Abstract
Purpose
Congenital urinary tract obstruction is a leading cause of renal maldevelopment and pediatric kidney disease. Nonetheless, few groups have examined its molecular pathogenesis in humans. We evaluated the role of BMP-7, a protein required for renal injury repair and nephrogenesis, in disease progression in patients with obstructive uropathy.
Materials and Methods
Whole kidney and cell specific BMP-7 expression was examined in a murine model of unilateral ureteral obstruction and in patients with congenital ureteropelvic junction obstruction. Findings were correlated with molecular markers of renal injury and clinical parameters.
Results
Unilateral ureteral obstruction led to a dramatic decrease in BMP-7 expression in the proximal and distal tubules before the onset of significant loss of renal architecture and fibrosis, suggesting that this is a critical molecular event that drives early stage disease progression. Loss of BMP-7 expression then extended to the collecting ducts and glomeruli in end stage kidney disease. When translating these findings to patients with ureteropelvic junction obstruction, global loss of BMP-7 expression correlated with a decreased number of nephrons, loss of renal architecture, severe renal fibrosis and loss of kidney function.
Conclusions
Given that BMP-7 has a critical role in renal injury repair and nephrogenesis, these findings show that cell specific changes in BMP-7 expression contribute to the onset of irreversible renal injury and impaired kidney development secondary to congenital urinary tract obstruction. Accordingly therapies that target these cell populations to restore BMP-7 activity may limit disease progression in patients with obstructive uropathy.
Keywords: kidney, ureteral obstruction, fibrosis, bone morphogenetic protein 7, growth and development
Obstructive uropathy is responsible for 16.5% of pediatric kidney transplants.1–3 Although the underlying etiology varies considerably, renal injury represents a common pathological end point of urinary tract dysfunction.4,5 Accordingly the development of therapies that limit the progression of renal injuries and preserve kidney function is an important goal to improve treatment in patients with obstructive uropathy. While progress has been made by studying animal models,4,5 few potential therapeutic targets have been validated in patients with obstructive uropathy.
In the last decade BMP-7 emerged as a critical renal protective protein that safeguards against various injurious conditions, including urinary tract dysfunction.6–18 The protective role of BMP-7 was initially attributed to its ability to inhibit transforming growth factor-β dependent profibrotic signaling pathways and the development of renal injuries.8,17–19 However, we and others recently found that BMP-7 also stimulates the repair of established renal injuries.6–9,16 Indeed, BMP-7 is required for the regeneration of renal architecture and the resolution of fibrosis during injury repair while treatment with recombinant BMP-7 stimulates kidney repair and reverses the progression of chronic renal injuries.7–9
Despite its importance little is known about the regulation of endogenous BMP-7 and its renal protective functions. However, we and others observed that chronic urinary obstruction leads to loss of BMP-7 in murine models of obstructive uropathy.7,11 Given that BMP-7 inhibits the pathogenesis of obstruction induced renal injury,6,7,11,13 these findings suggest that loss of BMP-7 expression has a critical role in the progression of renal injuries that are not effectively repaired by the kidney. These findings are particularly notable when considered in the context of congenital urinary obstruction. Since BMP-7 is required for nephrogenesis,20–22 BMP-7 loss may also contribute to renal maldevelopment secondary to congenital urinary obstruction.
In this study we identified the specific cell types that lose BMP-7 expression in the obstructed kidney. We examined their role in disease progression and determined the translational importance of this molecular event in human kidney disease.
MATERIALS AND METHODS
Obstructive Uropathy Murine Model
Complete UUO was created in 5, 8 to 10-week-old C57/BL6J mice by surgically occluding the proximal ureter with a microvascular clamp.23 All procedures were performed according to institutional animal care and use committee guidelines.
Patient Samples
Kidney samples were obtained from patients who underwent nephrectomy during treatment for Wilms tumor in 6 or kidney failure secondary to UPJ obstruction in 10. In control samples analysis was done on tumor-free tissue margins. All studies were performed according to institutional review board guidelines.
