinophil count, serum IgE, ESR and CRP values have substantial limitations as longitudinal biomarkers of disease activity or predictors of flare in EGPA. Similar to reports in other cohorts of patients with EGPA, median levels of eosinophils, IgE and ESR were associated with disease activity. However, at the patient level, the performance characteristics of these tests in association with disease activity were poor. In longitudinal analyses accounting for within-patient repeated measures, absolute eosinophil count and ESR were consistently associated with disease activity, but the associations were weak. Absolute eosinophil count and ESR also predicted disease relapse in EGPA, but the associations, while statistically significant, were again weak. These findings inform the interpretation of four laboratory tests that are routinely assessed in the clinical care of patients with EGPA.
For IgE, CRP and ESR, a pattern of low sensitivity and high specificity was observed in association with disease activity. The high specificity indicates that these tests tend to correctly identify patients without active disease as test negative (i.e. few false positives); however, the low sensitivity suggests that the test incorrectly identifies many patients with active disease as test negative (i.e. many false negatives). For absolute eosinophil count, both moderate sensitivity and specificity were observed. Given the fact that active disease tended to be mild in this cohort, it is possible that eosinophil count is a more sensitive measure of disease activity in situations of milder disease compared with IgE, ESR and CRP. In fact, ESR and CRP tended to perform better as markers of disease activity when a higher BVAS/WG threshold was imposed to define active disease. Clinically these findings parallel the observation that vasculitic manifestations (e.g. palpable purpura, neuropathy, GN, pulmonary haemorrhage, organ infarction, etc.) are more common during periods of severe disease activity, and asthma/allergy symptoms predominate during milder disease activity.
There are several potential explanations for the poor performance characteristics of these laboratory tests. Treatment effect and bias related to therapeutic decision making likely influenced the findings in this cohort. Treatment with prednisone profoundly altered the relationships among absolute eosinophil count, IgE, ESR and CRP (Fig. 1B). The association between each of these laboratory tests and disease activity was also mediated by treatment status (Fig. 2). In terms of performance characteristics, treatment likely increased the number of false negatives (normal laboratory tests during periods of active disease), leading to poor sensitivity. However, the overall performance characteristics of these tests did not significantly differ when assessed in the total cohort vs a cohort limited to patients not on treatment (Table 2). Therefore other explanations also likely contributed to the poor performance characteristics observed in this study. The weak associations between these tests and disease activity may reflect both the preponderance of mild disease activity in this cohort and the potential for misclassification of disease activity status in periods of milder disease. In addition, biomarkers derived from peripheral blood may not accurately reflect disease pathology at the tissue level, or these common laboratory tests may simply be poor longitudinal biomarkers in EGPA.
ANCA status has been associated with patterns of organ involvement and clinical outcome in EGPA. Differential organ involvement in ANCA-positive vs ANCA-negative disease was similar in this cohort compared with other large cohorts of EGPA [2, 4]. Although it is possible that ANCA status may reflect biologically different subgroups of EGPA, few differences in absolute eosinophil counts, IgE, ESR and CRP by ANCA status were observed in this cohort in association with clinical outcomes, and most differences were likely confounded by prednisone use. Only serum IgE differed by ANCA status, with higher levels seen in ANCA-positive patients. In an observational cohort of 383 patients with EGPA from France, there were no differences in absolute eosinophil count and ESR at the time of diagnosis by ANCA status, CRP was higher at diagnosis in ANCA-positive patients and serum IgE was not reported [4]. Similar to the French cohort, ∼50% of patients with EGPA in this study were taking other immunosuppressant medication in addition to glucocorticoids, and this percentage did not differ by ANCA status. The cumulative duration of prednisone use did not differ by ANCA status in the French cohort; however, data were not analysed on a per-visit basis and were only available for a subset of patients. In this study, the number of study visits in which a patient was taking prednisone was significantly higher for patients with ANCA-negative EGPA. Increased prednisone use in patients with ANCA-negative EGPA may reflect the higher burden of pulmonary disease manifestations observed in this subgroup.
A major strength of this study is that several commonly assessed clinical laboratory tests were critically evaluated simultaneously in a prospective observational cohort of patients with EGPA. The cohort included a large number of patients with a rare disease evaluated at multiple study centres by clinician investigators with expertise in the care and management of EGPA, and the data reported in this study reflect the challenges of assessing EGPA in everyday clinical practice. All data were recorded using standardized data collection forms. Multiple definitions of disease activity were studied in parallel. Laboratory tests were studied as both categorical and continuous variables, and sophisticated statistical models were used to account for repeated measures, treatment as a potential confounder and ANCA status as an effect modifier.
