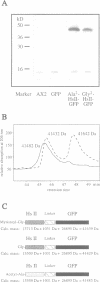
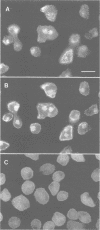
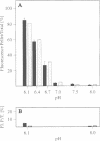
Abstract
Hisactophilins are myristoylated proteins that are rich in histidine residues and known to exist in Dictyostelium cells in a plasma membrane-bound and a soluble cytoplasmic state. Intracellular translocation of these proteins in response to pH changes was monitored using hisactophilin fusions with green fluorescent protein (GFP) and confocal laser scanning microscopy. Both the normal and a mutated non-myristoylated fusion protein shuffled within the cells in a pH-dependent manner. After lowering the pH, these proteins translocated within minutes between the cytoplasm, the plasma membrane and the nucleus. The role of histidine clusters on the surface of hisactophilin molecules in binding of the proteins to the plasma membrane and in their transfer to the nucleus is discussed on the basis of a pH switch mechanism.
Full text
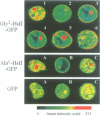
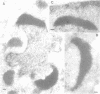
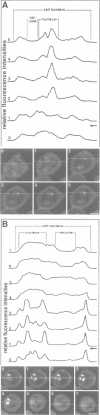
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aerts R. J., De Wit R. J., Van Lookeren Campagne M. M. Cyclic AMP induces a transient alkalinization in Dictyostelium. FEBS Lett. 1987 Aug 17;220(2):366–370. doi: 10.1016/0014-5793(87)80848-7. [DOI] [PubMed] [Google Scholar]
- Begg D. A., Rebhun L. I., Hyatt H. Structural organization of actin in the sea urchin egg cortex: microvillar elongation in the absence of actin filament bundle formation. J Cell Biol. 1982 Apr;93(1):24–32. doi: 10.1083/jcb.93.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrisch A., Dietrich C., Noegel A. A., Schleicher M., Sackmann E. The actin-binding protein hisactophilin binds in vitro to partially charged membranes and mediates actin coupling to membranes. Biochemistry. 1995 Nov 21;34(46):15182–15190. doi: 10.1021/bi00046a026. [DOI] [PubMed] [Google Scholar]
- Brink M., Gerisch G., Isenberg G., Noegel A. A., Segall J. E., Wallraff E., Schleicher M. A Dictyostelium mutant lacking an F-actin cross-linking protein, the 120-kD gelation factor. J Cell Biol. 1990 Oct;111(4):1477–1489. doi: 10.1083/jcb.111.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busa W. B. Mechanisms and consequences of pH-mediated cell regulation. Annu Rev Physiol. 1986;48:389–402. doi: 10.1146/annurev.ph.48.030186.002133. [DOI] [PubMed] [Google Scholar]
- Chalfie M., Tu Y., Euskirchen G., Ward W. W., Prasher D. C. Green fluorescent protein as a marker for gene expression. Science. 1994 Feb 11;263(5148):802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Knecht D., Lodish H. F., Loomis W. F. DNA sequences required for expression of a Dictyostelium actin gene. EMBO J. 1986 Dec 1;5(12):3361–3366. doi: 10.1002/j.1460-2075.1986.tb04651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward P., Nagai K., Chen D., Thomas H. D., Nagamine C. M., Lau Y. F. Polymorphism of a CAG trinucleotide repeat within Sry correlates with B6.YDom sex reversal. Nat Genet. 1994 Mar;6(3):245–250. doi: 10.1038/ng0394-245. [DOI] [PubMed] [Google Scholar]
- Davies L., Satre M., Martin J. B., Gross J. D. The target of ammonia action in dictyostelium. Cell. 1993 Oct 22;75(2):321–327. doi: 10.1016/0092-8674(93)80073-n. [DOI] [PubMed] [Google Scholar]
- Edmonds B. T., Murray J., Condeelis J. pH regulation of the F-actin binding properties of Dictyostelium elongation factor 1 alpha. J Biol Chem. 1995 Jun 23;270(25):15222–15230. doi: 10.1074/jbc.270.25.15222. [DOI] [PubMed] [Google Scholar]
- Faix J., Gerisch G., Noegel A. A. Overexpression of the csA cell adhesion molecule under its own cAMP-regulated promoter impairs morphogenesis in Dictyostelium. J Cell Sci. 1992 Jun;102(Pt 2):203–214. doi: 10.1242/jcs.102.2.203. [DOI] [PubMed] [Google Scholar]
