Abstract
Benign hereditary chorea (BHC) is a childhood-onset, hyperkinetic movement disorder normally with little progression of motor symptoms into adult life. The disorder is caused by mutations to the NKX2.1 (TITF1) gene and also forms part of the “brain–lung–thyroid syndrome”, in which additional developmental abnormalities of lung and thyroid tissue are observed. In this review, we summarize the main clinical findings in “classical” BHC syndrome and discuss more recently reported atypical features, including non-choreiform movement phenotypes. We highlight additional non-motor characteristics such as cognitive impairment and psychiatric symptoms, while discussing the evidence for BHC as a developmental disorder involving impaired neural migration and other multisystem developmental abnormalities. Finally, we will discuss the efficacy of available therapies in both affected pediatric and adult cohorts. Delineation of the BHC disease spectrum will no doubt expand our understanding of this disorder, facilitating better targeting of genetic testing and establish a framework for future clinical trials.
Keywords: Benign Hereditary Chorea, NKX2.1 mutations, Brain-Lung-Thyroid disorder
Introduction
Benign hereditary chorea (BHC) (MIM 118700) is a rare childhood-onset movement disorder characterized predominantly by non-progressive chorea. The first familial description reported a 32-member, five-generation African-American family from Mississippi, USA, in which the probands, two teenage brothers, presented with childhood-onset chorea and delayed motor development.1
A number of families with a similar phenotype to these original cases and an autosomal dominant pattern of inheritance were reported over subsequent years, although their descriptions were frequently heterogenous with atypical features such as dystonia, tremor, and myoclonus described.2–4 This led some to question the validity of BHC as single diagnosis and specific disease entity.5 Furthermore, 11 families, originally considered to have a clinical diagnosis of BHC, were later confirmed to have different genetically confirmed diagnoses, including Huntington’s disease (HD), myoclonus dystonia (MD), and ataxia telangiectasia (AT). This study also demonstrated the difficulties in classifying movement disorders, with significant discrepancy between experts when reviewing videotaped examinations.5
In 2000, genetic analysis of a four-generation Dutch family in tandem with another of the originally described BHC families, established linkage to a disease locus on the long arm of chromosome 14.2,6,7 Recombination analysis of six further families reduced the critical region to 8.4cM (14q13.1-q21.1). Using chromosomal markers and two-color fluorescence in situ hybridization (FISH) a de novo 1.2-Mb deletion involving the NKX2.1 gene, also known as TITF1, TTF1, and TEBP, was identified.8 Breedveld et al.9 confirmed further NKX2.1 variants in four additional unrelated BHC families, in whom whole gene deletion (WGD), frameshift, and missense mutations were identified. At the same time, NKX2.1 mutations were reported in a cohort of five pediatric patients with hypothyroidism and ongoing symptoms, despite adequate thyroxine replacement.10 The NKX2.1 clinical spectrum appeared to therefore extend beyond a pure neurological phenotype.
In this review, we discuss the spectrum of clinical, genetic, and pathological features considered to be typical of BHC, while also considering more recent reports that expand the spectrum of the disorder (Figure 1). We also discuss the current evidence for the physiological role of NKX2.1 protein, putative pathogenic disease mechanisms, and the relative efficacy of current treatment strategies.
Figure 1. Diagram of the “Classical” Features of Benign Hereditary Chorea and Expanding Clinical Phenotype. Each ring, brain (red), lung (green), and thyroid (blue), represents a body system involved in the classical brain–lung–thyroid triad. The most common clinical features are listed within each ring. Other clinical features increasingly recognized in association with NKX2.1 mutations are listed outside the rings. ADHD, Attention Deficit Hyperactivity Disorder; Brain–Lung–Thyroid, Overlapping clinical phenotypes involving the complete brain–lung–thyroid clinical spectrum; OCD, Obsessive–Compulsive Disorder.
Methods
We performed a systematic literature search of the PUBMED database using the key words “benign hereditary chorea”, “brain–lung–thyroid syndrome”, “NKX2.1”, “TITF1”, “TTF1”, and “TEBP ”. There was no restriction on year of publication but only those published in English and in peer-reviewed journals were included.
Epidemiology
A single epidemiological study of the Welsh population estimated the prevalence of BHC to be ∼1:500,000 in 1978, although this is widely considered to be an underestimate due to poor recognition of subtle choreiform phenotypes.11 Other studies have reported that, in addition to gender-dependent variation in penetrance (100% in males, 75% in females), there is significant inter- and intrafamilial variation of clinical phenotypes.12 Larger cohort studies have also suggested that females are more commonly affected than males (0.64:0.46), whether additional genetic or environmental factors contribute to this observed gender difference remains undetermined.13,14
Clinical description
Motor features
Classical motor symptoms in BHC include mild to moderate hypotonia with delayed motor milestones in infancy. Onset of chorea is usually in early childhood (median age 2.5–3 years), although this is likely to be younger due to relatively late recognition of the movement disorder phenotype.13–15 The chorea is often generalized, affecting all body parts (face, limbs, trunk) and tends to worsen with stress or excitement.16 In the majority of cases the chorea will remain stable, or improve during adolescence and early adulthood, with other movement disorders such as myoclonus becoming more prevalent and disabling.12,13
Other types of movement disorder have also been reported in NKX2.1 mutation-positive cases (either in isolation or in conjunction with chorea) and include ataxia, upper limb intention tremor, limb dystonia, and motor and vocal tics.13,14,17,18 Facial apraxia and axial dystonia have also been described, although in both cases, genetic confirmation was not possible as the clinical description pre-dated NKX2.1 gene identification.19,20 More recently, recurrent drop attacks and frequent falls have been reported, often resulting in significant trauma. In one of these cases, electroencephalography during a typical episode demonstrated that these features were not associated with ictal epileptiform activity, suggesting that they were a consequence of the movement disorder phenotype, rather than an epileptic event.17,21
Speech disturbance has been consistently reported in BHC patients. Dysarthria is most frequently reported, evident in 40% of cases from a single case series.14,22 Stammer as well as slow, slurred, stuttering, and explosive speech have also each been described in single cases.18,23,24 Interestingly, in the largest longitudinal cohort studied to date, no dysarthria was described but three unrelated cases were described as having vocal tics.13
Cognition
Cognitive status in those with BHC has historically been considered normal, and previously postulated to be an integral marker in differentiating this form of chorea from more neurodegenerative forms associated with cognitive impairment, e.g. Huntington’s disease.16 However, prior to NKX2.1 gene discovery, several reports documented reduced IQ in those with clinical BHC syndrome, with one study finding average IQ to be 10 points lower in motor affected individuals than unaffected family members.2,25,26 Subsequent studies have documented variable findings on cognitive assessment. Although some, using standardized scales (Mini Mental State Examination [MMSE] and Wechsler Adult Intelligence Scale - Revised [WAIS-R]), reported reduced levels of cognitive functioning, others have described children performing academically above that expected for their age.17,23 Two recent case series have also demonstrated conflicting results. We reported grossly normal levels of intellect, gauged by attendance in mainstream education, while Gras et al identified learning difficulties in 20 out of 28 reported cases.13,14 Of these 20 patients, 14 underwent formal assessment. Within this subgroup, three were considered to have mental retardation (IQ < 70), three had borderline IQ levels (70–80) and eight were found to have IQs within normal limits (IQ > 80). The other six cases were historically reported to have learning difficulties during the course of their education, though no formal cognitive assessment was performed. It therefore remains to be determined whether these deficits in cognitive function reflect an integral part of the BHC phenotype or a secondary consequence of social embarrassment and isolation resulting in reduced periods of education.
