Abstract
Background
Head and neck squamous cell carcinoma (HNSCC) is increasing in prevalence as society ages worldwide. However, there are no established treatment protocols for elderly patients, and the threshold for defining “elderly” is undetermined. In this study, we categorized elderly patients (65 years and older) with HNSCC into 2 groups: “young-old,” from 65 to 74 years old, and “old-old,” 75 years and older, and compared their treatment outcomes.
Methods
The subjects were 182 patients aged 65 years and older who visited our hospital for HNSCC from 2003 to 2009. We categorized them into 2 groups, young-old (65–74 years) and old-old (75 years and older), and compared the male-female ratio, ratio with underlying diseases, primary tumor sites, disease stage, applied treatments and curative rate. Additionally, for the curative treatment category in both groups, we compared recurrence rate, relationship between recurrence rate and use of concomitant chemotherapy, the 5-year relapse-free survival and the 5-year cause-specific survival.
Results
The ratio of patients with underlying diseases in the old-old group was significantly higher than in the young-old group, but there was no significant difference in curative rate between the 2 (old-old, 81.9%; young-old, 82.7%). The 5-year, cause-specific survival in curative treatment category was significantly lower in the old-old (66.1%) group than the young-old (94.1%) group.
Conclusion
Elderly patients of all ages should positively receive curative treatment. We suppose that concomitant chemotherapy is not acceptable in elderly patients. The 5-CSS of the curative treatment category in the old-old patients was significantly lower than in the young-old patients.
Keywords: elderly patients, head and neck squamous cell carcinoma, outcome
Globally, the population is rapidly aging and consequently the prevalence of patients with head and neck squamous cell carcinoma (HNSCC) is increasing. HNSCC is not a rare disease among elderly patients, as a previous study reported that the number of patients with HNSCC is increasing among patients aged 50 and older, and that 20% of HNSCC patients are aged 75 and older.1 We thus have more and more opportunities to conduct treatment decisions in elderly patients. However, the variety of such treatments is restricted in the elderly due to physical issues such as decreased organ function and increased prevalence of underlying diseases, as well as psychological and societal problems such as impaired cognition, tendency to accept death and the nearly absolute necessity of societal and financial supports. A separate issue is that no studies have clarified the threshold age defining “elderly.” A number of previous articles have used cutoff ages of 70 or 75 years. 2–7
In Japan, individuals aged 65 and older are referred to as “elderly.” The ratio of elderly people to the total population in Japan was 25.1% in April 2014, while the proportion of individuals aged 75 years and older was 12.3%.8 Both ratios are increasing. The same census found that in Tottori Prefecture (the location of our hospital), the ratio of elderly people to the total prefectural population was 28.2% and the ratio of those aged 75 years and older was 15.6%. Thus the population of Tottori Prefecture is aging more rapidly than Japan as a whole.
This study compared the treatment outcomes of elderly patients aged 65 and older seen at our hospital for HNSCC. We classified the patients into 2 groups: the young-old group, aged 65−74, and the old-old group, aged 75 and older.
MATERIALS AND METHODS
This study analyzed 182 patients aged 65 and older who visited our hospital for HNSCC from 2003 to 2009. First, we divided the patients into 2 groups based on the patients’ ages at their initial visits: the young-old group, aged 65 to 74 years, and the old-old group, aged 75 years and older.
We compared the male-female ratio, ratio of patients with underlying diseases, primary tumor sites, disease stage, applied treatments and curative rate. Underlying diseases were defined as conditions that could affect tumor treatment, such as cardiovascular disease, diabetes, cerebrovascular disease, respiratory disease, hepatic disease, kidney disease and psychiatric disorders. Patients with at least one of these disease types were considered to have an underlying disease.
Primary tumor sites were classified as larynx, oral cavity, hypopharynx, or others (epipharynx, oropharynx, acoustic organ, paranasal sinus and salivary gland), while tumor stage was classified as either early tumor (Stage I or II) or advanced tumor (Stage III or IV), according to the fifth edition of the Union for International Cancer Control classification of malignant tumors.
