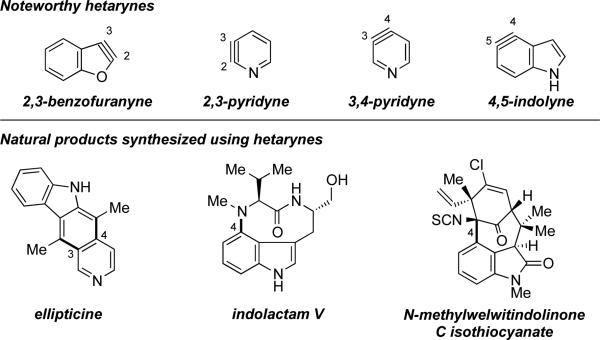
Over the past decade, there has been a rapidly expanding interest in the field of aryne chemistry. i The reactivity of benzyne and its derivatives has been well explored, with numerous trapping methodologies now available for the efficient assembly of functionalized arenes. In contrast, relatively fewer achievements have been made with respect to heterocyclic arynes, or hetarynes.ii The hetarynes have been a subject of interest and controversy since 1902, when Stoermer and coworkers proposed the intermediacy of a 2,3-benzofuranyne (Figure 1), iii which was later called into question.iia, iv Scattered reports of hetarynes have appeared over the past fifty years, but only recently have a few hetarynes been accessible and deemed useful in synthesis. Pyridynes and indolynes have been the most thoroughly studied from a methodological standpoint, and have also been tested in complex settings. v, vi For example, 3,4-pyridynes have been employed as key building blocks in syntheses of ellipticine,vb–g whereas 4,5-indolynes were recently used to construct key bonds in indolactam Vvig and N-methylwelwitindolinone C isothiocyanate.vih
Figure 1.
Noteworthy hetarynes and hetarynes in total synthesis.
Given the numerous synthetic advantages that arynes possess, along with their recent resurgence in the chemical literature,vii we sought to evaluate the potential synthetic utility of a variety of heterocyclic arynes. We report a computational approach to predict: a) the likelihood that a given hetaryne can be generated, and b) the degree of regioselectivity one may expect in a nucleophilic addition reaction involving the hetaryne intermediate. This approach makes the distortion viii model developed by Cheong et al.vie,f generally accessible through routine and rapid calculations. Moreover, we expect these studies will enable synthetic chemists to exploit heterocyclic arynes for the synthesis of natural products, compounds of medicinal value, and other functionalized heterocycles of practical importance.
We first established a computational means to assess if a given hetaryne species should be accessible.xiv Several computational studies of hetaryne energetics have been reported,ix including energy comparisons for pyridyne isomers.x However, to our knowledge, no method has been put forth to predict the accessibility of a hetaryne intermediate. The heat of hydrogenation is a well-established means to explore the strains and relative stabilities of alkynes,xi and we examined a simple method to estimate hetaryne stability involving the difference between electronic energies of arene and aryne.