Psychiatric symptoms
Depression, apathy, psychosis, and prolonged inpatient psychiatric admission were all described in early reports of patients with BHC clinical syndrome.2,27 Psychosis, schizophrenia, post-partum psychosis, obsessive–compulsive disorder (OCD), and attention deficit hyperactivity disorder (ADHD) have all been reported in patients with NKX2.1 mutations.14,18,28,29 The recent large, longitudinal French study found seven out of 28 mutation-positive cases to have a diagnosis of ADHD, of whom nearly all (six out of seven) had IQ levels within the normal range.13
Brain–lung–thyroid spectrum and other clinical features
The combination of brain, lung, and thyroid involvement was first described in a single case involving a contiguous NKX2.1 gene deletion in which the patient had neonatal respiratory failure, a high serum thyrotropin concentration, motor delay, hypotonia, and truncal ataxia.30 However, it wasn’t until description of a clinically similar case two decades later that the term brain–lung–thyroid syndrome was coined.31 Typical respiratory symptoms include neonatal or infant respiratory distress syndrome, frequently resulting in mechanical ventilation followed by recurrent pulmonary infections, development of obstructive airways disease, and chronic interstitial lung disease. Thyroid dysfunction usually takes the form of congenital hypothyroidism, which in a proportion of cases may be due to thyroid agenesis.16 Two larger case series have demonstrated 30–36% patients have symptoms involving all three organs, 32–40% with brain and thyroid involvement, and 20–21% with brain and lung disease alone.13,14 However, both cohorts only recruited participants from neurology/movement disorder outpatient clinics with likely ascertainment bias. In reality, the full triad of brain–lung–thyroid symptoms seems to be evident in up to 50% of cases with NKX2.1 mutations, while involvement of brain and thyroid appears to consistently involve 30% of all cases.15,32 It is postulated that brain–lung–thyroid syndrome and BHC may represent part of a continuous spectrum of disorders and that the associated lung and thyroid features were not recognized or investigated in early descriptions of the BHC clinical syndrome.12
Other clinical features have also been consistently reported, including developmental abnormalities of the urinary tract, such as hypospadias, vesico-urethral reflux, megabladder, duplex kidney, and recurrent urinary tract infections.3,14,28,33 Others have reported single cases manifesting hypo- or oligodontia,34 hypoparathyroidism,34 short stature, webbed neck,18 joint hypermobility,22 sensorineural hearing loss,35 seizures,36 optic nerve glioma,2 growth hormone deficiency, visual disturbance, pes cavus, and kyphosis.14 It is currently uncertain whether these features are disease related or incidental findings.
Links with malignancy
An increasing number of case reports describe malignancy in those with NKX2.1 mutations. Lung carcinoma is most frequently reported, often preceded by a history of pulmonary disease such as alveolar proteinosis, asthma, or recurrent respiratory tract infections.13,18,31,37 In addition, two terminal cases of leukemia and a single case of small cell bladder carcinoma have also been described.22,38 Interestingly in a mouse model of lung carcinoma, reduced TITF1 signaling contributes to tumor formation. Gene overexpression has also been recognized in a broad spectrum of malignancy subtypes including lung, thyroid, and prostate.39 More recently, copy number variant analysis in lung adenocarcinoma led to the suggestion of 14q13.3 amplification as a candidate proto-oncogene.40
Clinical indications for NKX2.1 testing and differential diagnosis
NKX2.1 testing should be considered in all those with a history of neonatal hypotonia, motor developmental delay, and early-onset hyperkinetic movement disorder with predominant chorea and/or dystonia. Clearly, early evidence of comorbid lung or thyroid symptoms would serve to reinforce the importance of genetic testing. However, these neurological features (in the presence of normal cerebral magnetic resonance imaging) are common to a number of other genetically determined movement disorders that would need to be considered in the clinical differential diagnosis. Those to consider would include myoclonus dystonia (particularly those with SGCE mutations), given the difficulties in discriminating distal low amplitude myoclonus and fine choreiform movements.41 Allan–Herndon–Dudley syndrome, caused by SLC16A2 mutations, is a rare X-linked hyperkinetic movement disorder. Its presentation frequently mirrors that of BHC with initial hypotonia and subsequent development of involuntary limb movements, frequently with dystonia.42 Finally, autosomal dominant inherited adenylate cyclase-5 (ADCY5) mutations have recently been identified in a number of families presenting with early-onset chorea and dystonia, and will become an increasingly important differential diagnosis in those with infancy-onset hyperkinetic movement disorders.43
Imaging
Although cerebral imaging is normal in the majority of NKX2.1 mutation-positive cases, a variety of structural and enhancement anomalies of uncertain significance have also been reported. These have included ventricular dilatation, empty sella, bilateral pallidal hyperintensity, and cystic masses in the pituitary and sella turcica.10,14,23,44 Volumetric analysis of two mutation-positive cases found bilateral reduction of striatal volumes, while magnetic resonance spectroscopy identified altered myoinositol, creatine, and N-acetyl-aspartate levels.45 Single-photon emission computed tomography scans of two pediatric cases found reduced technetium-99 uptake in the basal ganglia, while positron emission tomography (PET) studies of dopamine transmission demonstrated reduced post-synaptic D2 receptor function.37,46 Although neurodegeneration is not considered to occur in those with NKX2.1 mutations, fludeoxyglucose-PET findings have been inconsistent in a small number of cases demonstrating both normal and reduced cortico-striatal glucose metabolism, the latter often being a feature observed in neurodegenerative forms of chorea such as Huntington’s disease.28,38 Overall, these imaging findings are predominantly from single case reports or small case series, so their relevance to NKX2.1-related disease remain uncertain and may represent incidental findings. Future studies, involving larger cohorts, using standardized imaging techniques, are required before conclusions can be drawn regarding the specificity of radiological findings.