Histology and Immunostaining
For histology kidneys were fixed in HistoChoice®. Trichrome staining was performed with the Accustain® Trichrome Stain (Masson) Kit.
For immunostaining kidneys were fixed in HistoChoice and antigen retrieval was performed in boiling citrate buffer. Immunofluorescence was performed with antibodies to AQP2, BMP-7, type IV collagen, cleaved Caspase-3, CD68 (Abcam®), AQP1 or THP (Santa Cruz Biotechnology, Houston, Texas) and NL493 or NL557 conjugated secondary antibodies (R&D Systems®). Analysis was done with ImageJ (http://imagej.nih.gov/ij/).
Statistical Analysis
Statistical significance was determined by the t-test. Data are shown as the mean ± SD.
RESULTS
Obstruction Induced Renal Injury
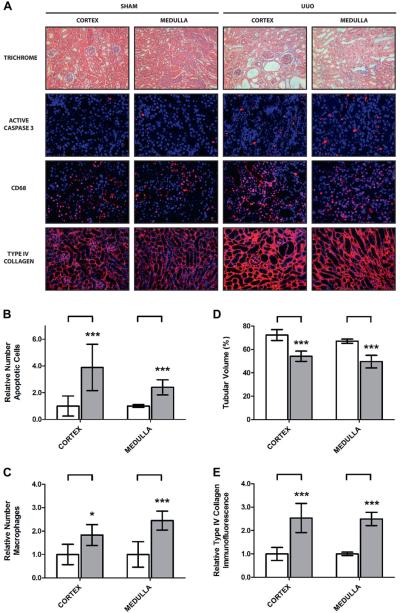
To examine the role of BMP-7 in obstructive uropathy we studied a murine model of UUO in which the proximal ureter was surgically occluded.23 The UUO model effectively mimicked the pathogenesis of obstruction induced renal injury and was characterized by tubular dilatation, apoptosis and renal inflammation (fig. 1, A to C).4,5 This culminated in a 25.6% loss of renal architecture and a 2.5-fold increase in interstitial fibrosis after 7 days of UUO (fig. 1, D and E).
Figure 1.
Five mice underwent sham operation or 7-day UUO. Kidneys were analyzed. A, Masson trichrome stain, reduced from ×200. Immunofluorescence for active/cleaved Caspase-3, CD-68 and type IV collagen, reduced from ×400. B, apoptotic cell quantification (A) Triple asterisks indicate p <0.005. C, macrophage infiltration quantification (A). Single asterisk indicates p <0.05. D, tubular volume quantification (A). E, type IV collagen quantification (A).
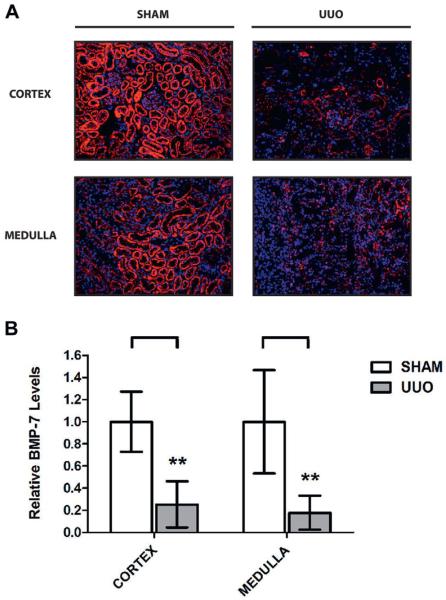
Importantly disease progression in the UUO model was accompanied by a 78.5% decrease in BMP-7 expression (fig. 2). We could not specifically attribute this to a particular region of the kidney because we observed a 74.7% and 82.2% decrease in the cortex and the medulla, respectively. Although BMP-7 loss occurred in a widespread manner, there were striking differences in cell type specific expression patterns of BMP-7 in control and UUO kidneys (fig. 2, A).
Figure 2.