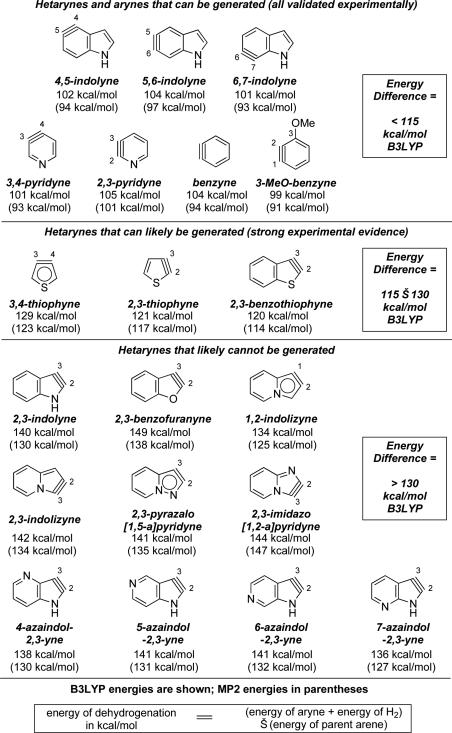
Geometry optimization of several known hetarynesv,vi and the corresponding parent heterocycles was conducted using DFT (B3LYP/6-31G*) and ab initio methods (MP2/6-311+G**).xii These results were also checked versus other functionals, as well as literature data. Using these optimized structures, and accounting for the energy of H2 in the hypothetical dehydrogenation reaction, the energy of dehydrogenation of arene to aryne can be calculated. This straightforward calculation provides a measure of relative stability for the hetarynes that were examined.xiii We found that both B3LYP and MP2 methods gave comparable results for energy of dehydrogenation (Figure 2). While the MP2 results were typically 4–10 kcal/mol lower than B3LYP, both methods predicted essentially the same relative stabilities.xiv Given that B3LYP calculations require less computing time, this method provides a rapid means to assess stabilities. Using B3LYP, the energy of dehydrogenation between an aryne and its parent heterocycle is on the order of 100 kcal/mol.xiv Benzyne and the frequently studied 3-methoxybenzyne were subjected to the same analysis and gave comparable energy differences. Numerous studies that validate the existence of indolynes,vi pyridynes,v benzyne,i and 3-methoxybenzynexv are available.
Figure 2.
Various arynes and a model for aryne accessibility.
Several 5-membered hetarynes were also evaluated. Interestingly, thiophynes, which have been suggested based on experimental evidence xvi are approximately 120–130 kcal/mol higher in energy compared to thiophene. The 2,3-indolyne and 2,3-benzofuranyne, neither of which has been conclusively accessed,ii,xvii are >140 kcal/mol higher in energy compared to their parent heterocycle.
Based on these results, an energy difference of approximately 115 kcal/mol or less suggests that the aryne can be readily generated.xviii An energy difference of roughly 115 to 130 kcal/mol suggests that an aryne may be accessible, although its preparation is likely to be challenging. Finally, an energy difference greater than approximately 130 kcal/mol reflects a hetaryne that is predicted to not be accessible experimentally. Several 5-membered hetarynes that fall into the latter two categories are depicted in Figure 2. In the system studied, 6-membered arynes are accessible, thiophynes are borderline, and most other 5-membered arynes are inaccessible.xix Importantly, the predictive capabilities of this model are independent of the method used for aryne generation,xx allowing experimentalists to pursue their method of choice based on precursor availability.i
With a general means available to assess the feasibility of hetaryne generation, we evaluated the prospects for a variety of hetarynes to react regioselectively in nucleophilic addition reactions using the aryne distortion model. This predictive model uses routine DFT calculations, but has also been shown to operate comparably well using other functionals or higher level calculations.vif A comparison of internal angles of the geometry-optimized hetaryne reveals the preferred site of attack. Specifically, the more linear terminus of the aryne is most electrophilic and is the preferred site of attack by nucleophiles. As shown by Cheong et al.,vie,f and as described above, this is due to less distortion being required to achieve the transition state when the nucleophile attacks the carbon with the larger internal angle. The degree of regioselectivity can be correlated to the difference in angles, with angle differences ≥ 4° generally suggesting synthetically useful regioselectivities and lower values giving low and variable levels of selectivity. For arynes with angle differences between the termini being < 4°, adjacent substituents, such as halides, may be used to modulate regioselectivities.vig
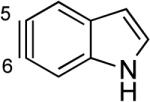
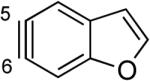
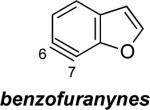
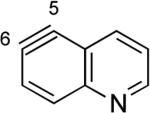
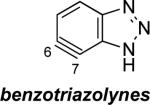
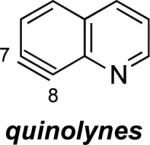
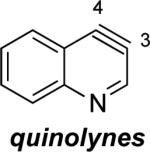
The structures of approximately 150 plausible hetarynes were optimized using rapid DFT (B3LYP/6-31G*) calculations. Selected results are depicted in Tables 1 and 2 with MP2 results for comparison, while the remaining data are available in the Supporting Information. Table 1 features a sampling of hetarynes where the triple bond is in the non-heterocyclic ring. For both indolynes and benzofuranynes, significant regioselectivities are predicted for most isomers, with the exception of the 5,6-arynes (entries 1 and 2). Replacing C2, C3, or both with N or O causes minimal perturbation of these trends, except additions to 4,5-hetarynes are expected to occur with improved regioselectivities. This is likely because of an inductive effect caused by the proximal heteroatoms. An example of this trend is shown in entry 3 for benzotriazolynes, although similar results were found for benzimidazolynes, benzoxazolynes, indazolynes, and other derivatives.xiv Finally, for 6,6-bicyclic hetarynes, regioselectivities are generally predicted to be less controlled, likely because of decreased ring strain present in the 6-membered ring, compared to the 5-membered ring. xxi For example, the aryne termini internal angles for each of the quinolyne isomers are nearly identical, suggesting modest regioselectivity in all cases (entry 4). Analogous trends were seen for an array of related hetarynes, including isoquinolynes, phthalazynes, and quinazolynes.xiv
Table 1.