Treatment
There are currently no consensus nor formal guidelines for the treatment of chorea in patients with BHC. Multiple reports have described single cases treated with a variety of agents including trihexyphenidyl, corticosteroids, sodium valproate, propranolol, ropinirole, and sulpiride, each with varying results.14,32,46 Levodopa has been the most consistent in providing symptomatic improvement (relatively high doses of 7–9 mg/kg/day), predominantly when given during childhood, and in conjunction with physiotherapy.21,22,47 Cases in which there has been little response to l-dopa have tended to use lower levodopa doses, principally limited due to tolerability.13,14 Tetrabenazine also led to motor improvements in five cases at doses of 0.5 mg/kg/day in children and 37.5 mg/day in adults. Cessation of a 75 mg/day dosage in one case led to worsening of chorea and disturbances to sleep and behavior.13,28 Olanzapine, for treatment of psychosis, improved chorea in a single NKX2.1 mutation-positive case, while in another the motor symptoms were left unchanged.18 Trihexyphenidyl and clonazepam have also been considered effective, each in single cases, although neither led to complete resolution of motor symptoms.14
Molecular genetics and pathophysiology
Gene function
NKX2.1 belongs to the natural killer gene family of highly conserved homeodomain-containing transcription factors. The gene comprises three coding exons giving rise to five transcripts. The two most common isoforms are 371 and 401 amino acids in length (ENST00000498187 and ENST00000354822) differing only in their N-terminal sequences.48 The majority of pathogenic mutations lead to protein truncation, either before or within the DNA-binding domain, preventing DNA binding, and lead to loss-of-function.9 In vitro studies have demonstrated that the mutant protein has a dominant-negative effect on transcriptional activity of the wild-type protein, suggesting that the clinical phenotype is due to NKX2.1 haploinsufficiency.15,29
NKX2.1 mutations
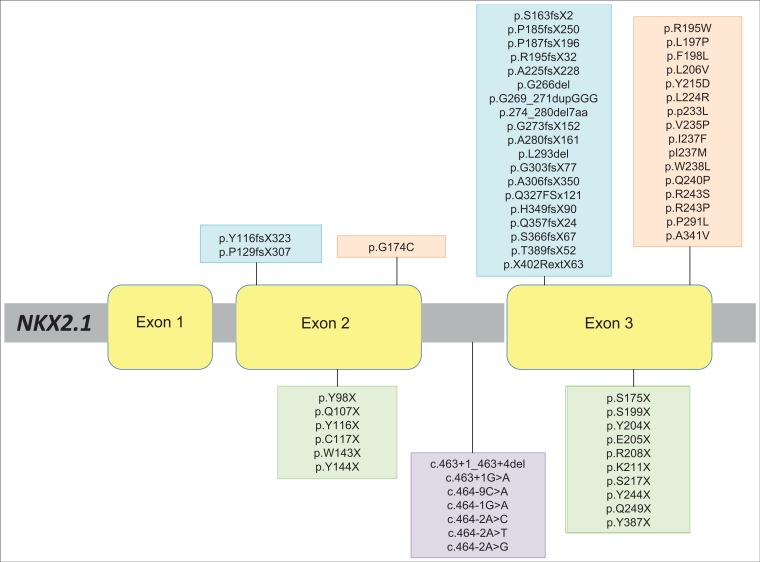
A broad spectrum of NKX2.1 mutations and diverse clinical phenotypes has been reported to date (Table 1, Figure 2). A correlation between phenotype severity and type of mutation has been postulated. Larger contiguous gene deletions have been associated with a more severe spectrum of the brain–lung–thyroid triad (often with additional clinical characteristics), while point nonsense mutations, affecting the terminal regions of the protein, are reported in those with a milder clinical phenotype.16 However, this genotype–phenotype association is not seen in all cases, suggesting that there may be other contributory environmental and/or epigenetic factors.13,14 NKX2.1 mutations also demonstrate considerable intrafamilial variability, with severity of the movement disorder ranging from significant to those with minimal functional impairment. There may also be individual heterogeneity in the expression of other motor and non-motor features between members of the same kindred.49 A full summary of all known mutations and clinical features of cases with contiguous gene deletions involving NKX2.1 is shown in Table 1 and Figure 2.
Table 1. Genotype and Clinical Phenotype of Reported Whole Gene Deletions Involving NKX2.1 .
| Authors | Year of Publication | Deletion size and Chromosomal Location* | Genes Involved1 | Organ Involvement | Additional Clinical Characteristics | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Brain | Lung | Thyroid | |||||
| Devriendt et al.30 | 1998 | <13cM deletion involving 14q13-q21 | NKX2.1, PAX9 | Hypotonia, truncal ataxia, motor DD | RDS, mechanical ventilation | Congenital hypothyroidism | Nil observed |
| Iwatani et al. (1)62 | 2000 | 14q12-q13.3 | NKX2.1, PAX9 | Motor DD | Bronchiolitis, recurrent RTI, RDS | Congenital hypothyroidism | Feeding difficulties, failure to thrive, microcephaly, hearing loss, cognitive impairment, dysmorphic facial features (e.g. hypertelorism, high arched palate), lower limb contractures |
| Iwatani et al. (2)62 | 2000 | 14q12-q13.3 | NKX2.1, PAX9 | Motor DD | Died of respiratory failure aged 3 years | Nil observed | Feeding difficulties, failure to thrive, microcephaly, hearing loss, cognitive impairment |
| Breedveld et al.9 | 2002 | 1.2Mb | NKX2.1, MBIP, NKX2.8, PAX9, SLC25A21 | Chorea | Nil observed | Nil observed | Nil observed |
| Krude et al.10 | 2002 | 14q11.2-q13.3 | NKX2.1 | Severe choreoathetosis | RDS, recurrent RTI | Thyroid hypoplasia | Nil observed |
| Devos et al. (1)34 | 2006 | 0.9Mb | NKX2.1, MBIP, NKX2.8, PAX9, SLC25A21, MIPOL1 | Chorea, hypotonia | Nil observed | Hypothyroidism | Diarrhea |