In our protocol, curative treatments were categorized as radiotherapy alone, surgery alone, or the combination of surgery and radiotherapy. Concomitant chemotherapy for curative treatments was classified as 4 patterns: neoadjuvant chemotherapy before surgery; neoadjuvant concurrent chemoradiotherapy before surgery; adjuvant concurrent chemoradiotherapy after surgery; and concurrent chemoradiotherapy alone. Chemotherapy dosages were decreased in patients aged 70 years or older or in those whose creatinine clearance was lower than 50 mL/min. Further, chemotherapy was not conducted in patients whose creatinine clearance was lower than 30 mL/min.
For both the young-old and old-old groups, we investigated the prevalence of patients in each of the following three categories: curative treatment category, in which patients received curative treatment and achieved locoregional control; palliative treatment category, in which patients received palliative treatment because they had distant metastasis at their initial visit or could not tolerate further curative treatments, or in which distant metastasis developed during treatment; and no-treatment category, in which no treatment was conducted due to patient refusal. We also examined the relationship between the frequency of underlying diseases and the curative rate.
Next, we investigated the recurrence rate, prevalence of concomitant chemotherapy use, relationship between recurrence rate and use of concomitant chemotherapy, the 5-year relapse-free survival (RFS) and the 5-year cause-specific survival (CSS) in the curative treatment category in each group.
The study was approved by the Ethics Committee of Tottori University Faculty of Medicine (approval number 2594).
When comparing the 2 groups, we used the chi-squared and Fisher’s exact test. We calculated the 5-year RFS and CSS using the Kaplan-Meier method, and detected the significant differences between groups using the log-rank test. A P value smaller then 0.05 was considered statistically significant.
RESULTS
Male-female ratio and ratio of patients with underlying diseases
Table 1 shows the male-female ratio and the ratio of patients with underlying diseases. The young-old group comprised 110 patients with a mean age of 69.5 years (65–74 years) and a male-female ratio of 5.1 (92 male, 18 female). The old-old group comprised 72 patients with a mean age of 80.0 years (75–93 years) and a male-female ratio of 3.2 (55 male, 17 female). The percentage of patients with underlying diseases was significantly higher in the old-old group (76.4%, 55 of 72 patients) than in the young-old group (58.2%, 64 of 110 patients) (P = 0.012).
Table 1. Male-female ratio and the percentage of patients with underlying diseases.
| Group | ||
| Young-old [n = 110] | Old-old [n = 72] | |
| Male:female ratio | 92:18 (5.1:1) | 55:17 (3.2:1) |
| Patients with underlying diseases | ||
| Number (%) | 64 (58.2%) | 55 (76.2%) |
Primary tumor sites and disease stage
As shown in Table 2, the primary tumor sites in the young-old group (110 patients) were: larynx (43 patients, 39.1%), hypopharynx (35 patients, 31.8%), oral cavity (18 patients, 16.4%) and others (14 patients, 12.7%). In the old-old group (72 patients) the sites were: larynx (30 patients, 41.7%), hypopharynx (11 patients, 15.3%), oral cavity (17 patients, 23.6%) and others (14 patients, 19.4%). Table 3 shows that in the young-old group, 51 patients (46.4%) were early stage (I or II) and 59 patients (53.6%) were advanced stage (III or IV). In the old-old group, 32 patients (44.4%) were early stage and 40 patients (55.6%) were advanced stage.
Table 2. Primary tumor site.
| Group | ||
| Young-old [n = 110] | Old-old [n = 72] | |
| Larynx | 43 (39.1%) | 30 (41.7%) |
| Oral cavity | 18 (16.4%) | 17 (23.6%) |
| Hypopharynx | 35 (31.8%) | 11 (15.3%) |
| Others | 14 (12.7%) | 14 (19.4%) |
Others: epipharynx, oropharynx, acoustic organ, paranasal sinus and salivary gland.