Predicted heterocyclic aryne regioselectivities.
| entry | site of attacka (angle difference) | hetaryne | entry | site of attacka (angle difference) | hetaryne |
|---|---|---|---|---|---|
| 1 |
C5 (4°) / B3LYP C5 (1°) / MP2 |
|
2 |
C5 (7°) / B3LYP C5 (3°) / MP2 |
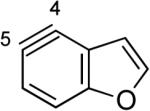
|
| C5 (3°) / B3LYP C5 (<1°) / MP2 |
|
C5 (2°) / B3LYP N/A (0°) / MP2 |
|
||
|
C6 (17°) / B3LYP
C6 (7°) / MP2 |
|
C6 (20°) / B3LYP
C6 (9°) / MP2 |
|
||
| 3 |
C5 (11°) / B3LYP
C5 (4°) / MP2 |
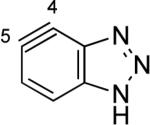
|
4 | C6 (2°) / B3LYP C6 (<1°) / MP2 |
|
| C5 (2°) / B3LYP C5 (1°) / MP2 |
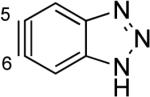
|
C7 (1°) / B3LYP C7 (1°) / MP2 |
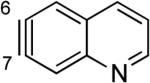
|
||
|
C6 (20°) / B3LYP
C6 (9°) / MP2 |
|
C7 (2°) / B3LYP C7 (3°) / MP2 |
|
||
Predicted site of attack based on the aryne distortion model.
Table 2.
Predicted heterocyclic aryne regioselectivities.
| entry | site of attacka (angle difference) | hetaryne | entry | site of attacka (angle difference) | hetaryne |
|---|---|---|---|---|---|
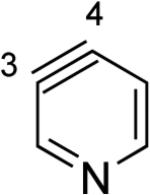
| 1 | C4 (1°) / B3LYP C4 (3°) / MP2 |
|
2 |
C3 (42°) / B3LYP
C3 (23°) / MP2 |
|
|
C2 (47°) / B3LYP
C2 (34°) / MP2 |
|
C4 (44°) / B3LYP
C4 (31°) / MP2 |

|
||
| 3 |
C5 (51°) / B3LYP
C5 (9°) / MP2 |
|
4 |
C3 (45°) / B3LYP
C3 (25°) / MP2 |
|
| 5 |
C2 (49°) / B3LYP
C2 (43°) / MP2 |
|
6 |
C5 (45°) / B3LYP
C5 (36°) / MP2 |
|
| C4 (3°) / B3LYP C4 (3°) / MP2 |
|
C6 (13°) / B3LYP C6 (2°) / MP2 |
|
||
| 7 |
C6 (53°) / B3LYP
C6 (49°) / MP2 |
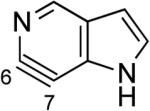
|
8 | N/A (0°) / B3LYP C4 (2°) / MP2 |
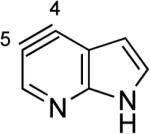
|
|
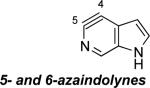
C5 (48°) / B3LYP
C5 (47°) / MP2 |
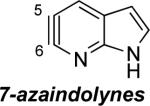
|
C6 (41°) / B3LYP
C6 (25°) / MP2 |
|
||
Predicted site of attack based on the aryne distortion model.