| Devos et al. (2)34 | 2006 | 0.9Mb | NKX2.1, MBIP, NKX2.8, PAX9, SLC25A21, MIPOL1 | Chorea, motor DD, hypotonia, cerebellar signs | Interstitial pneumonia | Hypothyroidism | Diarrhea, malabsorption, osteoporosis, hypoparathyroidism, dry eyes |
| Devos et al. (3)34 | 2006 | 0.9Mb | NKX2.1, MBIP, NKX2.8, PAX9, SLC25A21, MIPOL1 | Chorea, motor DD, hypotonia, cerebellar signs | Interstitial pneumonia | Hypothyroidism | Diarrhea, malabsorption, osteoporosis, dry eyes |
| Carre et al.15 | 2009 | 14q13 | NKX2.1 | Ataxia, motor DD | RDS, mechanical ventilation, Recurrent RTI | Congenital hypothyroidism | Mental retardation, impaired saccadic eye movements, absent corpus callosum |
| Accornero et al.63 | 2010 | 1.2Mb | NKX2.1, MBIP, NKX2.8, PAX9, SLC25A1 | Chorea | Nil observed | Subclinical hypothyroidism | Duplication of pituitary stalk |
| Uematsu et al.64 | 2012 | 2.6Mb deletion involving 14q12-q13 | NKX2.1 | Motor DD, choreoathetosis | Recurrent RTI | Nil observed | Bilateral reduced cerebral blood flow in basal ganglia (especially caudate nucleus) on ECD-SPECT imaging |
| Gras et al. (1)13 | 2012 | 13.8Mb deletion involving 14q13.2-q22.1 | NKX2.1, MBIP, PAX9, SLC25A21, MPOL1, FOXA1, SEC23A, GEMIN2, TRAPPC6B, CTAGE5, FBXO33, LRFN5, FSCB, C14orf28, KLHL28, FAM179B, PRPF39, FKBP3, FANCM, MDGA2 | Chorea, hypotonia | Nil observed | Nil observed | Nil observed |
| Gras et al. (2)13 | 2012 | 6.2-Mb deletion involving 14q13.2-q21.2 | NKX2.1, MBIP, PAX9, SLC25A21, MPOL1, FOXA1, SEC23A, GEMIN2, TRAPPC6B, CTAGE5, FBXO33 | Chorea, hypotonia | Nil observed | Hypothyroidism | Learning difficulties, ADHD |
| Gras et al. (3)13 | 2012 | 0.3-Mb deletion involving 14q13.3 | NKX2.1, MBIP, PAX9 | Chorea | Nil observed | Hypothyroidism | Learning difficulties, ADHD |
| Dale et al.65 | 2012 | 3.27-Mb deletion(Chr 14:35,327,739-38,602,335) | NKX2.1, PAX9, MIPOL1, SEC23A | Motor DD, gait disturbance | Nil observed | Nil observed | Mild intellectual disability, ligament laxity, oligodontia |
| Teissier et al.66 | 2012 | 14q13.3 | NKX2.1 | Chorea, dystonia, gait disturbance | COPD | Congenital hypothyroidism | Nil observed |
| Teissier et al.66 | 2012 | 14q13.3 | NKX2.1 | Chorea, hypotonia, ataxia | Nil observed | Congenital hypothyroidism | Dysmorphic features: hexadactyly, arched palate |
| Hamvas et al. (1)67 | 2013 | 14q13.1-q21.1 | NKX2.1 | Hypotonia, motor DD, ataxia | Hypoxia, recurrent RTI, pneumathoraces | Hypothyroidism | Absent corpus callosum |
| Hamvas et al. (2)67 | 2013 | 14q13.3 | NKX2.1 | Ataxia, motor DD | Recurrent infections | Hypothyroidism | Behavioral difficulties |
| Hamvas et al. (3)67 | 2013 | 14q13.3-q21.1 | NKX2.1 | Hypotonia, DD, ataxia | RDS, PH, RTI, oxygen therapy | Congenital hypothyroidism | Language delay |
| Hamvas et al. (4)67 | 2013 | 14q13.3-q21.1 | NKX2.1 | Hypotonia, developmental delay | RDS, PH | Congenital hypothyroidism | Microcephaly, language delay |
| Hamvas et al. (5)67 | 2013 | Deletion of exons 1 & 2 | NKX2.1 | Hypotonia, motor DD, ataxia | Severe RDS | Congenital hypothyroidism | Language delay |
| Peall et al. (1)14 | 2014 | 0.36Mb deletion(Chr14:36,924,171-37,283,221) | SFTA3, NKX2.1, BX161496, NKX2.8, PAX9, SLC25A21 | Hypotonia, dystonia, motor DD | Nil observed | Nil observed | Nil observed |
| Peall et al. (2)14 | 2014 | 4.7Mb deletion(Chr14:35,581,654-40,301,792) | RALGAPAI, BRMS1L, MBIP, SFTA3, NKX2.1, NKX2.8, PAX9, SLC25A21, MIPOL1, TTC6, SSTR1, CLEC14A, SEC23A, GEMIN2, TRAPPC6B, MIA2, CTAGE5, FBX033 | Hypotonia, motor DD | Recurrent RTI | Congenital hypothyroidism | Growth hormone deficiency, visual impairment |
ADHD, Attention Deficit Hyperactivity Disorder; COPD, Chronic Obstructive Pulmonary Disease; DD, Developmental delay; ECD-SPECT, Ethyl Cysteinate Dimer-Single-photon Emission Computed Tomography; PH, Pulmonary Hypertension; RDS, Respiratory Distress Syndrome; RTI, Respiratory Tract Infections.
Information regarding deletion size, chromosomal location and genes involved is given is as much detail as is available from each publication. The numbers in brackets in column one indicate individual, sequential cases when a single publication has reported multiple individuals.
Figure 2. Schematic Representation of NKX2.1 Missense, Frameshift, and Stop Codon Mutations Reported in the Published Literature to Date. Different reported NKX2.1 mutations are represented, and nucleotide changes and protein changes are displayed. Stop codon mutations (green) and splice-site mutations (purple) are located below the gene. Missense mutations (orange) and frameshift mutations (blue) are located above the gene.
