Abstract
The endocrine system employs peptide hormone signals to translate environmental changes into physiological responses. The diffuse endocrine system embedded in the gastrointestinal barrier epithelium is one of the largest and most diverse endocrine tissues. Furthermore, it is the only endocrine tissue in direct physical contact with the microbial environment of the gut lumen. However, it remains unclear how this sensory epithelium responds to specific pathogenic challenges in a dynamic and regulated manner. We demonstrate that the enteroendocrine cells of the adult Drosophila melanogaster midgut display a transient, sensitive, and systemic induction of the prosecretory factor dimmed (dimm) in response to the Gram-negative pathogen Pseudomonas entomophila (Pe). In enteroendocrine cells, dimm controls the levels of the targets Phm, dcat-4, and the peptide hormone, Allatostatin A. Finally, we identify dimm as a host factor that protects against Pe infection and controls the expression of antimicrobial peptides. We propose that dimm provides “gain” in enteroendocrine output during the adaptive response to episodic pathogen exposure.
Keywords: Drosophila, midgut, immunity, enteroendocrine, dimmed
The endocrine system mediates long-range peptide hormone signaling to broadcast environmental changes to target tissues via the circulatory system. Endocrine cells must therefore function as biological sensors that detect physiochemical stimuli and translate them into changes in peptide and amine signals. The diffuse enteroendocrine system of the gastrointestinal (GI) tract is notable for both its size and the diversity of its secretory products (Ahlman and Nilsson 2001; Rehfeld 2004). Embedded within the barrier epithelium of the GI tract, enteroendocrine cells are situated uniquely with respect to the complex microbial communities of the gut lumen. Secreted enteroendocrine peptide hormones regulate local processes such as peristalsis and intestinal secretion, as well as long-range effects on metabolism, immune response, and the nervous system (Nässel and Winther 2010; Helander and Fandriks 2012). Thus, enteroendocrine cells coordinate essential aspects of physiological homeostasis at the barrier epithelium.
Studies in mammals have demonstrated the ability of enteroendocrine cells to respond to bacterial challenge with secretion of peptides and changes in gene expression (reviewed in Furness et al. 2013). However, these studies were largely performed in vitro and focused on isolated enteroendocrine cell types. Precisely how the diffuse endocrine system responds to episodic challenge under physiological conditions and the molecular mechanisms that coordinate this adaptive endocrine response remain unknown.
The adult Drosophila gut is a useful model to investigate the function and regulation of the diffuse endocrine system. The population of endocrine cells can be readily detected along the entire anterior-posterior axis of the GI tract by the pan-enteroendocrine marker Prospero (Micchelli and Perrimon 2006; Ohlstein and Spradling 2006). The Drosophila midgut, like its mammalian counterpart, also expresses a diverse array of secretory peptide hormones that exhibit regional and local diversity along the GI tract (Veenstra 2009; Veenstra and Ida 2014; Beehler-Evans and Micchelli 2015). Many of these peptides are functionally conserved across species (Nässel and Winther 2010; Veenstra 2011). Importantly, the effects of microbes on the gut epithelium can also be studied in Drosophila. For example, Pseudomonas entomophila (Pe), a pathogenic Gram-negative bacteria isolated from the GI tract of wild Drosophila, is a potent stimulus promoting stem cell mediated regeneration of the adult midgut epithelium (Vodovar et al. 2005; Buchon et al. 2009; Jiang et al. 2009; Strand and Micchelli 2011). Although Pe has been shown to induce gene expression changes in some epithelial cell types, a response of the enteroendocrine cell population has not yet been described.
dimm encodes a NeuroD-related basic helix-loop-helix transcription factor that is expressed in a subpopulation of cells in the central and peripheral nervous system during development (Hewes et al. 2003). In dimm mutants, the levels of secretory neuropeptides in the central nervous system are diminished. Misexpression studies show that Dimm functions as a “master” regulator capable of conferring two essential properties of the regulated secretory pathway to cells that do not ordinarily display them; the ability to produce large dense core vesicles that store peptides and the enzymatic machinery necessary to posttranslationally process pro-forms into biologically active signals (Hamanaka et al. 2010). Consistent with this phenotype, genome-wide characterization of Dimm binding sites has led to the identification of a number of potential mediators of this process (Hadzic et al. 2015).
Here, we examine the adult Drosophila diffuse endocrine system and characterize the regulation and function of the prosecretory transcription factor dimm in response to pathogenic bacteria.
Materials and Methods
Fly stocks and culturing
The following stocks were used: Wild-type: w1118 and Canton-S; w; pros-LacZ, ry / TM3 ry, Sb, Ser; w, UAS-dcr2; NPFGal4 / CyO; tubGal80TS, UAS-GFP / TM6C; w; esgGal4, UAS-GFP, tubGal80TS (esgTS); w, UAS-dcr2; tubGal4 / CyO; UAS-GFP, tubGal80TS / TM6C (tubTS); w, UAS-rpr, UAS-hid; y w; P[EPgy2] dimmEY14636 (BL#21432); y w; dimmRev4 / CyO (Rev4, see Hewes et al. 2003); y v; P[TRiP.JF02093]attP2 (BL#26976, designated “dimmRNAi”); P[KK112513]VIE-260B (BL#v104028, designated “PhmRNAi”). For additional information, see FlyBase (http:flybase.org).
Wild-type flies of the genotype w1118 / Canton-S were analyzed in all wild-type data with the exception of Figure 1H, where w1118 and Canton-S were analyzed individually. The strong hypomorphic genotype dimmRev4 / dimmEY14636 was analyzed in all dimm loss of function experiments.
Figure 1.