UUO induced renal injury led to loss of BMP-7 expression in 5 mice with sham operation or 7-day UUO. Kidneys were analyzed. A, immunofluorescence for BMP-7, reduced from ×200. B, BMP-7 quantification (A). Asterisks indicate p <0.01.
Obstructed Kidney Localized Primarily to Proximal and Distal Tubules
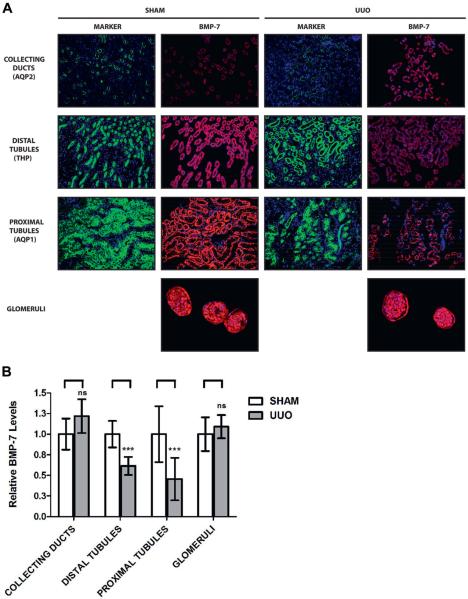
To further examine BMP-7 expression patterns we performed immunostaining for BMP-7 and markers of specific renal cell populations, including proximal tubules (AQP1),24 distal tubules (THP),25 collecting ducts (AQP2)26 and glomeruli. In control kidneys the highest BMP-7 levels were found in the distal and proximal tubules while moderate levels were found in collecting ducts, parietal epithelial cells and podocytes (fig. 3, A).
Figure 3.
BMP-7 expression spatial mapping in 5 mice with obstruction induced renal injury that underwent sham operation or 7-day UUO. A, kidneys were analyzed by immunofluorescence for BMP-7 along with molecular markers for AQP2, THP and AQP1. BMP-7 immunofluorescence images were digitally gated to show only cells positive for expression of specified marker protein. Reduced from ×200. B, relative BMP-7 expression quantification in specific kidney cell populations (A). ns, p >0.05. Asterisks indicate p <0.005.
We next examined BMP-7 expression patterns in the obstructed kidney and found a 54.6% and 38.4% decrease in the proximal and distal tubules, respectively. In contrast, we found no significant change in collecting ducts or glomeruli (fig. 3, B). These results likely underestimate the changes in BMP-7 expression since tubular dedifferentiation27,28 may lead to loss of marker expression in severely injured cells. Nonetheless, these findings clearly demonstrate that distal and proximal tubules have an important role in the loss of BMP-7 expression after chronic urinary obstruction.
Early Stage Progression of Kidney Disease in Obstructive Uropathy
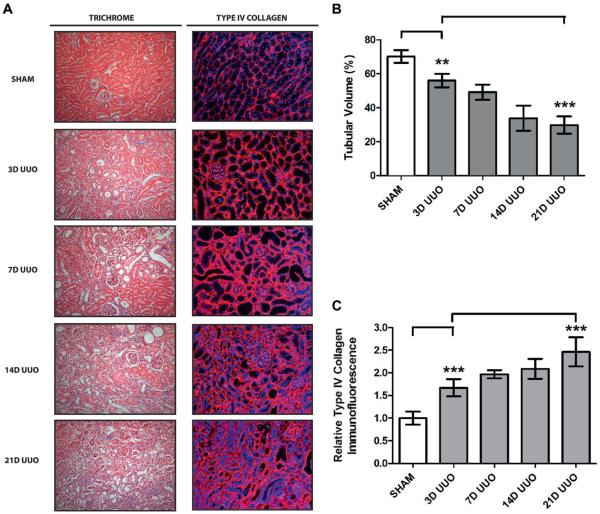
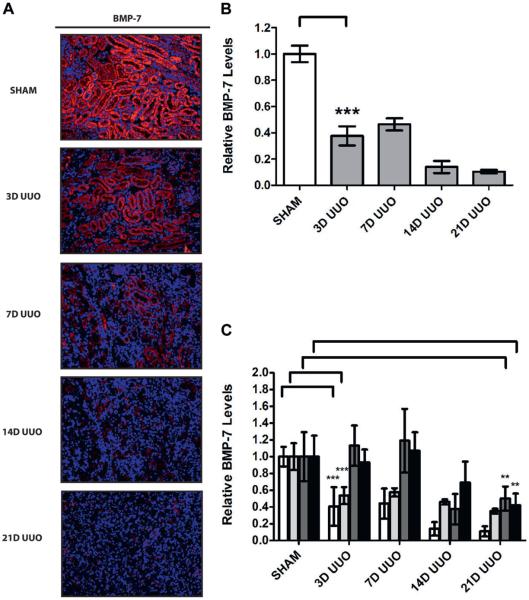
Given the critical renal protective functions of BMP-7, our findings strongly suggest that loss of BMP-7 expression in the proximal and distal tubules contributes to disease progression.6–9,16 Accordingly we determined whether BMP-7 loss is an early event that drives disease progression or a late event that results from renal dysfunction. To accomplish this we determined a detailed time course of disease progression after UUO. We detected hydronephrosis and tubular dilatation after 24 hours of UUO followed by onset of renal injury after 3 days, when there was increased apoptosis, a 20.1% decrease in tubular volume and a 1.6-fold increase in fibrosis. Nonetheless, most significant disease progression developed after 7 days of UUO and steadily continued until at least 21 days of UUO, when there was a 57.5% decrease in tubular volume and 2.5-fold increase in fibrosis (fig. 4).