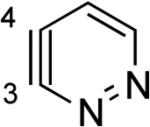
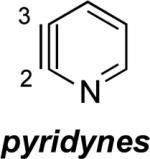
The 2,3-pyridyne and related compounds in Table 2 show enormous angular distortions, which have been noted previously.xxii In 2,3-pyridyne, resonance involving the in-plane lone pair on N causes even greater distortions than the inductive effects and angle restrictions from ring fusion that were noted earlier.vie–g However, the B3LYP calculations (and calculations with other density functionals, such as M06-2X) exaggerate the difference in angles for 2,3-pyridyne and 3,4-pyridizyne. Computational studies of 2,3-pyridine with SF-CCSD predict angles at C2 and C3 of 143° and 114°, respectively, while B3LYP predicts 151° and 104°, respectively, leading to an angle difference of 29° with SF-CCSD and 47° with B3LYP. Table 2 also contains results for geometry optimizations with MP2/6-311+G**; this method gives angles closer to the accurate SF-CCSD values in addition to energies of dehydrogenation (ΔED) that are 3–8 kcal/mol lower. While B3LYP exaggerates the angular distortions, the qualitative model proposed here gives rapid and important predictions about the magnitudes of regioselectivities of nucleophilic additions.
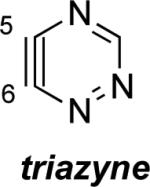
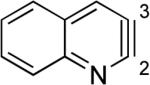
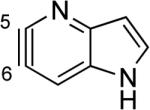
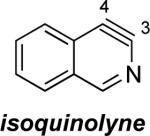
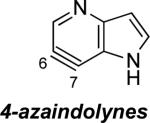
Aza and diaza analogues of the 2,3-pyridyne all possess similar properties and presumably would undergo attack by nucleophiles with excellent regioselectivity (entries 2 and 3). Quinolyne and isoquinolynes were also examined. Although the 3,4-quinolyne is predicted to display modest regioselectivity, the other isomers appear more promising (entries 4 and 5). Finally, a series of azaindolynes were surveyed (entries 6–8). With the exception of the 7-aza-4,5-indolyne, these species are likely to undergo attack by nucleophiles with excellent regioselectivity. Promising data regarding even more exotic hetarynes is presented in the Supporting Information.
Even though the model for predicting regioselectivities in nucleophilic additions to hetarynes is exceptionally efficient using routine computations, in some cases, computations are not necessary. Three general classes of hetarynes and their preferred site of attack by nucleophiles are shown in Figure 3. While not encompassing every hetaryne discussed in this manuscript, we expect these general guidelines will enable synthetic applications of hetaryne intermediates.
Figure 3.
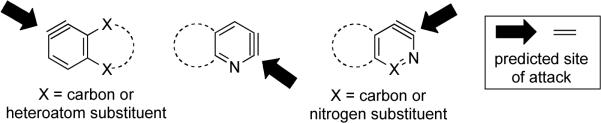
General predictions for hetaryne regioselectivity.