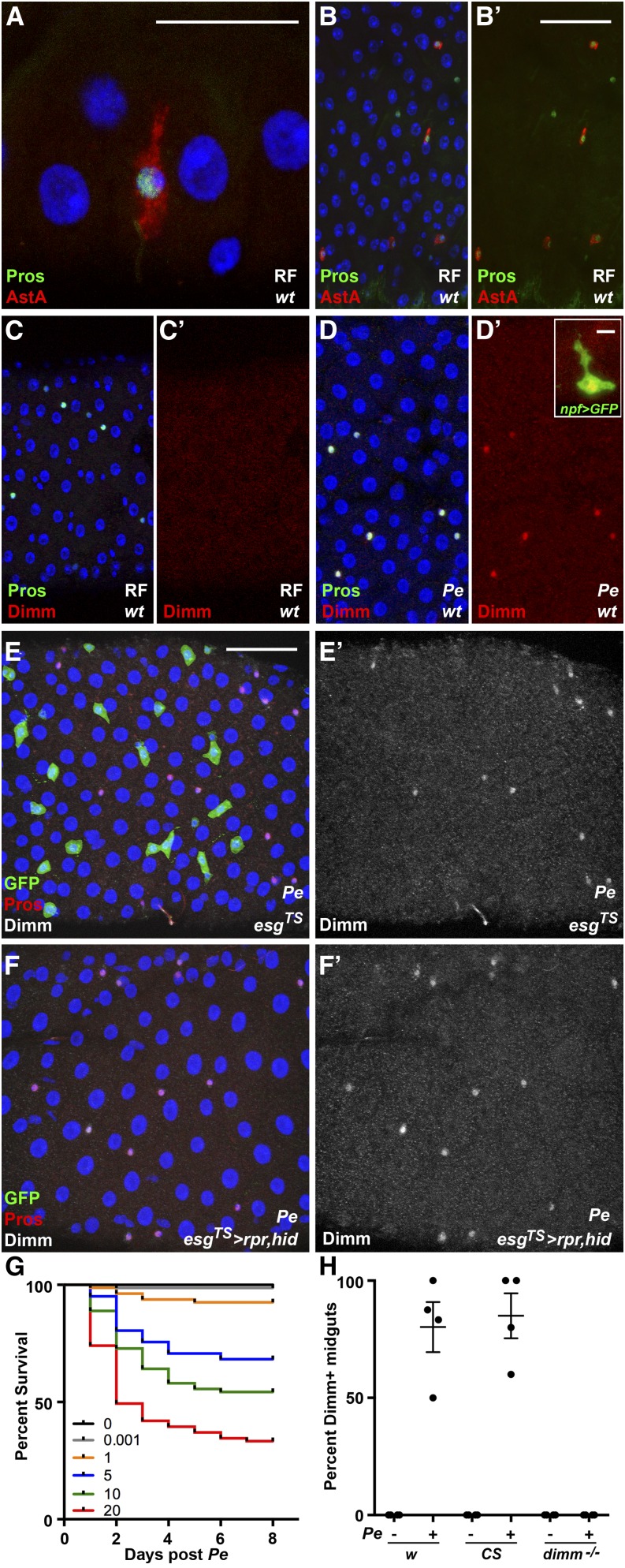
Adult enteroendocrine cells induce Dimm in response to the bacterial pathogen Pseudomonas entomophila (Pe). Gastrointestinal epithelium is shown under baseline and infected conditions. (A, B′) Confocal micrographs of the adult midgut epithelium stained for pros-LacZ and the peptide Allatostatin A (AstA) (DAPI, blue; anti-AstA, red; anti-βgal, green). (C, D′) Confocal micrographs of the adult midgut epithelium stained for Prospero (Pros) and Dimm after regular food (RF) (C, C′) or treatment with Pe (D, D′), (DAPI, blue; anti-Dimm, red; anti-Pros, green). Inset: representative cell from a Pe exposed midgut expressing npf > GFP (anti-Dimm, red; anti-GFP, green). (E, F′) Confocal micrographs of adult midguts temperature shifted to drive expression of GFP (E, E′) or the proapoptotic genes rpr and hid (F,F’) in esgTS expressing progenitor cells and subsequently exposed to Pe (DAPI, blue; anti-Pros, red; anti-GFP, green; anti-Dimm, white). Temperature shift to drive transgene expression was performed 3 d before exposure to Pe. RF controls are displayed in Figure S2. Scale bars: 10 µm (A), 50 µm (B, E), and 5 µm (D, inset). (G) Survival of wild-type females after a 24-hr exposure to a range of Pe doses (n = 4 trials, 80 females). (H) Quantification of the percentage of Dimm+ midguts per infection trial, comparing control genotypes and dimm mutants, Pe OD 5, 24 hr. Lines indicate mean values ± SEM.
Mated female flies were analyzed in all experiments. Females were collected the day of eclosion and aged 5 to 7 d before mock or Pe treatment. Females were 3 d of age in Supporting Information, Figure S1, C−E, where no mock or Pe treatments were administered. Flies were maintained on standard food media [i.e., regular food (RF); Lee and Micchelli 2013] and supplemented with yeast paste every 2−3 d in all RF conditions. Flies were cultured at 25° and transferred to 29° at the time of mock or Pe treatment and time points thereafter.
Antisera
Primary antibodies:
Primary antibodies included mouse anti-Allatostatin A (AstA) (1:20, DSHB); guinea pig anti-dCat-4 (CG13248) (1:500), (Park et al. 2011); guinea pig anti-Dimm (1:250), (Allan et al. 2005); chicken anti-GFP (1:10,000, Abcam); rabbit anti-Phm (1:750), (Kolhekar et al. 1997); mouse anti-Pros (1:100, DSHB); mouse anti-βGal (1:100, DSHB); and rabbit anti-βGal (1:2,000, Cappel).
Secondary antibodies:
Secondary antibodies used were goat anti-chicken Alexa Fluor 488 (1:2,000; Molecular Probes); goat anti-mouse Alexa Fluor 488 (1:2,000; Molecular Probes); goat anti-mouse Alexa Fluor 568 (1:2,000; Molecular Probes); goat anti-rabbit Alexa Fluor 568 (1:2,000; Molecular Probes); and goat anti-guinea pig Alexa Fluor 568 (1:2,000; Molecular Probes).