Figure 4.
Time course of disease progression after chronic UUO in 5 mice that underwent sham operation, or UUO for 3 (3D), 7 (7D), 14 (14D) or 21 days (21D). Kidneys were analyzed. A, Masson trichrome stain and immunofluorescence for type IV collagen, reduced from ×200. B, tubular volume quantification (A). Double asterisks indicate p <0.01. Triple asterisks indicate p <0.005. C, type IV collagen quantification (A).
When examining BMP-7 expression during disease progression, we noted a 62.4% decrease in total BMP-7 levels after only 3 days of UUO (fig. 5, A and B). Furthermore, we observed a 59.4% and 46.5% decrease in proximal and distal tubules, respectively. Loss of BMP-7 expression then steadily continued during the course of disease progression. Notably while there was no significant change in BMP-7 expression in glomeruli or collecting ducts after 7 days of UUO, there was a 58.3% decrease in glomeruli and a 49.9% decrease in collecting ducts after 21 days of UUO (fig. 5, C). Together these findings demonstrate that loss of BMP-7 expression in proximal and distal tubules occurs during the early stages of disease progression while loss of BMP-7 in glomeruli and collecting ducts occurs in end stage obstructive kidney disease. Notably this strongly suggests that cell type specific changes in BMP-7 expression are an important cause of disease progression in obstructive uropathy.
Figure 5.
BMP-7 expression temporal mapping in 5 mice with obstruction induced renal injury that underwent sham operation or UUO for 3 (3D), 7 (7D), 14 (14D) or 21 days (21D). Kidneys were analyzed. A, immunofluorescence for BMP-7, reduced from ×200. B, BMP-7 quantification (A). Triple asterisks indicate p <0.005. C, BMP-7 quantification in specific kidney cell populations (A). Double asterisks indicate p <0.01.
Important Cause of Kidney Disease Progression in Obstructive Uropathy
We examined the translational relevance of BMP-7 in patients with obstructive uropathy by obtaining kidney samples from 10 patients who underwent nephrectomy after renal failure developed due to UPJ obstruction, the most prevalent form of obstructive uropathy.3 As controls we obtained age and gender matched kidney samples from the tumor-free margins of 6 patients who underwent nephrectomy during treatment of Wilms tumor.