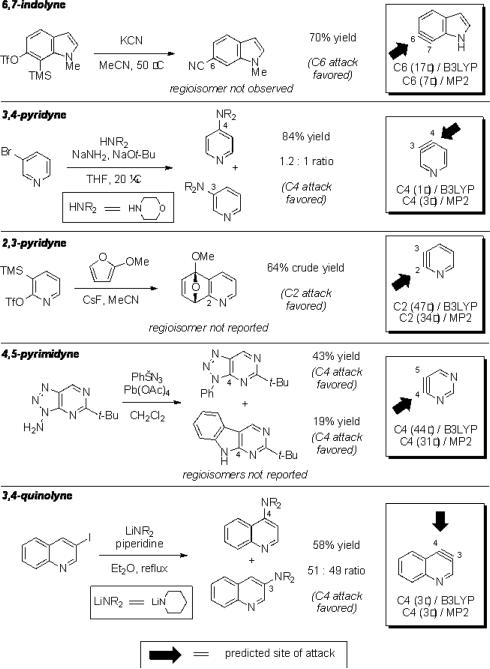
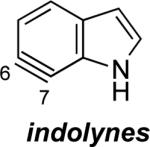
Although relatively few unsymmetrical hetarynes have been prepared, regioselectivity data is available for comparison to the computational predictions. In all cases where significant yields of product are obtained,xxiii the predictions and experimental results are in agreement (Figure 4). Indolynes have recently been studied by our laboratories, and each of the >20 examples that have been reported are in accord with the aryne distortion model.vie–g An example pertaining to a 6,7-indolyne is depicted. The remaining literature examples predominantly involve pyridynes and their derivatives. As shown, our findings for the 3,4-pyridynevh,i and 2,3-pyridynevj are also consistent with experimental data regarding the preferred site of attack and the degree of regioselectivity in each case.vb–g Comparisons of computational and experimental data for a 4,5-pyrimidyneixb and a 3,4-quinolynexxiv were also found to be in good accord. Several additional examples are depicted in the Supporting Information pertaining to 2,3-pyridyne-N-oxide, 4,5-benzofurazanyne, and 1,2-dibenzofuranyne.
Figure 4.
Known examples of indolynes, pyridynes, pyrimidynes, and quinolynes.
In summary, we have developed an efficient computational approach for evaluating the synthetic potential of heterocyclic arynes. DFT or ab initio calculations can be used to predict the likelihood that a given hetaryne can be generated, in addition to the degree of regioselectivity one may expect in a reaction between a given hetaryne and a nucleophilic trapping agent. Although both methods give comparable predictions, DFT requires less computing time. Our predictive tool requires only routine and rapid calculations, and further validates the aryne distortion model as a means to gauge nucleophilic regioselectivities. We expect that these findings will enable synthetic chemists to judiciously design syntheses of functionalized heterocycles using hetarynes as versatile intermediates.
Supplementary Material
Acknowledgments
The authors are grateful to NIH-NIGMS (R01 GM090007 to N.K.G), NSF (CHE-0548209 to K.N.H.), Boehringer Ingelheim (N.K.G.), DuPont (N.K.G.), Eli Lilly (N.K.G.), Amgen (N.K.G.), AstraZeneca (N.K.G.), the University of California, Los Angeles, Bristol-Myers Squibb (fellowship to J.D.C.), Pfizer (fellowship to J.D.C.), the Christopher S. Foote Fellowship (S.M.B.), the Stauffer Charitable Trust (S.M.B.), and the John Fell Oxford University Press Research Fund (R.S.P.), for financial support.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Contributor Information
Adam E. Goetz, Department of Chemistry and Biochemistry University of California, Los Angeles Los Angeles, CA 90095 (USA)
Sarah M. Bronner, Department of Chemistry and Biochemistry University of California, Los Angeles Los Angeles, CA 90095 (USA)
Jordan D. Cisneros, Department of Chemistry and Biochemistry University of California, Los Angeles Los Angeles, CA 90095 (USA)
Joshua M. Melamed, Department of Chemistry and Biochemistry University of California, Los Angeles Los Angeles, CA 90095 (USA)
Prof. Robert S. Paton, Department of Chemistry and Biochemistry University of California, Los Angeles Los Angeles, CA 90095 (USA) Chemistry Research Laboratory University of Oxford, Mansfield Road, Oxford OX1 3TA, UK.
Prof. K. N. Houk, Department of Chemistry and Biochemistry University of California, Los Angeles Los Angeles, CA 90095 (USA)
Prof. Neil K. Garg, Department of Chemistry and Biochemistry University of California, Los Angeles Los Angeles, CA 90095 (USA).