It is likely that BCH is genetically heterogeneous. In four families described in the original paper by Breedveld et al.,8 neither a NKX2.1 mutation nor linkage to chromosome 14 was identified. Genetic linkage analysis of two unrelated Japanese families, each demonstrating an autosomal dominant pattern of inherited, slowly progressive chorea, has suggested an alternative genetic locus on chromosome 8 (8q21.3-q23.3), and named benign hereditary chorea 2 (BHC2).50 Interestingly, the motor phenotype in both families differed somewhat from classical BHC, namely with onset of disease in adulthood and slowly progressive chorea. Furthermore, brain post-mortem analysis in one affected individual, who had died from a respiratory tract infection, revealed degeneration of the striatum and cerebral white matter together with evidence of a tauopathy similar to that seen in progressive supranuclear palsy.51
Animal models
Mouse expression studies reveal complex NKX2.1 expression during embryonic development, with transcription of the gene evident in the forebrain, thyroid, and developing lungs.52 NKX2.1 expression occurs early in brain development, in the progenitor and post-mitotic cells of the rostrobasal telencephalon, by the 11th somite stage.53 A NKX2.1 null knockout (KO) mouse model has no abnormal motor phenotype in its heterozygous form but NKX2.1–/– homozygous mice died at birth with absent lungs, pituitary, thyroid gland, and multiple abnormalities of the ventral forebrain.54
The rostrobasal telencephalon later develops into several structures including the medial ganglionic eminence (MGE), a precursor of the globus pallidus. NKX2.1 has been found to facilitate migration of striatal interneurons from the MGE to the lateral ganglionic eminence, which later forms the striatum. These neurons then migrate to the cortex and form predominantly gamma-aminobutyric acid and calbindin expressing neurons. Failure of development of the MGE prevents migration of cholinergic neurons to the pallidum and ultimately to the striatum.55 Butt et al.56 investigated the role of TTF1 during embryogenesis by using a conditional loss of function approach. They found that TTF1 function was necessary for differentiation of specific subtypes of interneurons with early loss resulting in ectopic production of medium spiny projection neurons while later loss resulted in near normal levels of cortical interneurons.56
During lung and thyroid development, NKX2.1 binds to the transcriptional regulatory elements of several proteins, e.g. secretoglobulin 1A and surfactant proteins in the lung and thyroglobulin and thyroperoxidase in thyroid tissue.57–59 Lung pathology results from disrupted branching of the bronchial tree during development, resulting in fewer alveoli, interstitial fibrosis, and cyst-like air spaces. Thyroid development is frequently interrupted resulting in incomplete development or agenesis.60
Neuropathology
Two patients with confirmed NKX2.1 mutations have undergone post-mortem examination, both having died from leukemia. In both, the gross macroscopic and microscopic appearances were considered normal.22,38 The original case was reviewed using immunohistochemical staining of striatal tissue, aimed at identifying the neurotransmitters of interneurons whose tangential migration is mediated by TITF1. This found reduced numbers of striatal interneurons and a reduced density of calbindin, met-enkephalin, and substance P immunoreactive fibers, corroborating a number of the findings from the NKX2.1 KO mouse model.61 These findings also support the idea that NKX2.1 mutation-positive BHC is a developmental disorder, manifesting during childhood with no further deterioration in adult life. This may also explain the few available reports of successful treatment are during childhood and adolescence with minimal change observed when treating adults.
Conclusion
The clinical features of NKX2.1 BHC have become more clearly delineated with increased reporting of mutation-positive cases, highlighting hypotonia, motor developmental delay, chorea, hypothyroidism, respiratory distress syndrome, and recurrent respiratory tract infections as the core features of brain–lung–thyroid syndrome. Our review illustrates that the full triad appears only to be present in approximately 30% of all mutation-positive cases, and therefore the absence of one or more disease-associated symptoms should not discourage genetic testing.
In this review, we have discussed the expanding phenotypic spectrum associated with NKX2.1 mutations, with some significant variation from original clinical descriptions. Long considered to be a disorder in which cognition was preserved, several standardized studies have now demonstrated that at least a proportion of cases are found to have reduced IQ. Furthermore, the reported developmental abnormalities of other organ systems (e.g. lung, thyroid, and possibly urinary tract and musculoskeletal system) provide evidence of the important role of NKX2.1 in normal development.
Future development of treatment strategies and novel therapies may also need to consider the role of NKX2.1 in early development. Symptomatic improvement with levodopa therapy in some cases may indicate a window of opportunity for treatment in underdeveloped neuronal networks. Improved understanding of the clinical spectrum and pathogenic mechanisms governing this condition will require future multicenter, longitudinal collaborative studies, new laboratory disease models, development of novel therapeutic approaches, and large-scale clinical trials.
Footnotes
Funding: None.
Financial Disclosures: None.
Conflict of Interest: The authors report no conflict of interest.
References
- 1.Haerer AF, Currier RD, Jackson JF. Hereditary nonprogressive chorea of early onset. N Engl J Med. 1967;276:1220–1224. doi: 10.1056/NEJM196706012762202. doi: http://dx.doi.org/10.1056/NEJM196706012762202. [DOI] [PubMed] [Google Scholar]