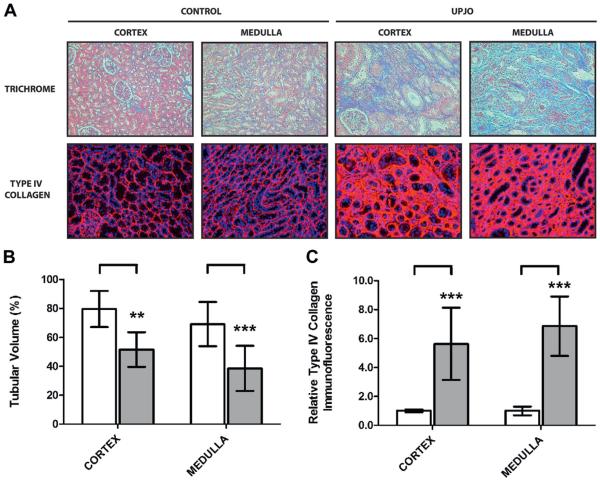
We first characterized disease progression in the UPJ obstruction group. Each patient was diagnosed with grade IV hydronephrosis on ultrasound and differential renal function less than 15% (mean 4.9% ± 5.5%) on diuretic renography. They also had renal pathology consistent with end stage obstructive kidney disease as characterized by a 39.7% decrease in tubular volume and a 6.2-fold increase in fibrosis (fig. 6).
Figure 6.
Renal injury assessment in kidney samples from 10 patients with obstructive uropathy (UPJO) and 6 controls. Kidneys were analyzed. A, Masson trichrome stain, reduced from ×200. Immunofluorescence for type IV collagen, reduced from ×200. B, tubular volume quantification (A). Double asterisks indicate p <0.01. Triple asterisks indicate p <0.005. C, type IV collagen quantification (A).
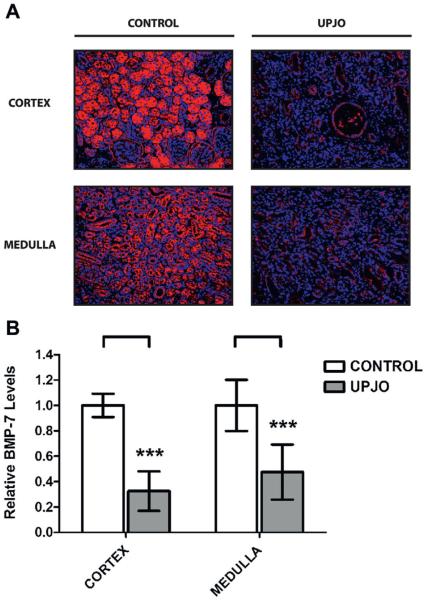
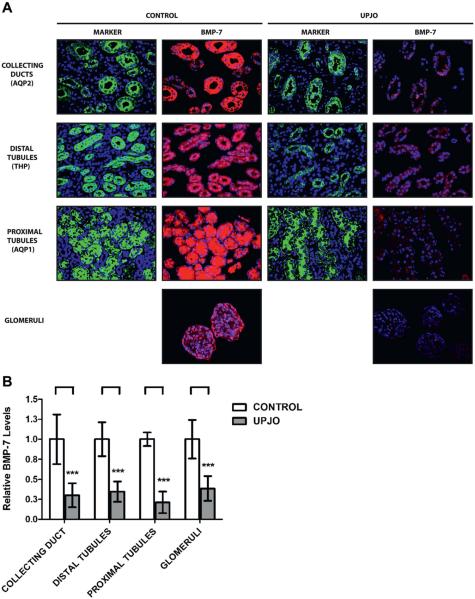
When examining renal BMP-7 expression, we found that patients in the UPJ obstruction group showed a 59.2% decrease in total BMP-7 levels (fig. 7). This was characterized by significant decreases in the proximal and distal tubules, collecting ducts and glomeruli (fig. 8). Global loss of BMP-7 in the UPJ obstruction group was consistent with the expression patterns observed in end stage disease in the murine UUO model (fig. 5). Together these findings demonstrate that loss of BMP-7 expression translates from mouse models of obstructive uropathy to human disease. When considered in the context of the renal protective functions of BMP-7,6–18 these findings strongly suggest that loss of BMP-7 expression is an important cause of disease progression in patients with obstructive uropathy.