References
- i.a Sanz R. Org. Prep. Proced. Int. 2008;40:215. doi: 10.1080/00304940809458083. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Peña D, Pérez D, Guitián E. Heterocycles. 2007;74:89. [Google Scholar]; c Dyke AM, Hester AJ, Lloyd-Jones GC. Synthesis. 2006:4093. [Google Scholar]; d Pellissier H, Santelli M. Tetrahedron. 2003;59:701. [Google Scholar]; e Wenk HH, Winkler M, Sander W. Angew. Chem. Int. Ed. 2003;42:502. doi: 10.1002/anie.200390151. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2003;115:518. [Google Scholar]
- ii.a Reinecke MG. Tetrahedron. 1982;38:427. [Google Scholar]; b Kauffmann T. Angew. Chem. Int. Ed. Engl. 1965;4:543. [Google Scholar]; Angew. Chem. 1965;77:557. [Google Scholar]; c Kauffmann T, Wirthwein R. Angew. Chem. Int. Ed. Engl. 1971;10:20. [Google Scholar]; Angew. Chem. 1971;83:21. [Google Scholar]
- iii.Stoermer R, Kahlert B. Ber. 1902;35:1633. [Google Scholar]
- iv.a Hoffmann RW. Dehydrobenzene and Cycloalkynes; Organic Chemistry; a Series of Monographs 11. Academic Press; New York: 1967. [Google Scholar]; b Wittig G. Angew. Chem. Int. Ed. 1962;1:415. [Google Scholar]; Angew. Chem. 1962;74:479. [Google Scholar]; c Gilman H, Melstrom DS. J. Am. Chem. Soc. 1948;70:1655. doi: 10.1021/ja01192a058. [DOI] [PubMed] [Google Scholar]
- v.For pyridynes, see: Levine R, Leake WW. Science. 1955;121:780. doi: 10.1126/science.121.3152.780.; Gribble GW, Saulnier MG, Sibi MP, Obaza-Nutaitis JA. J. Org. Chem. 1984;49:4518.; May C, Moody CJ. J. Chem. Soc., Chem. Commun. 1984:926.; May C, Moody CJ. J. Chem. Soc. Perkin Trans. 1988;1:247.; Sha C-K, Yang JF. Tetrahedron. 1992;48:10645.; Díaz MT, Cobas A, Guitián E, Castedo L. Synlett. 1998:157.; Díaz MT, Cobas A, Guitián E, Castedo L. Eur. J. Org. Chem. 2001:4543.; Tsukazaki M, Snieckus V. Heterocycles. 1992;33:533.; Vinter-Pasquier K, Jamart-Grégoire B, Caubère P. Heterocycles. 1997;45:2113.; Walters MA, Shay JJ. Synth. Commun. 1997;27:3573.; Enamorado MF, Ondachi PW, Comins DL. Org. Lett. 2010;12:4513. doi: 10.1021/ol101887b..