- 2.Bird TD, Carlson CB, Hall JG. Familial essential (“benign”) chorea. J Med Gen. 1976;13:357–362. doi: 10.1136/jmg.13.5.357. doi: http://dx.doi.org/10.1111/j.1399-0004.1978.tb02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns J, Neuhauser G, Tomasi L. Benign hereditary non-progressive chorea of early onset. Clinical genetics of the syndrome and report of a new family. Neuropadiatrie. 1976;7:431–438. doi: 10.1055/s-0028-1091643. doi: http://dx.doi.org/10.1055/s-0028-1091643. [DOI] [PubMed] [Google Scholar]
- 4.Nutting PA, Cole BR, Schimke RN. Benign, recessively inherited choreo-athetosis of early onset. J Med Genet. 1969;6:408–410. doi: 10.1136/jmg.6.4.408. doi: http://dx.doi.org/10.1136/jmg.6.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrag A, Quinn NP, Bhatia KP, Marsden CD. Benign hereditary chorea–entity or syndrome? Mov Disord. 2000;15:280–288. doi: 10.1002/1531-8257(200003)15:2<280::aid-mds1011>3.0.co;2-q. doi: http://dx.doi.org/10.1002/1531-8257(200003)15:2<280::AID-MDS1011>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 6.de Vries BB, Arts WF, Breedveld GJ, Hoogeboom JJ, Niermeijer MF, Heutink P. Benign hereditary chorea of early onset maps to chromosome 14q. Am J Human Genet. 2000;66:136–142. doi: 10.1086/302725. doi: http://dx.doi.org/10.1086/302725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez M, Raskind W, Matsushita M, Wolff J, Lipe H, Bird T. Hereditary benign chorea: Clinical and genetic features of a distinct disease. Neurology. 2001;57:106–110. doi: 10.1212/wnl.57.1.106. [DOI] [PubMed] [Google Scholar]
- 8.Breedveld GJ, Percy AK, MacDonald ME, et al. Clinical and genetic heterogeneity in benign hereditary chorea. Neurology. 2002;59:579–584. doi: 10.1212/wnl.59.4.579. [DOI] [PubMed] [Google Scholar]
- 9.Breedveld GJ, van Dongen JW, Danesino C, et al. Mutations in TITF-1 are associated with benign hereditary chorea. Hum Mol Gen. 2002;11:971–979. doi: 10.1093/hmg/11.8.971. [DOI] [PubMed] [Google Scholar]
- 10.Krude H, Schutz B, Biebermann H, et al. Choreoathetosis, hypothyroidism, and pulmonary alterations due to human NKX2-1 haploinsufficiency. J Clin Invest. 2002;109:475–480. doi: 10.1172/JCI14341. doi: http://dx.doi.org/10.1172/JCI14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harper PS. Benign hereditary chorea. Clinical and genetic aspects. Clin Gen. 1978;13:85–95. doi: 10.1111/j.1399-0004.1978.tb04133.x. doi: http://dx.doi.org/10.1111/j.1399-0004.1978.tb04133.x. [DOI] [PubMed] [Google Scholar]
- 12.Kleiner-Fisman G, Lang AE. Benign hereditary chorea revisited: A journey to understanding. Mov Disord. 2007;22:2297–2305. doi: 10.1002/mds.21644. quiz 2452, doi: http://dx.doi.org/10.1002/mds.21644. [DOI] [PubMed] [Google Scholar]
- 13.Gras D, Jonard L, Roze E, et al. Benign hereditary chorea: Phenotype, prognosis, therapeutic outcome and long term follow-up in a large series with new mutations in the TITF1/NKX2-1 gene. J Neurol, Neurosurg, Psychiatry. 2012;83:956–962. doi: 10.1136/jnnp-2012-302505. doi: http://dx.doi.org/10.1136/jnnp-2012-302505. [DOI] [PubMed] [Google Scholar]
- 14.Peall KJ, Lumsden D, Kneen R, et al. Benign hereditary chorea related to NKX2.1: Expansion of the genotypic and phenotypic spectrum. Dev Med Child Neurol. 2014;56:642–648. doi: 10.1111/dmcn.12323. doi: http://dx.doi.org/10.1111/dmcn.12323. [DOI] [PubMed] [Google Scholar]
- 15.Carre A, Szinnai G, Castanet M, et al. Five new TTF1/NKX2.1 mutations in brain-lung-thyroid syndrome: Rescue by PAX8 synergism in one case. Hum Mol Gen. 2009;18:2266–2276. doi: 10.1093/hmg/ddp162. doi: http://dx.doi.org/10.1093/hmg/ddp162. [DOI] [PubMed] [Google Scholar]
- 16.Inzelberg R, Weinberger M, Gak E. Benign hereditary chorea: An update. Parkinsonism Relat Disord. 2011;17:301–307. doi: 10.1016/j.parkreldis.2011.01.002. doi: http://dx.doi.org/10.1016/j.parkreldis.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 17.McMichael G, Haan E, Gardner A, et al. NKX2-1 mutation in a family diagnosed with ataxic dyskinetic cerebral palsy. Eur J Med Genet. 2013;56:506–509. doi: 10.1016/j.ejmg.2013.07.003. doi: http://dx.doi.org/10.1016/j.ejmg.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Glik A, Vuillaume I, Devos D, Inzelberg R. Psychosis, short stature in benign hereditary chorea: A novel thyroid transcription factor-1 mutation. Mov Disord. 2008;23:1744–1747. doi: 10.1002/mds.22215. doi: http://dx.doi.org/10.1002/mds.22215. [DOI] [PubMed] [Google Scholar]
- 19.Suchowersky O, Hayden MR, Martin WR, Stoessl AJ, Hildebrand AM, Pate BD. Cerebral metabolism of glucose in benign hereditary chorea. Mov Disord. 1986;1:33–44. doi: 10.1002/mds.870010105. doi: http://dx.doi.org/10.1002/mds.870010105. [DOI] [PubMed] [Google Scholar]
- 20.Schady W, Meara RJ. Hereditary progressive chorea without dementia. J Neurol Neurosurg Psychiatry. 1988;51:295–297. doi: 10.1136/jnnp.51.2.295. doi: http://dx.doi.org/10.1136/jnnp.51.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosati A, Berti B, Melani F, Cellini E, Procopio E, Guerrini R. Recurrent drop attacks in early childhood as presenting symptom of benign hereditary chorea caused by TITF1 gene mutations. Dev Med Child Neurol. 2014 Nov 20; doi: 10.1111/dmcn.12644. doi: http://dx.doi.org/10.1111/dmcn.12644. [DOI] [PubMed] [Google Scholar]
- 22.Asmus F, Horber V, Pohlenz J, et al. A novel TITF-1 mutation causes benign hereditary chorea with response to levodopa. Neurology. 2005;64:1952–1954. doi: 10.1212/01.WNL.0000164000.75046.CC. [DOI] [PubMed] [Google Scholar]
- 23.Costa MC, Costa C, Silva AP, et al. Nonsense mutation in TITF1 in a Portuguese family with benign hereditary chorea. Neurogenetics. 2005;6:209–215. doi: 10.1007/s10048-005-0013-1. doi: http://dx.doi.org/10.1007/s10048-005-0013-1. [DOI] [PubMed] [Google Scholar]