There are some important potential limitations of this study to consider. Accurately defining disease activity in EGPA is challenging, in part because the disease has both an asthma/allergic component and a vasculitic component. A standardized asthma-specific disease activity index was not incorporated into the definitions of disease activity. Persistence of asthma and allergic symptoms in EGPA during periods of mild disease activity and/or remission from vasculitis is common and could contribute to the finding of poor performance characteristics of absolute eosinophil count and IgE in this cohort where disease activity was minimal. Despite this limitation, it is common practice to exclude isolated asthma or sinusitis from the definition of disease activity in EGPA given the lack of specificity of these symptoms. Variation in laboratory assay techniques across the different centres is another potential limitation; however, the multicentre study design was useful in reducing potential selection bias. Treatment status was considered as a categorical variable because information related to drug dosage was not recorded in the dataset. This could lead to residual confounding in statistical models that adjust for treatment effect. Investigators were not blinded to laboratory test results, which could influence disease activity assessment; however, the disease assessment tools are independent of laboratory data. Lack of blinding may explain why the overall performance characteristics of absolute eosinophil count differed from the other laboratory tests, as eosinophil levels may have more influence in the assessment of disease activity in EGPA. Theoretically, unblinding of investigators should create bias in favour of finding an association and proposing value as a biomarker, but performance was poor despite the potential for such bias.
This work highlights that commonly used laboratory tests have significant limitations as clinical biomarkers of disease activity and predictors of relapse in EGPA, an observation that has both clinical and research implications. Absolute eosinophil count, serum IgE, ESR and CRP are associated with disease activity in EGPA and can help inform the diagnosis. In contrast, the use of these tests as markers of disease activity over time or as predictors of disease flare has limitations when applied to the assessment of an individual patient. Clinicians who monitor these tests longitudinally should be aware of their performance characteristics and should consider the potential for confounding by treatment status when interpreting test results. There are several novel candidate biomarkers of disease activity in EGPA [13], including eosinophil granule proteins [14, 15], eotaxins [16, 17], IL-5 [18] and various other cytokines/chemokines [19–24]. Findings from this study emphasize the importance of studying novel candidate biomarkers in observational cohorts of EGPA before their clinical utility as longitudinal biomarkers is accepted.
Rheumatology key messages.
Commonly measured clinical laboratory tests have significant limitations as longitudinal biomarkers in EGPA.
Performance characteristics of biomarkers in EGPA are affected by disease severity and treatment status.
Novel biomarkers in EGPA are needed and should be assessed longitudinally in observational cohorts.
Funding: This work was sponsored by the Vasculitis Clinical Research Consortium, which has received support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS; U54 AR057319 and U01 AR5187404), the National Center for Research Resources (U54 RR019497), the National Center for Advancing Translational Science and the Office of Rare Diseases Research. This work was also supported through the Intramural Research Program at NIAMS.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Vaglio A, Buzio C, Zwerina J. Eosinophilic granulomatosis with polyangiitis (Churg–Strauss): state of the art. Allergy. 2013;68:261–73. doi: 10.1111/all.12088. [DOI] [PubMed] [Google Scholar]
- 2.Sinico RA, Di Toma L, Maggiore U, et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg–Strauss syndrome. Arthritis Rheum. 2005;52:2926–35. doi: 10.1002/art.21250. [DOI] [PubMed] [Google Scholar]
- 3.Sable-Fourtassou R, Cohen P, Mahr A, et al. Antineutrophil cytoplasmic antibodies and the Churg–Strauss syndrome. Ann Intern Med. 2005;143:632–8. doi: 10.7326/0003-4819-143-9-200511010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Comarmond C, Pagnoux C, Khellaf M, et al. Eosinophilic granulomatosis with polyangiitis (Churg–Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 2013;65:270–81. doi: 10.1002/art.37721. [DOI] [PubMed] [Google Scholar]