Figure 7.
Kidney samples were obtained from 10 patients with obstructive uropathy (UPJO) and 6 controls. Disease progression in patients was associated with loss of BMP-7 expression. Kidneys were analyzed. A, immunofluorescence for BMP-7, reduced from ×200. B, BMP-7 quantification (A). Asterisks indicate p <0.005.
Figure 8.
BMP-7 expression spatial mapping in kidney samples from 10 patients with obstructive uropathy (UPJO) and 6 controls. A, kidneys were analyzed by immunofluorescence for BMP-7 along with molecular markers for AQP2, THP and AQP1. BMP-7 immunofluorescence images were digitally gated to show only cells positive for expression of specified marker protein. Reduced from ×200. B, relative BMP-7 expression quantification in specific kidney cell populations (A). Results in each cell population represent independent immunostaining experiment. Asterisks indicate p <0.005.
DISCUSSION
In working toward therapies for kidney disease BMP-7 has emerged as a promising target. BMP-7 treatment inhibits the pathogenesis of renal injury in response to a wide range of acute and chronic stimuli.6–18 The therapeutic effects of BMP-7 also extend beyond inhibiting the pathogenesis of renal injuries since it can stimulate injury repair and in some cases even reverse disease progression.6–9,16
Although most studies have focused on the therapeutic effects of recombinant BMP-7, it has become evident that endogenous BMP-7 also has an important role in the response to renal injury. We previously reported that BMP-7 is required to repair obstruction induced renal injuries.7 However, loss of BMP-7 occurs in obstructive uropathy and other conditions that lead to chronic renal injury.7,10–12,29 Together these findings strongly suggest that BMP-7 loss contributes to the dysregulation of kidney repair after chronic renal injury.
The current study extends these findings by revealing that loss of BMP-7 is an early event in disease progression that develops within 3 days of onset of urinary obstruction in the murine UUO model. This is notable since the most significant changes in renal pathology do not occur until after 7 days of obstruction. When considered in addition to the renal protective effects of BMP-7,6,7,11,13 these findings strongly suggest that loss of BMP-7 is a critical molecular event that drives disease progression in obstructive uropathy and other conditions that lead to chronic kidney disease.
When translating these findings to human disease, we found that BMP-7 loss also occurred in patients with UPJ obstruction during progression to chronic kidney disease. A limitation of our study methodology is that we could only examine BMP-7 expression in patients with end stage disease. Future studies to measure BMP-7 in serum or urine may provide additional information on the role of BMP-7 in the early stages of disease progression in patients with obstructive uropathy.
Nonetheless, our study definitively demonstrates that loss of BMP-7 occurs in human kidney disease and provides further evidence that targeting BMP-7 may be an effective therapeutic approach to limit the progression of chronic renal injuries. Renal protective therapies may be particularly useful for obstructive uropathy since treatment in these patients is often characterized by watchful waiting while the clinician assesses the need for surgical intervention.5,30 Additionally, targeting BMP-7 may have additional benefits in these patients since BMP-7 also has an important role in nephrogenesis in the developing kidney20–22 and renal maldevelopment contributes to disease progression after congenital urinary obstruction.2,5
The development of therapies targeting BMP-7 could be advanced by better understanding the regulation of BMP-7 expression. Our study shows that BMP-7 loss in the obstructed kidney occurs in a cell type specific manner in proximal and distal tubules in early stage disease and in collecting ducts and glomeruli in end stage disease. There are several possible explanations of these expression patterns. The first explanation is that it reflects differences in the pathophysiology of urinary obstruction. However, this does not appear likely since hydrostatic pressure in the obstructed kidney is greater in collecting ducts and distal tubules. A more likely explanation is that renal cell types have different mechanisms for regulating BMP-7 expression. In contrast to the mature kidney, BMP-7 expression in the developing kidney is highest in distal tubules and collecting ducts.20,21 This observation supports the idea that BMP-7 expression is subject to complex mechanisms of regulation that can independently regulate BMP-7 expression in different segments of the nephron.