Table 3. Disease stage.
| Stage | Group | |
| Young-old [n = 110] | Old-old [n = 72] | |
| Early (I–II) | 51 (46.4%) | 32 (44.4%) |
| Advanced (III–IV) | 59 (53.6%) | 40 (55.6%) |
Applied treatments
Table 4 shows the details of the applied treatments in each category. The curative treatment category comprised 91 of 110 patients (82.7%) in the young-old group and 59 of 72 patients (81.9%) in the old-old group. The palliative treatment category comprised 14 of 110 patients (12.7%) in the young-old group and 8 of 72 patients (11.1%) in the old-old group. The no treatment category comprised 5 of 110 patients (4.5%) in the young-old and 5 of 72 patients (6.9%) in the old-old group.
Table 4. Applied treatments.
| Treatment | Group | |
| Young-old [n = 110] | Old-old [n = 72] | |
| Radical treatment | 91 (82.7%) | 59 (81.9%) |
| Radiotherapy | 25 | 18 |
| Surgery | 35 | 28 |
| Surgery + radiotherapy | 31 | 13 |
| Palliative treatment | 14 (12.7%) | 8 (11.1%) |
| No treatment | 5 (4.5%) | 5 (6.9%) |
Relationship between underlying disease and curative rate
Table 5 shows the curative rates in patients with or without underlying diseases. In both groups, curative treatments were possible in a higher percentage of patients without underlying diseases than in those who had such conditions: in the young-old group, 89.1% versus 78.1%, and in the old-old group, 88.2% versus 80.0%. In each group, the curative rate of patients without underlying diseases was slightly higher but not significantly so.
Table 5. Relationship between the existence of underlying disease and cure rate.
| Underlying disease | Group | |||
| Young-old [n = 110] | Old-old [n = 72] | |||
| Curative rate | P value | Curative rate | P value | |
| Positive | 50/64 (78.6%) | 0.132 | 44/55 (80.0%) | 0.355 |
| Negative | 41/46 (89.1%) | 15/17 (88.2%) | ||
Frequency of recurrence
Table 6 shows the number of patients who developed recurrence in the curative treatment category. Recurrence occurred in 18 of 91 patients (19.8%) in the young-old group and 19 of 59 patients (32.3%) in the old-old group. The recurrence rate was higher in the old-old group than the young-old group, but the difference was not significant.
Table 6. Number of recurrent patients.
| Recurrent patients | Group | ||
| Young-old [n = 91] | Old-old [n = 59] | P value | |
| Number (%) | 18 (19.8%) | 19 (32.2%) | 0.084 |
Relationship between use of concomitant chemotherapy and recurrence rates
The percentage of the curative treatment category in which concomitant chemotherapy was performed was significantly higher in the young-old group than in the old-old group: 58.2% (53 of 91 patients) versus 40.7% (24of 59 patients), respectively (P = 0.035). In both groups, the recurrence rates were higher when concomitant chemotherapy was used than when it was not, but not significantly so: in the young-old group, 26.4% (14 of 53 patients) versus 10.5% (4 of 38 patients), and in the old old group, 33.3% (8 of 24 patients) versus 31.4% (11 of 35 patients), respectively.
Survival rate (5-year RFS and CSS)
As shown in Fig. 1, the 5-year RFS of the curative treatment category in the young-old group (91 patients) was 76.4%, while that in the old-old group (59 patients) was 61.3%, a significant difference (P = 0.027). As shown in Fig. 2, the 5-year CSS of the curative treatment category in the young-old group (91 patients) was 94.1%, while that in the old-old group (59 patients) was 66.1%, a significant difference (P = 0.0016).
Fig. 1.
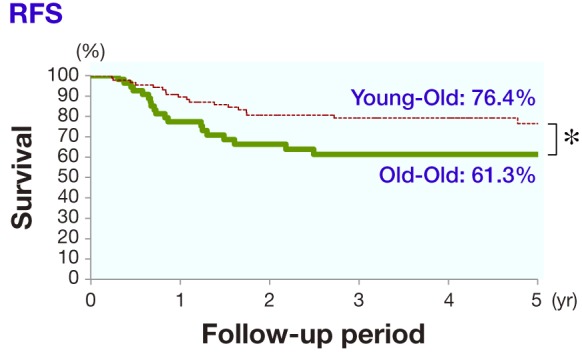
In the curative treatment category, the difference between the 5-year RFS of the young-old group (76.4%) and old-old group (61.3%) was significant (*P = 0.027). RFS, relapse-free survival.