- Fukui Y. Intranuclear actin bundles induced by dimethyl sulfoxide in interphase nucleus of Dictyostelium. J Cell Biol. 1978 Jan;76(1):146–157. doi: 10.1083/jcb.76.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch G., Albrecht R., Heizer C., Hodgkinson S., Maniak M. Chemoattractant-controlled accumulation of coronin at the leading edge of Dictyostelium cells monitored using a green fluorescent protein-coronin fusion protein. Curr Biol. 1995 Nov 1;5(11):1280–1285. doi: 10.1016/s0960-9822(95)00254-5. [DOI] [PubMed] [Google Scholar]
- Gross J. D., Bradbury J., Kay R. R., Peacey M. J. Intracellular pH and the control of cell differentiation in Dictyostelium discoideum. Nature. 1983 May 19;303(5914):244–245. doi: 10.1038/303244a0. [DOI] [PubMed] [Google Scholar]
- Habazettl J., Gondol D., Wiltscheck R., Otlewski J., Schleicher M., Holak T. A. Structure of hisactophilin is similar to interleukin-1 beta and fibroblast growth factor. Nature. 1992 Oct 29;359(6398):855–858. doi: 10.1038/359855a0. [DOI] [PubMed] [Google Scholar]
- Hanakam F., Eckerskorn C., Lottspeich F., Müller-Taubenberger A., Schäfer W., Gerish G. The pH-sensitive actin-binding protein hisactophilin of Dictyostelium exists in two isoforms which both are myristoylated and distributed between plasma membrane and cytoplasm. J Biol Chem. 1995 Jan 13;270(2):596–602. doi: 10.1074/jbc.270.2.596. [DOI] [PubMed] [Google Scholar]
- Harwood A. J., Drury L. New vectors for expression of the E.coli lacZ gene in Dictyostelium. Nucleic Acids Res. 1990 Jul 25;18(14):4292–4292. doi: 10.1093/nar/18.14.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. D., Epel D. Intracellular pH and activation of sea urchin eggs after fertilisation. Nature. 1976 Aug 19;262(5570):661–664. doi: 10.1038/262661a0. [DOI] [PubMed] [Google Scholar]
- Kim J., Blackshear P. J., Johnson J. D., McLaughlin S. Phosphorylation reverses the membrane association of peptides that correspond to the basic domains of MARCKS and neuromodulin. Biophys J. 1994 Jul;67(1):227–237. doi: 10.1016/S0006-3495(94)80473-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Mosior M., Chung L. A., Wu H., McLaughlin S. Binding of peptides with basic residues to membranes containing acidic phospholipids. Biophys J. 1991 Jul;60(1):135–148. doi: 10.1016/S0006-3495(91)82037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S., Aderem A. The myristoyl-electrostatic switch: a modulator of reversible protein-membrane interactions. Trends Biochem Sci. 1995 Jul;20(7):272–276. doi: 10.1016/s0968-0004(00)89042-8. [DOI] [PubMed] [Google Scholar]
- Melchior F., Gerace L. Mechanisms of nuclear protein import. Curr Opin Cell Biol. 1995 Jun;7(3):310–318. doi: 10.1016/0955-0674(95)80084-0. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H. Effects of growth factors on intracellular pH regulation. Annu Rev Physiol. 1986;48:363–376. doi: 10.1146/annurev.ph.48.030186.002051. [DOI] [PubMed] [Google Scholar]
- Nellen W., Silan C., Firtel R. A. DNA-mediated transformation in Dictyostelium discoideum: regulated expression of an actin gene fusion. Mol Cell Biol. 1984 Dec;4(12):2890–2898. doi: 10.1128/mcb.4.12.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasher D. C., Eckenrode V. K., Ward W. W., Prendergast F. G., Cormier M. J. Primary structure of the Aequorea victoria green-fluorescent protein. Gene. 1992 Feb 15;111(2):229–233. doi: 10.1016/0378-1119(92)90691-h. [DOI] [PubMed] [Google Scholar]
- Randazzo P. A., Terui T., Sturch S., Fales H. M., Ferrige A. G., Kahn R. A. The myristoylated amino terminus of ADP-ribosylation factor 1 is a phospholipid- and GTP-sensitive switch. J Biol Chem. 1995 Jun 16;270(24):14809–14815. doi: 10.1074/jbc.270.24.14809. [DOI] [PubMed] [Google Scholar]
- Resh M. D. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994 Feb 11;76(3):411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Scheel J., Ziegelbauer K., Kupke T., Humbel B. M., Noegel A. A., Gerisch G., Schleicher M. Hisactophilin, a histidine-rich actin-binding protein from Dictyostelium discoideum. J Biol Chem. 1989 Feb 15;264(5):2832–2839. [PubMed] [Google Scholar]