While this research raises many important questions, it has become increasingly evident that the molecular regulation of endogenous BMP-7 and its renal protective functions are important future directions for research with potential therapeutic implications.
CONCLUSIONS
Our results demonstrate that disease progression in obstructive uropathy is driven by a cell type specific decrease in the expression of BMP-7, a protein required for renal injury repair and nephrogenesis. Loss of BMP-7 expression correlates with reduced nephron number, loss of renal architecture, severe renal fibrosis and loss of kidney function in patients with congenital UPJ obstruction. Together these findings validate BMP-7 as a potential therapeutic target to prevent the onset of irreversible renal injury and impaired kidney development in patients with obstructive uropathy. Furthermore, since loss of BMP-7 was noted in various other conditions that lead to renal injury,7,10–12,29 it is likely that we are only beginning to realize the importance of BMP-7 during the clinical progression of chronic kidney disease across a broad spectrum of etiologies.
Acknowledgments
Supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant 1R01DK096177 (PFA), and the National Kidney Foundation (SRM).
Abbreviations and Acronyms
- AQP
aquaporin
- BMP-7
bone morphogenic protein-7
- THP
Tamm-Horsfall protein
- UPJ
ureteropelvic junction
- UUO
unilateral ureteral obstruction
REFERENCES
- 1.Roth KS, Koo HP, Spottswood SE, et al. Obstructive uropathy: an important cause of chronic renal failure in children. Clin Pediatr (Phila) 2002;41:309. doi: 10.1177/000992280204100503. [DOI] [PubMed] [Google Scholar]
- 2.Schedl A. Renal abnormalities and their developmental origin. Nat Rev Genet. 2007;8:791. doi: 10.1038/nrg2205. [DOI] [PubMed] [Google Scholar]
- 3.Becker A, Baum M. Obstructive uropathy. Early Hum Dev. 2006;82:15. doi: 10.1016/j.earlhumdev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Chevalier RL, Thornhill BA, Forbes MS, et al. Mechanisms of renal injury and progression of renal disease in congenital obstructive nephropathy. Pediatr Nephrol. 2010;25:687. doi: 10.1007/s00467-009-1316-5. [DOI] [PubMed] [Google Scholar]
- 5.Rosen S, Peters CA, Chevalier RL, et al. The kidney in congenital ureteropelvic junction obstruction: a spectrum from normal to nephrectomy. J Urol. 2008;179:1257. doi: 10.1016/j.juro.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 6.Manson SR, Niederhoff RA, Hruska KA, et al. The BMP-7-Smad1/5/8 pathway promotes kidney repair after obstruction induced renal injury. J Urol. 2011;185:2523. doi: 10.1016/j.juro.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manson SR, Niederhoff RA, Hruska KA, et al. Endogenous BMP-7 is a critical molecular determinant of the reversibility of obstruction-induced renal injuries. Am J Physiol Renal Physiol. 2011;301:F1293. doi: 10.1152/ajprenal.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeisberg M, Hanai J, Sugimoto H, et al. BMP-7 counteracts TGF-beta1-induced epithelial-tomesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 9.Zeisberg M, Shah AA, Kalluri R. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J Biol Chem. 2005;280:8094. doi: 10.1074/jbc.M413102200. [DOI] [PubMed] [Google Scholar]
- 10.Zeisberg M, Bottiglio C, Kumar N, et al. Bone morphogenic protein-7 inhibits progression of chronic renal fibrosis associated with two genetic mouse models. Am J Physiol Renal Physiol. 2003;285:F1060. doi: 10.1152/ajprenal.00191.2002. [DOI] [PubMed] [Google Scholar]