Fig. 2.
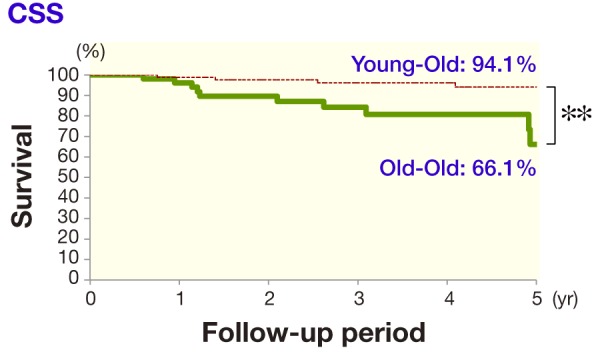
In the curative treatment category, the difference between the 5-year CSS of the young-old group (94.1%) and old-old group (66.1%) was significant (**P = 0.0016). CSS, cause-specific survival.
DISCUSSION
The treatment decision was dedicated to elderly patients because of their physical or social circumstances. In our study, we investigated the outcomes of treatment for HNSCC in elderly patients.
Prior studies reported that increased patient age was associated with a significantly higher ratio of females to males,2, 9 and our results showed a similar trend. In addition, the prevalence of larynx and oral cavity tumors was found to be higher in older patients while that of hypopharynx tumors was lower, 2, 9, 10 which mirrored our findings.
Regarding the ratio of early- to advanced-stage tumors, various results have been reported. For example, one study found that in patients aged 75 years and older, this ratio did not differ greatly between elderly and young adult patients.2 Another study reported that elderly patients had more early-stage tumors than younger patients.4 In our study, advanced-stage tumors were observed slightly more often than early-stage tumors in both groups.
Derks et al. reported that in 3 patients groups divided by age (ages 45−60, 70−79 and 80 years and older), the percentages of patients who received curative treatment were 89%, 75% and 36%, respectively.3 Huang et al. 4 reported that patients under 75 years old and those 75 years and older received curative treatment in 93% and 79% of patients, respectively. As shown in these reports, older patients were less likely to be candidates for curative treatments. In our study, the percentages of patients in the curative treatment category were 82.7% in the young-old group and 81.9% in the old-old group.
The percentages of patients with underlying diseases were 58.2% in the young-old group and 76.2% in the old-old group. However, there were no significant correlations between the existence of underlying disease and the curative rate, and curative treatments can often be conducted despite the presence of these conditions. One previous study reported that the presence of underlying diseases did not affect treatment completion,11 and another found that there was no correlation between the existence of underlying disease and either co-morbidity or complications in elderly patients.12 As reported in some studies,2, 3, 6, 13 treatment choices in elderly patients with HNSCC should not be based on patients’ ages. We suppose that both young-old and old-old patients can achieve nearly equivalent curative rates as a result of curative treatments.
In our study, the use of concomitant chemotherapy did not diminish the recurrence rate in either group, although the recurrence rate tended to be higher in the old-old group than the young-old group. Previous studies reported that reducing chemotherapy dosages undermined the effectiveness of treatments14 and that the concomitant use of chemotherapy did not suppress local tumor recurrence or distant metastasis. 15 We suppose that concomitant chemotherapy is not acceptable in elderly patients, since chemotherapy doses must be reduced in most of them.
It was reported that the survival rate was lower or almost equivalent in an elderly group compared to a younger group.9 In our study, the 5-year RFS of the curative treatment category in the young-old group was 76.4%, which was significantly higher than the 61.3% in the old-old group. Further the 5-year CSS of the curative treatment category in the young-old group was 94.1%, which was significantly higher than the 66.1% in the old-old group.
In conclusion, elderly patients of all ages should absolutely receive curative treatment. We believe that concomitant chemotherapy is not acceptable in elderly patients. The 5-CSS of the curative treatment category in the old-old patients was significantly lower than in the young-old patients.