Histology
Isolation and whole-mount immunostaining of adult midguts was performed as previously described in detail (Micchelli 2014). In summary, adult female flies were dissected in 1× phosphate-buffered saline (PBS; Sigma-Aldrich). The midgut was removed and fixed in 0.5× PBS, 4% electron microscopy-grade formaldehyde (Polysciences) overnight at 4°. Samples were washed in 1× PBS, 0.1% Triton X-100 (PBST) for a minimum of 2 hr, and then incubated in primary antisera overnight. Samples were subsequently washed for 2 hr in PBST, incubated in secondary antisera for 3 hr, and washed a minimum of 2 hr in PBST. DAPI containing Vectashield mounting media (Vector Laboratories) was used for mounting.
Bacterial infection
Flies were infected ad libitum with Pseudomonas entomophila (Pe). Infected flies were fed on Tegosept-free food supplemented with 0.5mL of Pe. Pe bacterial culture was concentrated by centrifugation and resuspended in 5% sucrose. OD600 was measured and the concentrated Pe was subsequently diluted to the designated experimental OD. Mock-infected flies were placed on Tegosept-free food supplemented with 0.5 mL of 5% sucrose. Flies were shifted to and maintained at 29° throughout the course of the experiment after mock or Pe treatment. Exposure to mock or Pe food was 24 hr. Flies were returned to RF after treatment. The number of Pe colony-forming units per 0.5 mL of applied volume was estimated by counting the colony-forming units from a serial dilution of OD 20 resuspended Pe culture.
In lethality assays, adult female flies were collected in the first 24 hr of eclosion and aged 5−7 d. Flies were cultured at 25° at the ratio of 3 males + 20 females per vial. Survival of males was not included in the analysis. Flies were transferred to mock or Pe laced food of the designated OD and cultured at 29°. Flies were transferred to RF supplemented with yeast paste after 24h of Pe exposure. Lethality was assayed daily for 8 d.
Imaging acquisition and quantification
Confocal images were collected using a Leica TCS SP5 microscope. Photoshop CS (Adobe) and ImageJ (National Institutes of Health) were used to process images for brightness and contrast. To analyze the percent of Dimm+ midguts, samples were classified as positive when they displayed >2 anti-Dimm+ cells in the superficial side of the entire anterior-posterior length. Dimm+, Pros+ values were obtained by analyzing projected maximum micrographs collected at 40× magnification along the entire anterior-posterior axis of each gut analyzed. Regional boundaries were defined by morphologic constrictions present in the medial portion. ImageJ software was used to measure mean intensity values. Specifically, using ImageJ, we defined particle areas using the anti-Pros channel and then measured mean intensity of the anti-Dimm channel in that area. Dimm levels were normalized for each micrograph. Levels were normalized using the following equation (R − B) / B, where R = mean intensity in the particle area and B = mean intensity of a midgut region outside particle areas. A similar method was used to collect AstA levels (Figure 4P), with the exception that particle areas were defined manually by tracing the AstA+ cytoplasmic area.
Figure 4.
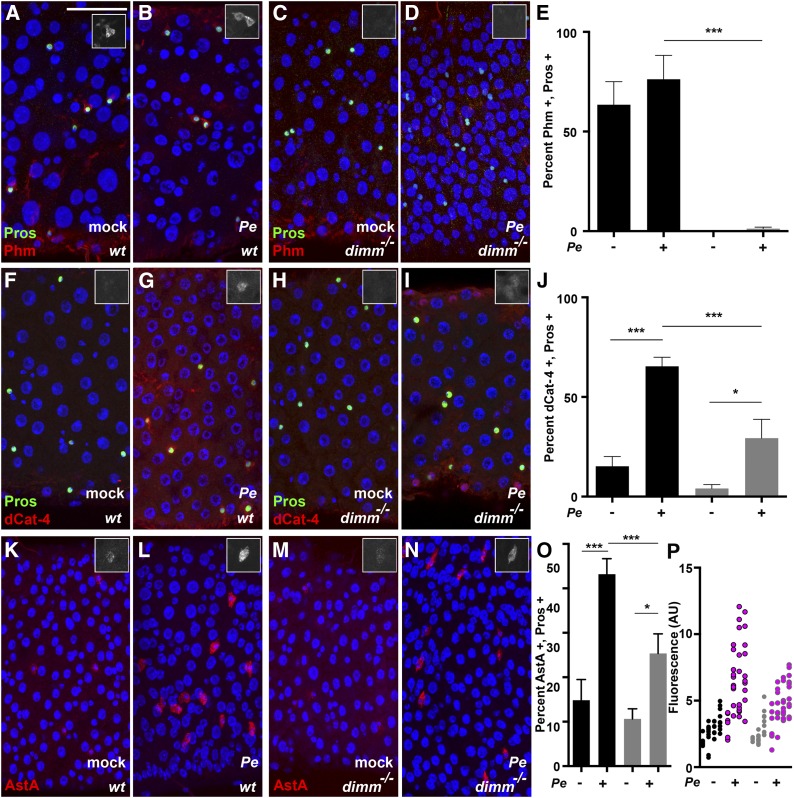
dimm target gene expression and peptide regulation in enteroendocrine cells. Analysis of dimm target genes. Insets show representative cells in gray scale to best permit comparison between samples. (A−E) Phm staining in wild-type and dimm mutant midguts after mock or Pe treatment. (DAPI, blue; anti-Phm, red; anti-Pros, green). (F−J) dCat-4 staining in wild-type and dimm mutant midguts after mock or Pe treatment. (DAPI, blue; anti-dCat-4, red; anti-Pros, green). (K−P) Allatostatin A (AstA) staining in wild-type and dimm mutant midguts after mock or Pe treatment. (DAPI, blue; anti-AstA, red). (E, J, and O) Percent positive cells of indicated antibody. Black bars, wild-type; gray bars, dimm mutants. Bars indicate mean values ± SEM (n = 2−3 trials, 12−18 midguts). (P) Quantification of mean fluorescence of anti-AstA staining in the AstA+ cell population after mock or Pe treatment. Wild-type mock, black; wild-type Pe, pink with black border; dimm mutant mock, gray; dimm mutant Pe, pink with gray border, (n = 1 trial, 3−4 midguts, 3−11 AstA+ cells per midgut). Pe dose was OD 5 and time of collection 24 hr in all experiments. Scale bar: 50 µm.