- 11.Hruska KA, Guo G, Wozniak M, et al. Osteogenic protein-1 prevents renal fibrogenesis associated with ureteral obstruction. Am J Physiol Renal Physiol. 2000;279:F130. doi: 10.1152/ajprenal.2000.279.1.F130. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, de Caestecker M, Kopp J, et al. Renal bone morphogenetic protein-7 protects against diabetic nephropathy. J Am Soc Nephrol. 2006;17:2504. doi: 10.1681/ASN.2006030278. [DOI] [PubMed] [Google Scholar]
- 13.Morrissey J, Hruska K, Guo G, et al. Bone morphogenetic protein-7 improves renal fibrosis and accelerates the return of renal function. J Am Soc Nephrol, suppl. 2002;13:S14. [PubMed] [Google Scholar]
- 14.Wang S, Chen Q, Simon TC, et al. Bone morphogenic protein-7 (BMP-7), a novel therapy for diabetic nephropathy. Kidney Int. 2003;63:2037. doi: 10.1046/j.1523-1755.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- 15.Vukicevic S, Basic V, Rogic D, et al. Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J Clin Invest. 1998;102:202. doi: 10.1172/JCI2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhen-Qiang F, Bing-Wei Y, Yong-Liang L, et al. Localized expression of human BMP-7 by BMMSCs enhances renal repair in an in vivo model of ischemia-reperfusion injury. Genes Cells. 2012;17:53. doi: 10.1111/j.1365-2443.2011.01572.x. [DOI] [PubMed] [Google Scholar]
- 17.Meng XM, Chung AC, Lan HY. Role of the TGF-beta/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond) 2013;124:243. doi: 10.1042/CS20120252. [DOI] [PubMed] [Google Scholar]
- 18.Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol. 2001;187:265. doi: 10.1002/jcp.1080. [DOI] [PubMed] [Google Scholar]
- 19.Bottinger EP. TGF-beta in renal injury and disease. Semin Nephrol. 2007;27:309. doi: 10.1016/j.semnephrol.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- 21.Luo G, Hofmann C, Bronckers AL, et al. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- 22.Kazama I, Mahoney Z, Miner JH, et al. Podocyte-derived BMP7 is critical for nephron development. J Am Soc Nephrol. 2008;19:2181. doi: 10.1681/ASN.2007111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cochrane AL, Kett MM, Samuel CS, et al. Renal structural and functional repair in a mouse model of reversal of ureteral obstruction. J Am Soc Nephrol. 2005;16:3623. doi: 10.1681/ASN.2004090771. [DOI] [PubMed] [Google Scholar]
- 24.Maunsbach AB, Marples D, Chin E, et al. Aquaporin-1 water channel expression in human kidney. J Am Soc Nephrol. 1997;8:1. doi: 10.1681/ASN.V811. [DOI] [PubMed] [Google Scholar]
- 25.Sikri KL, Foster CL, MacHugh N, et al. Localization of Tamm-Horsfall glycoprotein in the human kidney using immuno-fluorescence and immuno-electron microscopical techniques. J Anat. 1981;132:597. [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto T, Sasaki S, Fushimi K, et al. Expression of AQP family in rat kidneys during development and maturation. Am J Physiol. 1997;272:F198. doi: 10.1152/ajprenal.1997.272.2.F198. [DOI] [PubMed] [Google Scholar]
- 27.Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol, suppl. 2003;14:S55. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 28.Ishibe S, Cantley LG. Epithelial-mesenchymal-epithelial cycling in kidney repair. Curr Opin Nephrol Hypertens. 2008;17:379. doi: 10.1097/MNH.0b013e3283046507. [DOI] [PubMed] [Google Scholar]
- 29.Wang SN, Lapage J, Hirschberg R. Loss of tubular bone morphogenetic protein-7 in diabetic nephropathy. J Am Soc Nephrol. 2001;12:2392. doi: 10.1681/ASN.V12112392. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen HT, Herndon CD, Cooper C, et al. The Society for Fetal Urology consensus statement on the evaluation and management of antenatal hydronephrosis. J Pediatr Urol. 2010;6:212. doi: 10.1016/j.jpurol.2010.02.205. [DOI] [PubMed] [Google Scholar]