- 5.Guillevin L, Cohen P, Gayraud M, et al. Churg–Strauss syndrome. Clinical study and long-term follow-up of 96 patients. Medicine. 1999;78:26–37. doi: 10.1097/00005792-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Solans R, Bosch JA, Perez-Bocanegra C, et al. Churg–Strauss syndrome: outcome and long-term follow-up of 32 patients. Rheumatology. 2001;40:763–71. doi: 10.1093/rheumatology/40.7.763. [DOI] [PubMed] [Google Scholar]
- 7.Manger BJ, Krapf FE, Gramatzki M, et al. IgE-containing circulating immune complexes in Churg–Strauss vasculitis. Scandinavian J Immunol. 1985;21:369–73. doi: 10.1111/j.1365-3083.1985.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 8.Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of Churg–Strauss syndrome (allergic granulomatosis and angiitis) Arthritis Rheum. 1990;33:1094–100. doi: 10.1002/art.1780330806. [DOI] [PubMed] [Google Scholar]
- 9.Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM. 1994;87:671–8. [PubMed] [Google Scholar]
- 10.Stone JH, Hoffman GS, Merkel PA, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. Arthritis Rheum. 2001;44:912–20. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Merkel PA, Cuthbertson DD, Hellmich B, et al. Comparison of disease activity measures for anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis. Ann Rheum Dis. 2009;68:103–6. doi: 10.1136/ard.2008.097758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SAS FAQ. http://www.ats.ucla.edu/stat/sas/faq/survival_repeated_events.htm (13 September 2013, date last accessed) [Google Scholar]
- 13.Khoury P, Grayson PC, Klion AD. Eosinophils in vasculitis: characteristics and roles in pathogenesis. Nat Rev Rheumatol. 2014;10:474–83. doi: 10.1038/nrrheum.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guilpain P, Guillevin L, Mouthon L. [Eosinophil granule cationic proteins: eosinophil activation markers] Rev Med Interne. 2006;27:406–8. doi: 10.1016/j.revmed.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Guilpain P, Auclair JF, Tamby MC, et al. Serum eosinophil cationic protein: a marker of disease activity in Churg–Strauss syndrome. Ann N Y Acad Sci. 2007;1107:392–9. doi: 10.1196/annals.1381.041. [DOI] [PubMed] [Google Scholar]
- 16.Polzer K, Karonitsch T, Neumann T, et al. Eotaxin-3 is involved in Churg–Strauss syndrome—a serum marker closely correlating with disease activity. Rheumatology. 2008;47:804–8. doi: 10.1093/rheumatology/ken033. [DOI] [PubMed] [Google Scholar]
- 17.Zwerina J, Bach C, Martorana D, et al. Eotaxin-3 in Churg–Strauss syndrome: a clinical and immunogenetic study. Rheumatology. 2011;50:1823–7. doi: 10.1093/rheumatology/keq445. [DOI] [PubMed] [Google Scholar]
- 18.Jakiela B, Szczeklik W, Plutecka H, et al. Increased production of IL-5 and dominant Th2-type response in airways of Churg–Strauss syndrome patients. Rheumatology. 2012;51:1887–93. doi: 10.1093/rheumatology/kes171. [DOI] [PubMed] [Google Scholar]
- 19.Dallos T, Heiland GR, Strehl J, et al. CCL17/thymus and activation-related chemokine in Churg–Strauss syndrome. Arthritis Rheum. 2010;62:3496–503. doi: 10.1002/art.27678. [DOI] [PubMed] [Google Scholar]
- 20.Terrier B, Bieche I, Maisonobe T, et al. Interleukin-25: a cytokine linking eosinophils and adaptive immunity in Churg–Strauss syndrome. Blood. 2010;116:4523–31. doi: 10.1182/blood-2010-02-267542. [DOI] [PubMed] [Google Scholar]
- 21.Hellmich B, Csernok E, Gross WL. Proinflammatory cytokines and autoimmunity in Churg–Strauss syndrome. Ann N Y Acad Sci. 2005;1051:121–31. doi: 10.1196/annals.1361.053. [DOI] [PubMed] [Google Scholar]
- 22.Kiene M, Csernok E, Muller A, et al. Elevated interleukin-4 and interleukin-13 production by T cell lines from patients with Churg–Strauss syndrome. Arthritis Rheum. 2001;44:469–73. doi: 10.1002/1529-0131(200102)44:2<469::AID-ANR66>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Khoury P, Zagallo P, Talar-Williams C, et al. Serum biomarkers are similar in Churg–Strauss syndrome and hypereosinophilic syndrome. Allergy. 2012;67:1149–56. doi: 10.1111/j.1398-9995.2012.02873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schonermarck U, Csernok E, Trabandt A, Hansen H, Gross WL. Circulating cytokines and soluble CD23, CD26 and CD30 in ANCA-associated vasculitides. Clin Exp Rheumatol. 2000;18:457–63. [PubMed] [Google Scholar]