Quantitative polymerase chain reaction (qPCR) analysis
Tissue was collected from whole bodies (n = 10 females per treatment per trial) and midguts (n = 20 females per treatment per trial) and was collected and analyzed from two to three infection trials. Head, leg, and wing tissue was excluded from whole body collection. Crop and malpighian tubule tissue was excluded from midgut collection. Total RNA was extracted using the RNeasy Mini Kit (QIAGEN) according to the manufacturer’s instructions. On-column DNase digestion with RNase-Free DNase set (QIAGEN) was performed to remove genomic DNA contamination. The quality and quantity of RNA were assessed using a NanoDrop 1000 Spectrophotometer (Thermo Scientific). cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit according to the manufacturer’s protocol (Applied Biosystems). cDNA was subsequently treated with RNase H (New England Biolabs) for 30 min at 37°. qPCR was performed using an Applied Biosystems StepOnePlus System and SYBR Green PCR Master Mix (Applied Biosystems). The 2−ΔΔCT method was used to analyze fold change between mock and Pe treated samples (Livak and Schmittgen 2001). RpL32 was used as a calibrator and verified in our experiments as a reliable standard unaffected by Pe treatment (Figure S3).
Primers
Primer sequences are listed in Table S1. The dimm primers generated in this study were designed with the consideration that they span an exon−exon junction and were validated by serial dilution of cDNA and tested for melt curve specificity.
Statistical analyses and sample sizes
Graphs and statistical analyses were generated using Prism GraphPad Software. Samples sizes below are listed as n = number of trials, number of samples analyzed in total per treatment.
For survival analysis:
Log-rank (Mantel-Cox) test was performed. Figure 1G and Table S2: n = 4 trials, 80 females. Figure 5A and Table S3: n = 4 trials, 80 females.
Figure 5.
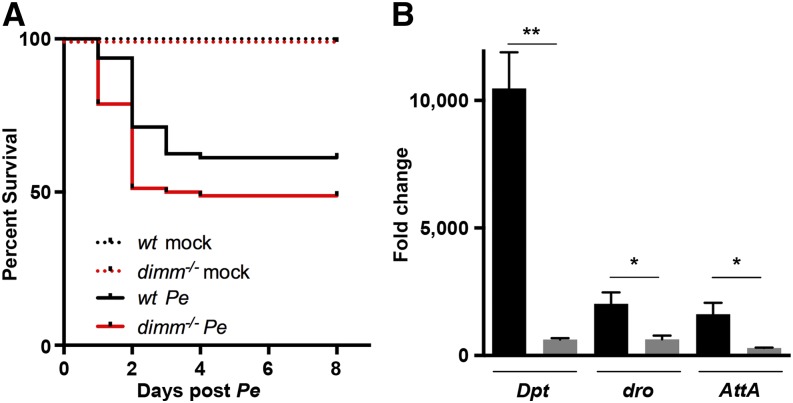
dimm is a host factor that protects against Pseudomonas entomophila (Pe) infection. Survival and immune response of dimm mutants after Gram-negative Pe infection. (A) Survival of wild-type and dimm mutants after exposure to mock or Pe treatment. Flies were exposed to Pe OD 10 for 24 hr (n = 4 trials, 80 females). (B) Quantitative polymerase chain reaction analysis of mRNA in whole-body tissue of a subset of antimicrobial peptides in wild-type and dimm mutant genotypes, Pe OD 5, collected 24 hr after Pe (wild-type, black bars; dimm mutant, gray bars). Fold change represents Pe compared with mock using the 2−ΔΔCT method (n = 3 trials, 30 flies). Bars indicate mean values ± SEM.
For percent expression analysis:
One-way analysis of variance (ANOVA) was performed, followed by Tukey’s multiple comparisons. Percent Dimm+ midguts and Dimm+ cells (Figure 1, Figure 2, and Figure 3): n = 3−4 trials, 18−24 midguts. Percent Phm+ cells (Figure 4E): n = 3 trials, 18 midguts. Percent dCat-4+ cells (Figure 4J): n = 2 trials, 12 midguts. Percent AstA+ cells (Figure 4O): n = 3 trials, 18 midguts.
Figure 2.
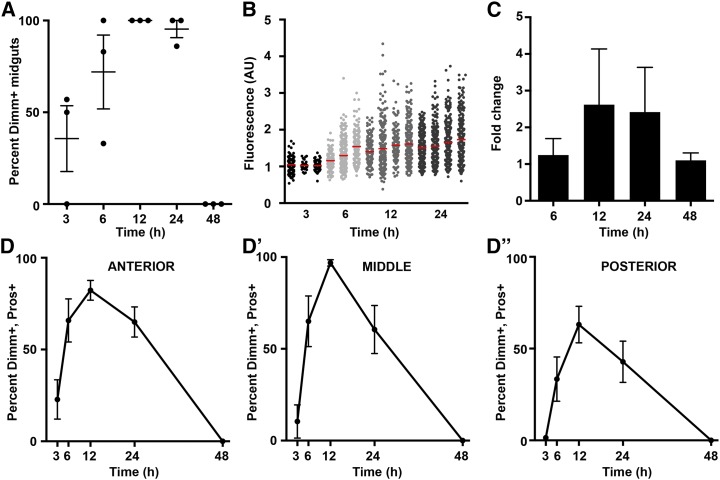
Dimm is transiently induced after Pseudomonas entomophila (Pe). Time course of dimm induction. (A) Quantification of the percentage of individuals in a given trial that displayed at least two positive anti-Dimm enteroendocrine cells over time after Pe (n = 3 trials, 18 midguts). (B) Quantification of fluorescence of anti-Dimm staining in individual Pros+ cells over time. Each column represents enteroendocrine cells from an individual midgut (n = 3−4 midguts, 35−345 enteroendocrine cells per midgut). Arbitrary units (AU). (C) Quantitative polymerase chain reaction analysis of dimm mRNA expression in midgut tissue over time (n = 2 trials, 40 midguts). Fold change represents Pe compared with mock using the 2−ΔΔCT method. (D-D′′) Regional analysis of the percentage of Dimm+ enteroendocrine cells per midgut over time (n = 3 trials, 18 midguts). Pe dose was OD 5 and time of collection as indicated. Mean values ± SEM are plotted.