- 24.Doyle DA, Gonzalez I, Thomas B, Scavina M. Autosomal dominant transmission of congenital hypothyroidism, neonatal respiratory distress, and ataxia caused by a mutation of NKX2-1. J Pediatr. 2004;145:190–193. doi: 10.1016/j.jpeds.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Sleigh G, Lindenbaum RH. Benign (non-paroxysmal) familial chorea. Paediatric perspectives. Arch Dis Child. 1981;56:616–621. doi: 10.1136/adc.56.8.616. doi: http://dx.doi.org/10.1136/adc.56.8.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leli DA, Furlow TW, Jr, Falgout JC. Benign familial chorea: An association with intellectual impairment. J Neurol Neurosurg Psychiatry. 1984;47:471–474. doi: 10.1136/jnnp.47.5.471. doi: http://dx.doi.org/10.1136/jnnp.47.5.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hageman G, Ippel PF, van Hout MS, Rozeboom AR. A Dutch family with benign hereditary chorea of early onset: Differentiation from Huntington’s disease. Clin Neurol Neurosurg. 1996;98:165–170. doi: 10.1016/0303-8467(96)00015-7. [DOI] [PubMed] [Google Scholar]
- 28.Salvatore E, Di Maio L, Filla A, et al. Benign hereditary chorea: Clinical and neuroimaging features in an Italian family. Mov Disord. 2010;25:1491–1496. doi: 10.1002/mds.23065. doi: http://dx.doi.org/10.1002/mds.23065. [DOI] [PubMed] [Google Scholar]
- 29.Moya CM, Perez de Nanclares G, Castano L, et al. Functional study of a novel single deletion in the TITF1/NKX2.1 homeobox gene that produces congenital hypothyroidism and benign chorea but not pulmonary distress. J Clin Endocrinol Metab. 2006;91:1832–1841. doi: 10.1210/jc.2005-1497. doi: http://dx.doi.org/10.1210/jc.2005-1497. [DOI] [PubMed] [Google Scholar]
- 30.Devriendt K, Vanhole C, Matthijs G, de Zegher F. Deletion of thyroid transcription factor-1 gene in an infant with neonatal thyroid dysfunction and respiratory failure. N Engl J Med. 1998;338:1317–1318. doi: 10.1056/NEJM199804303381817. doi: http://dx.doi.org/10.1056/NEJM199804303381817. [DOI] [PubMed] [Google Scholar]
- 31.Willemsen MA, Breedveld GJ, Wouda S, et al. Brain-Thyroid-Lung syndrome: A patient with a severe multi-system disorder due to a de novo mutation in the thyroid transcription factor 1 gene. Eur J Pediatr. 2005;164:28–30. doi: 10.1007/s00431-004-1559-x. doi: http://dx.doi.org/10.1007/s00431-004-1559-x. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura K, Sekijima Y, Nagamatsu K, Yoshida K, Ikeda S. A novel nonsense mutation in the TITF-1 gene in a Japanese family with benign hereditary chorea. J Neurolog Sci. 2012;313:189–192. doi: 10.1016/j.jns.2011.09.013. doi: http://dx.doi.org/10.1016/j.jns.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Ferrara AM, De Michele G, Salvatore E, et al. A novel NKX2.1 mutation in a family with hypothyroidism and benign hereditary chorea. Thyroid. 2008;18:1005–1009. doi: 10.1089/thy.2008.0085. doi: http://dx.doi.org/10.1089/thy.2008.0085. [DOI] [PubMed] [Google Scholar]
- 34.Devos D, Vuillaume I, de Becdelievre A, et al. New syndromic form of benign hereditary chorea is associated with a deletion of TITF-1 and PAX-9 contiguous genes. Mov Disord. 2006;21:2237–2240. doi: 10.1002/mds.21135. doi: http://dx.doi.org/10.1002/mds.21135. [DOI] [PubMed] [Google Scholar]
- 35.Damasio H, Antunes L, Damasio AR. Familial nonprogressive involuntary movements of childhood. Ann Neurol. 1977;1:602–603. doi: 10.1002/ana.410010619. doi: http://dx.doi.org/10.1002/ana.410010619. [DOI] [PubMed] [Google Scholar]
- 36.Landrieu P, Benchet ML, Tardieu M, Lapresle J. Sex-linked, nonprogressive, familial chorea. Revue Neurologique. 1984;140:432–433. [PubMed] [Google Scholar]
- 37.Konishi T, Kono S, Fujimoto M, et al. Benign hereditary chorea: Dopaminergic brain imaging in patients with a novel intronic NKX2.1 gene mutation. J Neurol. 2013;260:207–213. doi: 10.1007/s00415-012-6618-z. doi: http://dx.doi.org/10.1007/s00415-012-6618-z. [DOI] [PubMed] [Google Scholar]
- 38.Kleiner-Fisman G, Rogaeva E, Halliday W, et al. Benign hereditary chorea: Clinical, genetic, and pathological findings. Ann Neurol. 2003;54:244–247. doi: 10.1002/ana.10637. doi: http://dx.doi.org/10.1002/ana.10637. [DOI] [PubMed] [Google Scholar]
- 39.Kang Y, Hebron H, Ozbun L, Mariano J, Minoo P, Jakowlew SB. Nkx2.1 transcription factor in lung cells and a transforming growth factor-beta1 heterozygous mouse model of lung carcinogenesis. Mol Carcinog. 2004;40:212–231. doi: 10.1002/mc.20034. [DOI] [PubMed] [Google Scholar]
- 40.Weir BA, Woo MS, Getz G, et al. Characterizing the cancer genome in lung adenocarcinoma. Nature. 2007;450:893–898. doi: 10.1038/nature06358. doi: http://dx.doi.org/10.1038/nature06358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peall KJ, Kurian MA, Wardle M, et al. SGCE and myoclonus dystonia: Motor characteristics, diagnostic criteria and clinical predictors of genotype. J Neurol. 2014;261:2296–2304. doi: 10.1007/s00415-014-7488-3. doi: http://dx.doi.org/10.1007/s00415-014-7488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matheus MG, Lehman RK, Bonilha L, Holden KR. Redefining the pediatric phenotype of X-linked monocarboxylate transporter 8 (MCT8) deficiency: Implications for diagnosis and therapies. J Child Neurol. 2015 Apr 21; doi: 10.1177/0883073815578524. [DOI] [PubMed] [Google Scholar]
- 43.Carapito R, Paul N, Untrau M, et al. A de novo ADCY5 mutation causes early-onset autosomal dominant chorea and dystonia. Mov Disord. 2015;30:423–427. doi: 10.1002/mds.26115. doi: http://dx.doi.org/10.1002/mds.26115. [DOI] [PubMed] [Google Scholar]
- 44.Veneziano L, Parkinson MH, Mantuano E, Frontali M, Bhatia KP, Giunti P. A novel de novo mutation of the TITF1/NKX2-1 gene causing ataxia, benign hereditary chorea, hypothyroidism and a pituitary mass in a UK family and review of the literature. Cerebellum. 2014;13:588–595. doi: 10.1007/s12311-014-0570-7. doi: http://dx.doi.org/10.1007/s12311-014-0570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maccabelli G, Pichiecchio A, Guala A, et al. Advanced magnetic resonance imaging in benign hereditary chorea: Study of two familial cases. Mov Disord. 2010;25:2670–2674. doi: 10.1002/mds.23281. doi: http://dx.doi.org/10.1002/mds.23281. [DOI] [PubMed] [Google Scholar]
- 46.Mahajnah M, Inbar D, Steinmetz A, Heutink P, Breedveld GJ, Straussberg R. Benign hereditary chorea: Clinical, neuroimaging, and genetic findings. J Child Neurol. 2007;22:1231–1234. doi: 10.1177/0883073807306261. doi: http://dx.doi.org/10.1177/0883073807306261. [DOI] [PubMed] [Google Scholar]