The authors declare no conflict of interest.
REFERENCES
- 1. Sikora AG, Toniolo P, DeLacure MD. The changing demographics of head and neck squamous cell carcinoma in the United States. Laryngoscope. 2004; 114: 1915-23. . [DOI] [PubMed] [Google Scholar]
- 2.Sarini J, Fournier C, Lefebvre JL, Bonafos G, Van JT, Coche-Dequéant B. Head and neck squamous cell carcinoma in elderly patients: a long-term retrospective review of 273 cases. Arch Otolaryngol Head Neck Surg. 2001; 127: 1089-92. . [DOI] [PubMed] [Google Scholar]
- 3. Derks W, de Leeuw JRJ, Hordijk GJ, Winnubst JAM. Reasons for non-standard treatment in elderly patients with advanced head and neck cancer. Eur Arch Otorhinolaryngol. 2005; 262: 21-6. . [DOI] [PubMed] [Google Scholar]
- 4.Huang SH, O’Sullivan B, Waldron J. Patterns of care in elderly head and neck cancer radiation oncology patients: a singlecenter cohort study. Int J Radiat Oncol Biol Phys. 2011; 79: 46-51. . [DOI] [PubMed] [Google Scholar]
- 5. Barzan L, Veronesi A, Caruso G. Head and neck cancer and aging: a retrospective study in 438 patients. J Laryngol Otol. 1990; 104: 634-40. . [DOI] [PubMed] [Google Scholar]
- 6. Bhattacharyya N. A matched survival analysis for squamous cell carcinoma of the head and neck in the elderly. Laryngoscope. 2003; 113: 368-72. . [DOI] [PubMed] [Google Scholar]
- 7. Vaccher E, Talamini R, Franchin G, Tirelli U, Barzan L. Elderly head and neck (H-N) cancer patients: a monoinstitutional series. Tumori. 2003; 113: 368-72. . [DOI] [PubMed] [Google Scholar]
- 8. Statistics Bureau: Population Estimates 2014 [Internet]. Tokyo: Minisry of Internal Affairs and Communications; c1996. [cited 2014 Oct 1]. Available from: http://www.stat.go.jp/. [Google Scholar]
- 9. Gugic J, Strojan P. Squamous cell carcinoma of the head and neck in the elderly. Rep Pract Oncol Radiother. 2013; 18: 16-25. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Italiano A, Ortholan C, Dassonville O, Poissonnet G, Thariat J, Benezery K. Head and neck squamous cell carcinoma in patients aged > or = 80 years: patterns of care and survival. Cancer. 2008; 113: 3160-8. . [DOI] [PubMed] [Google Scholar]
- 11. Sharma A, Madan R, Kumar R, Sagar P, Kamal VK, Thakar A. Compliance to Therapy-Elderly Head and Neck Carcinoma Patients. Can Geriatr J. 2014; 17: 83-7. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peters TT, van der Laan BF, Plaat BE, Wedman J, Langendijk JA, Halmos GB. The impact of comorbidity on treatmentrelated side effects in older patients with laryngeal cancer. Oral Oncol. 2011; 47: 56-61. . [DOI] [PubMed] [Google Scholar]
- 13. Lalami Y, de Castro G, Jr, Bernard-Marty C, Awada A. Management of head and neck cancer in elderly patients. Drugs Aging. 2009; 26: 571-83. . [DOI] [PubMed] [Google Scholar]
- 14. Schnider M, Thyss A, Ayela P, Gaspard MH, Otto J, Creisson A. Chemotherapy for patients aged over 80 In: Fentiman IS Monfardini S, editors. Cancer in the elderly. New York: Oxford University Press; 1994. p. 53-60. [Google Scholar]
- 15. Chen H, Zhou L, Chen D, Luo J. Clinical efficacy of neoadjuvant chemotherapy with platinum-based regimen for patients with locoregionally advanced head and neck squamous cell carcinoma: an evidence-based meta-analysis. Ann Saudi Med. 2011; 31: 502-12. . [DOI] [PMC free article] [PubMed] [Google Scholar]