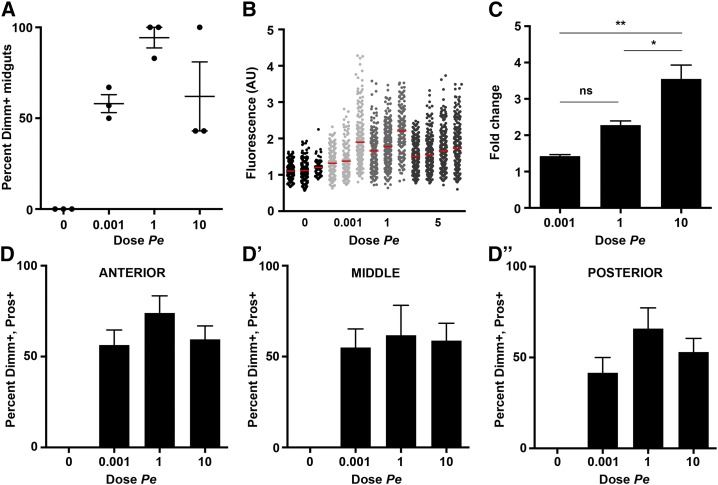
Figure 3.
Dimm induction is sensitive to the dose of Pseudomonas entomophila (Pe). Dose response of dimm induction. (A) Quantification of the percentage of individuals in a given trial that displayed at least two positive anti-Dimm enteroendocrine cells over varying doses of Pe (n = 3 trials, 18 midguts). (B) Quantification of fluorescence of anti-Dimm staining in individual Pros+ cells over Pe dose. Each column represents enteroendocrine cells from an individual midgut (n = 3−4 midguts, 70−330 enteroendocrine cells per midgut). (C) Quantitative polymerase chain reaction analysis of dimm mRNA expression in midgut tissue over Pe dose (n = 3 trials, 60 midguts). Fold change represents Pe compared with mock using the 2−ΔΔCT method. (D-D′′) Regional analysis of the percentage of Dimm+ enteroendocrine cells per midgut over Pe dose (n = 3 trials, 18 midguts). Time of collection was 24 hr after Pe and dose as indicated. Mean values ± SEM are plotted.
For fluorescence intensity:
Dimm (Figure 2B and Figure 3B): n = 1 trial, 3−4 midguts, 35−345 enteroendocrine cells. AstA (Figure 4P): n = 1 trial, 3−4 midguts, 3−11 enteroendocrine cells.
For qPCR analysis:
One-way ANOVA (Figure 2C and Figure 3C) and unpaired t-test (Figures 5B and Figure S1) were performed. dimm expression (Figure 2C): n = 2 trials, 40 midguts, (Figure 3C): n = 3 trials, 60 midguts, (Figure S1): n = 3 trials, 30 whole bodies. AMP expression (Figures 5B and Figure S3): n = 3 trials, 30 whole bodies. upd3 expression (Figure S3): n = 3 trials, 60 midguts. The qPCR data presented in Figure 3C was analyzed from samples that met a quality control criterion for Pe infectivity. Specifically, a parallel survival assay group displayed significant lethality at 48h following Pe OD 20 exposure.
For mutant analysis:
For mutant analysis (Figure S1), an unpaired t-test was performed. Body mass: n = 3 trials, 70-90 females. Midgut area: n = 2 trials, 14−19 midguts. Prospero cell density: n = 2 trials, 16 midguts.
Results
Mature enteroendocrine cells induce Dimm in response to Pe infection
We hypothesized that cells of the diffuse endocrine system would stain positively for Dimm protein, because dimm is a prosecretory factor and enteroendocrine cells are secretory cells known to express a variety of peptide hormones (Nassel and Winther 2010). The pan-enteroendocrine marker Prospero (Pros) was used to visualize cells of the diffuse endocrine system along the length of the adult midgut. Pros+ staining is readily detected in individual endocrine cells, as are secretory neuropeptides, such as AstA, which is expressed in class I enteroendocrine cells of the posterior midgut (Figure 1, A and B′) (Beehler-Evans and Micchelli 2015). To test whether Dimm is normally present in wild-type adult enteroendocrine cells under baseline conditions, we examined the colocalization of anti-Dimm in Pros+ cells. Yet, Dimm protein was not detected in any cells of the adult midgut under our experimental conditions (Figure 1, C and C′), despite the fact that identical antiserum has been used to detect Dimm in other tissues (Hewes et al. 2003; Park et al. 2008).
We next examined Dimm protein distribution in the gut after exposure to the Gram-negative bacteria Pe. We first measured the survival of wild-type flies after exposure to Pe ad libitum at different culture densities (Figure 1G). We selected an intermediate value of OD 5 as the experimental exposure, because it was associated with high survival of wild-type flies during the first 48 hr after exposure. We estimate that OD 5 contained 2.3 × 109 cells applied per vial (Table S4). In contrast to mock-treated controls, exposure to Pe at OD 5 was sufficient to induce anti-Dimm staining in Pros+ cells (Figure 1, D and D′). Upon induction, Dimm colocalized with nuclear DAPI, consistent with its characterized role as a transcription factor (Hewes et al. 2003; Park et al. 2011; Hadzic et al. 2015) and with markers of specific enteroendocrine cell subtypes (Figure 1D′ inset). When two different wild-type genotypes were used, Dimm induction was highly reproducible across independent trials (Figure 1H). Furthermore, adult midguts from dimm mutants did not display Dimm+ staining after exposure to Pe, despite the presence and normal density of Pros+ cells (Figure 1H and Figure S1). These results indicate that the immunoreactivity observed in wild-type enteroendocrine cells after bacterial challenge reflects changes in Dimm protein.