- Schmidt-Zachmann M. S., Dargemont C., Kühn L. C., Nigg E. A. Nuclear export of proteins: the role of nuclear retention. Cell. 1993 Aug 13;74(3):493–504. doi: 10.1016/0092-8674(93)80051-f. [DOI] [PubMed] [Google Scholar]
- Schmidt J. M., Robson R. M., Zhang J., Stromer M. H. The marked pH dependence of the talin-actin interaction. Biochem Biophys Res Commun. 1993 Dec 15;197(2):660–666. doi: 10.1006/bbrc.1993.2530. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Cragoe E. J., Jr Regulation of human neutrophil chemotaxis by intracellular pH. J Biol Chem. 1986 May 15;261(14):6492–6500. [PubMed] [Google Scholar]
- Spudich J. A. Biochemical and structural studies of actomyosin-like proteins from non-muscle cells. II. Purification, properties, and membrane association of actin from amoebae of Dictyostelium discoideum. J Biol Chem. 1974 Sep 25;249(18):6013–6020. [PubMed] [Google Scholar]
- Sternfeld J., David C. N. Ammonia plus another factor are necessary for differentiation in submerged clumps of Dictyostelium. J Cell Sci. 1979 Aug;38:181–191. doi: 10.1242/jcs.38.1.181. [DOI] [PubMed] [Google Scholar]
- Swierczynski S. L., Blackshear P. J. Membrane association of the myristoylated alanine-rich C kinase substrate (MARCKS) protein. Mutational analysis provides evidence for complex interactions. J Biol Chem. 1995 Jun 2;270(22):13436–13445. doi: 10.1074/jbc.270.22.13436. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Ames J. B., Harvey T. S., Stryer L., Ikura M. Sequestration of the membrane-targeting myristoyl group of recoverin in the calcium-free state. Nature. 1995 Aug 3;376(6539):444–447. doi: 10.1038/376444a0. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Use of poly(vinylpyrrolidone) and poly(vinyl alcohol) for cryoultramicrotomy. Histochem J. 1989 Mar;21(3):163–171. doi: 10.1007/BF01007491. [DOI] [PubMed] [Google Scholar]
- Town C. D., Dominov J. A., Karpinski B. A., Jentoft J. E. Relationships between extracellular pH, intracellular pH, and gene expression in Dictyostelium discoideum. Dev Biol. 1987 Aug;122(2):354–362. doi: 10.1016/0012-1606(87)90300-9. [DOI] [PubMed] [Google Scholar]
- Van Duijn B., Inouye K. Regulation of movement speed by intracellular pH during Dictyostelium discoideum chemotaxis. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4951–4955. doi: 10.1073/pnas.88.11.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergères G., Manenti S., Weber T., Stürzinger C. The myristoyl moiety of myristoylated alanine-rich C kinase substrate (MARCKS) and MARCKS-related protein is embedded in the membrane. J Biol Chem. 1995 Aug 25;270(34):19879–19887. doi: 10.1074/jbc.270.34.19879. [DOI] [PubMed] [Google Scholar]
- Weissman J. S., Kim P. S. The pro region of BPTI facilitates folding. Cell. 1992 Nov 27;71(5):841–851. doi: 10.1016/0092-8674(92)90559-u. [DOI] [PubMed] [Google Scholar]
- Whiteside S. T., Goodbourn S. Signal transduction and nuclear targeting: regulation of transcription factor activity by subcellular localisation. J Cell Sci. 1993 Apr;104(Pt 4):949–955. doi: 10.1242/jcs.104.4.949. [DOI] [PubMed] [Google Scholar]
- Williams G. B., Elder E. M., Sussman M. Modulation of the cAMP relay in Dictyostelium discoideum by ammonia and other metabolites: possible morphogenetic consequences. Dev Biol. 1984 Oct;105(2):377–388. doi: 10.1016/0012-1606(84)90294-x. [DOI] [PubMed] [Google Scholar]
- Williams J. G. Regulation of cellular differentiation during Dictyostelium morphogenesis. Curr Opin Genet Dev. 1991 Oct;1(3):358–362. doi: 10.1016/s0959-437x(05)80300-4. [DOI] [PubMed] [Google Scholar]
- Zheng J., Knighton D. R., Xuong N. H., Taylor S. S., Sowadski J. M., Ten Eyck L. F. Crystal structures of the myristylated catalytic subunit of cAMP-dependent protein kinase reveal open and closed conformations. Protein Sci. 1993 Oct;2(10):1559–1573. doi: 10.1002/pro.5560021003. [DOI] [PMC free article] [PubMed] [Google Scholar]