- vi.For indolynes, see: Julia M, Huang Y, Igolen J, Acad CR. Sci., Ser. C. 1967;265:110.; Igolen J, Kolb A, Acad CR. Sci., Ser. C. 1969;269:54.; Julia M, Goffic FL, Igolen J, Baillarge M. Tetrahedron Lett. 1969;10:1569.; For more recent accounts, see: Bronner SM, Bahnck KB, Garg NK. Org. Lett. 2009;11:1007. doi: 10.1021/ol802958a.; Cheong PH-Y, Paton RS, Bronner SM, Im G-YJ, Garg NK, Houk KN. J. Am. Chem. Soc. 2010;132:1267. doi: 10.1021/ja9098643.; Im G-YJ, Bronner SM, Goetz AE, Paton RS, Cheong PH-Y, Houk KN, Garg NK. J. Am. Chem. Soc. 2010;132:17933. doi: 10.1021/ja1086485.; Bronner SM, Goetz AE, Garg NK. J. Am. Chem. Soc. 2011;133:3832. doi: 10.1021/ja200437g.; Huters AD, Quasdorf KW, Styduhar ED, Garg NK. J. Am. Chem. Soc. 2011;133:15797. doi: 10.1021/ja206538k.; Buszek KR, Luo D, Kondrashov M, Brown N, VanderVelde D. Org. Lett. 2007;9:4135. doi: 10.1021/ol701595n.; Brown N, Luo D, VanderVelde D, Yang S, Brassfield A, Buszek KR. Tetrahedron Lett. 2009;50:63. doi: 10.1016/j.tetlet.2008.10.086.; Buszek KR, Brown N, Luo D. Org. Lett. 2009;11:201. doi: 10.1021/ol802425m.; Brown N, Luo D, Decapo JA, Buszek KR. Tetrahedron Lett. 2009;50:7113. doi: 10.1016/j.tetlet.2009.09.083.; Garr AN, Luo D, Brown N, Cramer CJ, Buszek KR, VanderVelde D. Org. Lett. 2010;12:96. doi: 10.1021/ol902415s.; Thornton PD, Brown N, Hill D, Neuenswander B, Lushington GH, Santini C, Buszek KR. ACS Combi. Sci. 2011;13:443. doi: 10.1021/co2000289..
- vii.Arynes are highly reactive, inherently electrophilic (umpolung of common arene reactivity), and can provide access to polysubstituted aromatic compounds. See ref i.
- viii.For the application of distortion energies to regioselectivity of cycloaddition reactions, see: Ess DH, Houk KN. J. Am. Chem. Soc. 2007;129:10646. doi: 10.1021/ja0734086.; Ess DH, Houk KN. J. Am. Chem. Soc. 2008;130:10187. doi: 10.1021/ja800009z.; Lam Y.-h., Cheong PH-Y, Blasco Mata JM, Stanway SJ, Gouverneur V, Houk KN. J. Am. Chem. Soc. 2009;131:1947. doi: 10.1021/ja8079548.; Hayden AE, Houk KN. J. Am. Chem. Soc. 2009;131:4084. doi: 10.1021/ja809142x.; Schoenebeck F, Ess DH, Jones GO, Houk KN. J. Am. Chem. Soc. 2009;131:8121. doi: 10.1021/ja9003624.; For the application of distortion energies to regioselectivity of palladium-catalyzed cross-coupling reactions, see: Legault CY, Garcia Y, Merlic CA, Houk KN. J. Am. Chem. Soc. 2007;129:12664. doi: 10.1021/ja075785o.; Garcia Y, Schoenebeck F, Legault CY, Merlic CA, Houk KN. J. Am. Chem. Soc. 2009;131:6632. doi: 10.1021/ja9004927.; For a discussion of activation strain theory, see: van Zeist W-J, Bickelhaupt FM. Org. Biomol. Chem. 2010;8:3118. doi: 10.1039/b926828f.; For a discussion of the application of distortion/interaction theory to stereoselectivity, see: Kolakowski RV, Williams LJ. Nat. Chem. 2010;2:303. doi: 10.1038/nchem.577..
- ix.a Radom L, Nobes RH, Underwood DJ, Li W-K. Pure Appl. Chem. 1986;58:75. [Google Scholar]; b Tielemans M, Areschka V, Colomer J, Promel R, Langenaeker W, Geerlings P. Tetrahedron. 1992;48:10575. [Google Scholar]; c Pangamte HN, Lyngdoh RHD. J. Phys. Chem. A. 2010;114:2710. doi: 10.1021/jp911593y. [DOI] [PubMed] [Google Scholar]
- x.a Adam W, Grimison A, Hoffmann R. J. Am. Chem. Soc. 1969;91:2590. [Google Scholar]; b Mariet N, Ibrahim-Ouali M, Parrain J-L, Santelli M. J. Mol. Struct. (Theochem) 2004;679:53. [Google Scholar]; c Liu R, Tate DR, Clark JA, Moody PR, Van Buren AS, Krauser JA. J. Phys. Chem. 1996;100:3430. [Google Scholar]; d Sparrapan R, Mendes MA, Carvalho M, Eberlin MN. Chem. Eur. J. 2000;6:321. doi: 10.1002/(SICI)1521-3765(20000117)6:2<321::AID-CHEM321>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- xi.This hydrogenation energy approach is often used to measure or predict alkene and alkyne stabilities; for examples, see: Anslyn EA, Dougherty DA. Modern Physical Organic Chemistry. University Science Books; Sausalito, CA: 2006. pp. 112–113.; Turner RB, Jarrett AD, Goebel P, Mallon BJ. J. Am. Chem. Soc. 1973;95:790.; Bach RD. J. Am. Chem. Soc. 2009;131:5233. doi: 10.1021/ja8094137.; This method has previously been used to test the influence of substituents on benzyne energetics; see: Maurin P, Ibrahim-Ouali M, Parrain J-L, Santelli M. J. Mol. Struct. (Theochem) 2003;637:91. Also, see ref 10b.