Exposure to Pe can lead to rapid production of new epithelial cells in the gut through a stem cell-mediated regenerative response (Jiang et al. 2009). It is therefore possible that the increase in Dimm signal reflects a part of the differentiation program of newly generated enteroendocrine cells. Alternatively, Dimm induction after Pe exposure could occur homeostatically in extant endocrine cells. To distinguish these possibilities, we examined Dimm immunostaining in the context of an adult midgut epithelium depleted of escargot (esg) expressing progenitor cells. We performed a conditional genetic ablation by initiating transgene expression of proapoptotic genes in adult progenitor cells (UAS-rpr, UAS-hid; esgGal4, UAS-GFP; tubGal80TS). Ablation was confirmed by the absence of GFP+ progenitor cells and this manipulation alone was not associated with accumulation of Dimm (Figure S2). After exposure to Pe, midguts depleted of esg+ cells were capable of robust Dimm induction in Pros+ cells (Figure 1, E−F′). Taken together, we conclude that Dimm protein is normally low under baseline conditions, and is induced in mature enteroendocrine cells following exposure to Pe.
Dimm induction in the diffuse endocrine system is transient and systemic
To further characterize Dimm induction in enteroendocrine cells, we performed a time course analysis ranging from 0 to 48 hr following initial Pe exposure. Four parameters were measured: (1) the percentage of individuals within an experimental group that displayed Dimm+ staining (percent Dimm+ midguts); (2) the percentage of Dimm+ endocrine cells per midgut (percent Dimm+, Pros+); (3) the anti-Dimm fluorescence intensity of individual enteroendocrine cells within a midgut; and (4) the fold induction of dimm mRNA measured by qPCR (Figure 2). Individuals displayed detectable Dimm+ cells between 3 and 24 hr after initial Pe exposure, with the percentage of positive midguts highest at 12 and 24 hr (Figure 2A). Dimm+ cells were not detectable in midguts from the 48-hr time point. Analysis of the percentage of Dimm+, Pros+ cells within midguts revealed that a majority of Pros+ cells become Dimm+ after Pe treatment (Figure 2, D and D′′). We independently examined data collected from the anterior, middle, and posterior midgut regions to identify any potential differences in either the timing or extent of Dimm induction. Regions did not significantly differ from each other within time points, with the exception of the 12-hr time point when the middle region was significantly higher than the posterior region (**ANOVA, F = 6.503). Consistent with this statistical result, we noted a general trend that the posterior midgut displayed lower percent Dimm+ values and that endocrine cells in the anterior region most proximal to the middle midgut were most consistently bright and of high percent induction. Fluorescence intensity of anti-Dimm staining in individual Pros+ cells was consistent with percent cell induction and showed higher values at the 12- and 24-hr time points (Figure 2B). Finally, to determine whether dimm is transcriptionally induced after Pe exposure, we compared dimm mRNA levels between mock- and Pe-treated flies by qPCR (Figure 2C). An increase in dimm transcript was detected at the 12- and 24-hr time points but not at 6- or 48-hr. Transcriptional induction was likewise reduced in dimm mutants (Figure S1). Taken together, we conclude that dimm is induced at the transcriptional and protein levels measured, and exhibit a similar time course showing highest values 12 and 24 hr after Pe exposure.
Dimm induction in enteroendocrine cells is sensitive to low doses of Pe
We next asked whether Dimm induction varies with Pe dose. We exposed flies to Pe ranging from OD 0.001 to OD 10 and quantified the same set of parameters described previously (Figure 3). Although Pe doses of OD 0.001 and OD 1 were associated with very low lethality in wild-type flies (Figure 1G and Table S2), these doses were nevertheless sufficient to induce Dimm+ midguts at comparable frequency to OD 10 (Figure 3A). Similarly, Pe dose did not affect the percentage of Dimm+ endocrine cells assayed in different regions of the midgut (Figure 3, D−D′′). Thus, the percentage of Dimm+ enteroendocrine cells can reach high values at sublethal doses of Pe. Fluorescence intensity of anti-Dimm staining in individual Pros+ cells also reached high values at the relatively low dose of OD 0.001 (Figure 3B). Finally, examination of dimm mRNA levels by qPCR revealed that increasing Pe dose correlated with increased fold change in transcript (Figure 3C). Two transcripts previously characterized to be responsive to Pe infection, unpaired 3 (upd3) and Diptericin (Dpt) (Vodovar et al. 2005; Jiang et al. 2009), showed a similar trend, suggesting that dose dependent transcriptional induction may be a general aspect of the response to Pe (Figure S3). Taken together, we conclude that independent measures of Dimm protein induction are sensitive to low doses of Pe, and that dimm transcript induction is dose dependent.
Enteroendocrine cells express Dimm target genes in a dimm-dependent manner
The biosynthetic enzyme peptidylglycine alpha-monooxygenase (Phm), and dcat-4, which encodes a putative amino acid transporter, are direct transcriptional targets of Dimm in the embryonic central nervous system (Park et al. 2011). We therefore examined these targets in the diffuse endocrine system by immunostaining (Figure 4). After mock treatment, wild-type flies have detectable Phm in Pros+ cells despite a lack of Dimm staining in mock-treated flies (Figure 4A). Phm was also detectable in wild-type endocrine cells after Pe exposure (Figure 4B). However, Phm was not detectable in enteroendocrine cells of dimm mutants under mock- or Pe-infected conditions, despite the presence of Pros+ cells in mutant midguts (Figure 4, C and D and Figure S1, see the Materials and Methods section for description of genotype used). To test whether Phm is increased in midguts exposed to Pe, we quantified the percentage of Phm+, Pros+ cells after mock and Pe treatment. The percentage of Phm+, Pros+ cells was not significantly changed in response to Pe exposure (Figure 4E). We conclude that dimm is necessary for detection of Phm protein in adult midgut endocrine cells, and that Phm is detectable at similar levels in endocrine cells independent of Pe exposure.