- 47.Fons C, Rizzu P, Garcia-Cazorla A, et al. TITF-1 gene mutation in a case of sporadic non-progressive chorea. Response to levodopa treatment. Brain Dev. 2012;34:255–257. doi: 10.1016/j.braindev.2011.04.007. doi: http://dx.doi.org/10.1016/j.braindev.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 48.Ikeda K, Clark JC, Shaw-White JR, Stahlman MT, Boutell CJ, Whitsett JA. Gene structure and expression of human thyroid transcription factor-1 in respiratory epithelial cells. J Biol Chem. 1995;270:8108–8114. doi: 10.1074/jbc.270.14.8108. doi: http://dx.doi.org/10.1074/jbc.270.14.8108. [DOI] [PubMed] [Google Scholar]
- 49.Sempere AP, Aparicio S, Mola S, Perez-Tur J. Benign hereditary chorea: Clinical features and long-term follow-up in a Spanish family. Parkinsonism Relat Disord. 2013;19:394–396. doi: 10.1016/j.parkreldis.2012.08.006. doi: http://dx.doi.org/10.1016/j.parkreldis.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Shimohata T, Hara K, Sanpei K, et al. Novel locus for benign hereditary chorea with adult onset maps to chromosome 8q21.3 q23.3. Brain. 2007;130:2302–2309. doi: 10.1093/brain/awm036. doi: http://dx.doi.org/10.1093/brain/awm036. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida Y, Nunomura J, Shimohata T, Nanjo H, Miyata H. Benign hereditary chorea 2: Pathological findings in an autopsy case. Neuropathology. 2012;32:557–565. doi: 10.1111/j.1440-1789.2011.01288.x. doi: http://dx.doi.org/10.1111/j.1440-1789.2011.01288.x. [DOI] [PubMed] [Google Scholar]
- 52.Lazzaro D, Price M, de Felice M, Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113:1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- 53.Shimamura K, Hartigan DJ, Martinez S, Puelles L, Rubenstein JL. Longitudinal organization of the anterior neural plate and neural tube. Development. 1995;121:3923–3933. doi: 10.1242/dev.121.12.3923. [DOI] [PubMed] [Google Scholar]
- 54.Kimura S, Hara Y, Pineau T, et al. The T/ebp null mouse: Thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. doi: http://dx.doi.org/10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 55.Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: Evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- 56.Butt SJ, Sousa VH, Fuccillo MV, et al. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031. doi: http://dx.doi.org/10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kleinlein B, Griese M, Liebisch G, et al. Fatal neonatal respiratory failure in an infant with congenital hypothyroidism due to haploinsufficiency of the NKX2-1 gene: Alteration of pulmonary surfactant homeostasis. Arch Dis Child Fetal Neonatal Ed. 2011;96:F453–F456. doi: 10.1136/adc.2009.180448. doi: http://dx.doi.org/10.1136/adc.2009.180448. [DOI] [PubMed] [Google Scholar]
- 58.Bruno MD, Bohinski RJ, Huelsman KM, Whitsett JA, Korfhagen TR. Lung cell-specific expression of the murine surfactant protein A (SP-A) gene is mediated by interactions between the SP-A promoter and thyroid transcription factor-1. J Biol Chem. 1995;270:6531–6536. doi: 10.1074/jbc.270.12.6531. [DOI] [PubMed] [Google Scholar]
- 59.Bachurski CJ, Yang GH, Currier TA, Gronostajski RM, Hong D. Nuclear factor I/thyroid transcription factor 1 interactions modulate surfactant protein C transcription. Mol Cell Biol. 2003;23:9014–9024. doi: 10.1128/MCB.23.24.9014-9024.2003. doi: http://dx.doi.org/10.1128/MCB.23.24.9014-9024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galambos C, Levy H, Cannon CL, et al. Pulmonary pathology in thyroid transcription factor-1 deficiency syndrome. Am J Respir Crit Care Med. 2010;182:549–554. doi: 10.1164/rccm.201002-0167CR. doi: http://dx.doi.org/10.1164/rccm.201002-0167CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kleiner-Fisman G, Calingasan NY, Putt M, Chen J, Beal MF, Lang AE. Alterations of striatal neurons in benign hereditary chorea. Mov Disord. 2005;20:1353–1357. doi: 10.1002/mds.20577. [DOI] [PubMed] [Google Scholar]
- 62.Iwatani N, Mabe H, Devriendt K, Kodama M, Miike T. Deletion of NKX2.1 gene encoding thyroid transcription factor-1 in two siblings with hypothyroidism and respiratory failure. J Pediatr. 2000;137:272–276. doi: 10.1067/mpd.2000.107111. doi: http://dx.doi.org/10.1067/mpd.2000.107111. [DOI] [PubMed] [Google Scholar]
- 63.Accornero S, Danesino C, Bastianello S, D’Errico I, Guala A, Chiovato L. Duplication of the pituitary stalk in a patient with a heterozygous deletion of chromosome 14 harboring the thyroid transcription factor-1 gene. J Clin Endocrinol Metab. 2010;95:3595–3596. doi: 10.1210/jc.2010-0621. doi: http://dx.doi.org/10.1210/jc.2010-0621. [DOI] [PubMed] [Google Scholar]
- 64.Uematsu M, Haginoya K, Kikuchi A, et al. Hypoperfusion in caudate nuclei in patients with brain-lung-thyroid syndrome. J Neurol Sci. 2012;315:77–81. doi: 10.1016/j.jns.2011.11.025. doi: http://dx.doi.org/10.1016/j.jns.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 65.Dale RC, Grattan-Smith P, Nicholson M, Peters GB. Microdeletions detected using chromosome microarray in children with suspected genetic movement disorders: A single-centre study. Dev Med Child Neurol. 2012;54:618–623. doi: 10.1111/j.1469-8749.2012.04287.x. doi: http://dx.doi.org/10.1111/j.1469-8749.2012.04287.x. [DOI] [PubMed] [Google Scholar]
- 66.Teissier R, Guillot L, Carre A, et al. Multiplex ligation-dependent probe amplification improves the detection rate of NKX2.1 mutations in patients affected by brain-lung-thyroid syndrome. Horm Res Paediatr. 2012;77:146–151. doi: 10.1159/000337214. doi: http://dx.doi.org/10.1159/000337214. [DOI] [PubMed] [Google Scholar]
- 67.Hamvas A, Deterding RR, Wert SE, et al. Heterogeneous pulmonary phenotypes associated with mutations in the thyroid transcription factor gene NKX2-1. Chest. 2013;144:794–804. doi: 10.1378/chest.12-2502. doi: http://dx.doi.org/10.1378/chest.12-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]