- xii.a) All computations were carried out using Spartan ‘06 on an iMac computer. Spartan ’06. Wavefunction, Inc; Irvine, CA: 2006. ; b) Comparable results were obtained using higher levels of theory; see Supporting Information for details.
- xiii.Correcting for zero-point energy has a negligible effect on the results.
- xiv.See Supporting Information for details.
- xv.For select recent studies involving 3-methoxybenzyne, see: Liu Z, Larock RC. J. Org. Chem. 2006;71:3198. doi: 10.1021/jo0602221.; Rogness DC, Larock RC. J. Org. Chem. 2010;75:2289. doi: 10.1021/jo1000687.; Allan KM, Gilmore CD, Stoltz BM. Angew. Chem. Int. Ed. 2011;50:4488. doi: 10.1002/anie.201100911.; Gebara KS, Casagrande GA, Raminelli C. Tetrahedron Lett. 2011;52:2849.
- xvi.a Reinecke MG, Newsom JG. J. Am. Chem. Soc. 1976;98:3021. [Google Scholar]; b Reinecke MG, Newsom JG, Chen L-J. J. Am. Chem. Soc. 1981;103:2760. [Google Scholar]; c Ye X-S, Li W-K, Wong HNC. J. Am. Chem. Soc. 1996;118:2511. [Google Scholar]
- xvii.Hoffman RW. Dehydrobenzene and Cycloalkynes. Academic Press; New York: 1967. ; Conway SC, Gribble GW. Heterocycles. 1992;34:2095.; Bergman J, Eklund N. Tetrahedron. 1980;36:1439..
- xviii.While this value corresponds to energy differences of known aryne intermediates, it does not account for potential side reactions (i.e. ring fragmentations) that could potentially compete with aryne formation. Therefore, it is possible that arynes within this category may be inaccessible due to other competing reaction pathways.
- xix.Some evidence is available for the existence of 3,4-pyrrolyne, see: Liu J-H, Chan H-W, Xue F, Wang Q-G, Mak TCW, Wong HNC. J. Org. Chem. 1999;64:1630. doi: 10.1021/jo982129l..
- xx.Although the predictive capabilities of this model function regardless of the method of hetaryne generation, certain methods (e.g., those that liberate gases, such as CO2, CO, and N2) are likely to be more promising than others.
- xxi.Hamura T, Ibusuki Y, Sato K, Matsumoto T, Osamura Y, Suzuki K. Org. Lett. 2003;5:3551. doi: 10.1021/ol034877p. [DOI] [PubMed] [Google Scholar]
- xxii.Rau NJ, Wenthold PG. J. Phys. Chem. A. 2011;115:10353. doi: 10.1021/jp2051068.. Also, see refs 19, 63, and 64 therein.
- xxiii.Of the hetaryne data available, one example involving a 7,8-quinolyne cycloaddition does not fit the proposed model. However, the reported yield is 35%, with no discussion of the remainder of the mass. See: Collis GE, Burrell AK. Tetrahedron Lett. 2005;46:3653..
- xxiv.Kauffman T, Boettcher F-P, Hansen J. Liebigs Ann. Chem. 1962;659:102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.