Analysis of dCat-4 revealed detectable dCat-4 in a portion of wild-type Pros+ cells after mock and Pe treatment (Figure 4, F and G). In contrast to Phm, the percentage of dCat-4+, Pros+ cells was significantly increased as an effect of Pe (Figure 4J). dimm mutants produced detectable levels of dCat-4 protein; however, the percentage of dCat-4+ Pros+ cells did not increase as significantly as an effect of Pe in the absence of dimm (Figure 4, H, I, and J). We conclude that dCat-4 is a regulated target during the midgut response to Pe, and that dimm is not absolutely necessary for dCat-4 expression, but is necessary for dCat-4 induction in response to Pe. Taken together, we conclude that two previously identified direct transcriptional targets also show dimm dependent expression and induction in midgut endocrine cells.
dimm is required for increased levels of AstA hormone after Pe infection
We hypothesized that Dimm induction in enteroendocrine cells could help support changes in peptide hormone levels after Pe. To test this possibility, we examined the protein AstA after mock and Pe treatment. AstA was detectable in both wild-type and dimm mutants under mock conditions (Figure 4, K and M). AstA also was detectable in both genotypes after Pe exposure (Figure 4, L and N). We noted a visual increase in the intensity of AstA staining in samples exposed to Pe, and therefore quantified the percentage of AstA+, Pros+ cells, as well as the fluorescence intensity of AstA antibody staining in individual cells (Figure 4, O and P). We observed that wild-type midguts significantly increased the percentage and levels of AstA after exposure to Pe. dimm mutants displayed a similar trend of AstA induction in response to Pe, however both the percentage of AstA+, Pros+ cells and the fluorescence intensity of individual enteroendocrine cells were less pronounced than that of wild-type flies. We conclude that AstA is a regulated peptide during the midgut response to Pe and that dimm is not absolutely necessary for AstA expression, but is necessary for normal AstA induction in response to Pe exposure. We note that in contrast to Phm and dcat-4, profiling studies in other tissues have not identified peptide hormones as direct targets (Park et al. 2011; Hadzic et al. 2015). Therefore, it remains unclear whether AstA is a direct transcriptional target in the gut or whether this regulation is indirect.
dimm is a host factor that protects Drosophila against Pe infection
To test the functional requirement for dimm, we first examined the survival of dimm mutants after Pe challenge. Before treatment, dimm mutants displayed reductions in mass and midgut area (Figure S1, C and D). However, no significant effect on the density of Pros+ cells was detected (Figure S1E). After Pe exposure, survival of dimm mutants was significantly decreased compared with wild-type controls (Figure 5A and Table S3). Similar results were also observed following conditional expression of RNAi targeting dimm or Phm (Figure S4). To more specifically examine the involvement of dimm in the host immune response, we next compared wild-type and dimm mutant flies for their expression of a subset of antimicrobial peptides known to be involved in the innate immune response to Pe (Vodovar et al. 2005). The AMPs Diptericin (Dpt), drosocin (dro), and Attacin A (AttA) were highly induced in wild-type flies exposed to Pe (Figure 5B). However, dimm mutant flies displayed a significantly lower fold change in response to Pe for each AMP measured. Taken together, we conclude that dimm is a protective host factor that is necessary for the induction of antimicrobial peptides following infection by the Gram-negative pathogen Pe.
Discussion
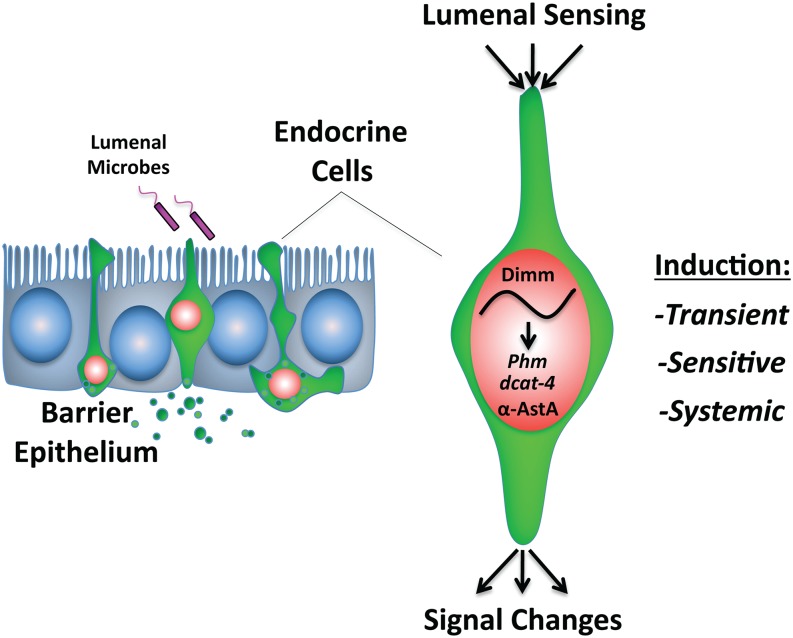
We used the Drosophila midgut to investigate how the diffuse endocrine system responds to pathogenic infection by a Gram-negative bacterium. Our studies establish an important new link between pathogenic infection and the coordinated induction of a prosecretory program in cells of the diffuse endocrine system. They also suggest a molecular model explaining how changes in the lumenal environment might result in a dynamically altered organismal physiology (Figure 6). Mature enteroendocrine cells respond to the pathogenic bacterium Pe with induction of dimm, a prosecretory basic helix-loop-helix transcription factor. This response shows a defined signature in several variables, including time, dose and region of enteroendocrine induction. In the diffuse endocrine system, dimm is necessary for normal levels of the important peptide hormone AstA and an enzyme necessary for the processing of peptide hormones more generally. Thus, Dimm may function to provide “gain” in the adaptive response of the diffuse endocrine system (Mills and Taghert 2012). Finally, we show that dimm is an essential host factor that protects the organism against pathogenic challenge and controls the induction of antimicrobial peptides. Future studies will determine the extent to which changes in the diffuse endocrine system directly regulate immune function or whether these processes occur in parallel.
Figure 6.
Model.
Acknowledgments
We thank Dr. Bruno Lemaitre for Pseudomonas entomophila strains; the Drosophila Bloomington stock center; the VDRC stock center; the Developmental Studies Hybridoma Bank; Drs. Ross Cagan, Jan Veenstra, and Ping Shen for reagents; and members of the Micchelli and Taghert laboratories for helpful discussions. K.B.B. is an Olin Fellow. C.A.M. is a Pew Biomedical Scholar and American Cancer Society Research Scholar. Additional funding for this research was provided by the National Institutes of Health; R01 NS021749 (P.H.T.) and RO1 DK095871 (C.A.M.).
Footnotes
Supporting information is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.115.019117/-/DC1
Communicating editor: A. Gasch
Literature Cited
- Ahlman H., Nilsson, 2001. The gut as the largest endocrine organ in the body. Ann. Oncol. 12(Suppl 2): S63–S68. [DOI] [PubMed] [Google Scholar]
- Allan D. W., Park D., St Pierre S. E., Taghert P. H., Thor S., 2005. Regulators acting in combinatorial codes also act independently in single differentiating neurons. Neuron 45: 689–700. [DOI] [PubMed] [Google Scholar]
- Beehler-Evans R., Micchelli C. A., 2015. Generation of enteroendocrine cell diversity in midgut stem cell lineages. Development 142: 654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N., Broderick N. A., Chakrabarti S., Lemaitre B., 2009. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 23: 2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B., Rivera L. R., Cho H. J., Bravo D. M., Callaghan B., 2013. The gut as a sensory organ. Nat Rev Gastroenterol Hepatol 10: 729–740. [DOI] [PubMed] [Google Scholar]
- Hadzic T., Park D., Abruzzi K. C., Yang L., Trigg J. S., et al. , 2015. Genome-wide features of neuroendocrine regulation in Drosophila by the basic helix-loop-helix transcription factor DIMMED. Nucleic Acids Res. 43: 2199–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka Y., Park D., Yin P., Annangudi S. P., Edwards T. N., et al. , 2010. Transcriptional orchestration of the regulated secretory pathway in neurons by the bHLH protein DIMM. Curr. Biol. 20: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander H. F., Fandriks L., 2012. The enteroendocrine “letter cells”—time for a new nomenclature? Scand. J. Gastroenterol. 47: 3–12. [DOI] [PubMed] [Google Scholar]
- Hewes R. S., Park D., Gauthier S. A., Schaefer A. M., Taghert P. H., 2003. The bHLH protein Dimmed controls neuroendocrine cell differentiation in Drosophila. Development 130: 1771–1781. [DOI] [PubMed] [Google Scholar]
- Jiang H., Patel P. H., Kohlmaier A., Grenley M. O., McEwen D. G., et al. , 2009. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137: 1343–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolhekar A. S., Roberts M. S., Jiang N., Johnson R. C., Mains R. E., et al. , 1997. Neuropeptide amidation in Drosophila: separate genes encode the two enzymes catalyzing amidation. J. Neurosci. 17: 1363–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. C., Micchelli C. A., 2013. Development and characterization of a chemically defined food for Drosophila. PLoS One 8: e67308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Micchelli C. A., 2014. Whole-mount immunostaining of the adult Drosophila gastrointestinal tract. Methods 68: 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli C. A., Perrimon N., 2006. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439: 475–479. [DOI] [PubMed] [Google Scholar]
- Mills J. C., Taghert P. H., 2012. Scaling factors: transcription factors regulating subcellular domains. BioEssays 34: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassel D. R., Winther A. M., 2010. Drosophila neuropeptides in regulation of physiology and behavior. Prog. Neurobiol. 92: 42–104. [DOI] [PubMed] [Google Scholar]
- Ohlstein B., Spradling A., 2006. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439: 470–474. [DOI] [PubMed] [Google Scholar]
- Park D., Hadzic T., Yin P., Rusch J., Abruzzi K., et al. , 2011. Molecular organization of Drosophila neuroendocrine cells by Dimmed. Curr. Biol. 21: 1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D., Shafer O. T., Shepherd S. P., Suh H., Trigg J. S., et al. , 2008. The Drosophila basic helix-loop-helix protein DIMMED directly activates PHM, a gene encoding a neuropeptide-amidating enzyme. Mol. Cell. Biol. 28: 410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld J. F., 2004. A centenary of gastrointestinal endocrinology. Horm. Metab. Res. 36: 735–741. [DOI] [PubMed] [Google Scholar]
- Strand M., Micchelli C. A., 2011. Quiescent gastric stem cells maintain the adult Drosophila stomach. Proc. Natl. Acad. Sci. USA 108: 17696–17701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra J. A., 2009. Peptidergic paracrine and endocrine cells in the midgut of the fruit fly maggot. Cell Tissue Res. 336: 309–323. [DOI] [PubMed] [Google Scholar]
- Veenstra J. A., 2011. Neuropeptide evolution: neurohormones and neuropeptides predicted from the genomes of Capitella teleta and Helobdella robusta. Gen. Comp. Endocrinol. 171: 160–175. [DOI] [PubMed] [Google Scholar]
- Veenstra J. A., Ida T., 2014. More Drosophila enteroendocrine peptides: Orcokinin B and the CCHamides 1 and 2. Cell Tissue Res. 357: 607–621. [DOI] [PubMed] [Google Scholar]
- Vodovar N., Vinals M., Liehl P., Basset A., Degrouard J., et al. , 2005. Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc. Natl. Acad. Sci. USA 102: 11414–11419. [DOI] [PMC free article] [PubMed] [Google Scholar]