Abstract
Background
Mesenchymal stem cells (MSCs) hold promise for application in adult stem cell-mediated regenerative medicine in bone remodeling and fracture repair. MSCs in vitro can be directed to osteogenic lineage by dexamethasone (DEX); however, the use of DEX is not practical in clinical settings because of adverse side effects such as glucocorticoid-induced osteoporosis. For identifying substances that facilitate osteogenesis, a monitoring system, which detects the osteogenic differentiation stage of MSCs accurately and easily, is required.
Methods
By focusing on the human osteocalcin (OC) gene whose expression profile is described along with osteogenic differentiation, we constructed the luciferase (Luc) reporter gene driven by the enhancer/promoter sequence of the human OC gene (OC-Luc) utilizing a mammalian artificial chromosome. Mammalian artificial chromosome is a suitable platform for loading reporter constructs, because of its stable episomal maintenance in host cells, transferability into any cell and assurance of long-term physiological transgene expression. We loaded the OC-Luc on a mammalian artificial chromosome vector engineered from mouse chromosome (designated as mouse artificial chromosome, MAC) in Chinese hamster ovary cells (OC-Luc/MAC) and transferred this into human MSC cells via chromosome transfer.
Results
OC-Luc/MAC in human MSC cells are responsive to positive and negative stimulation by 1 alpha,25-dihydroxyvitamin D3 and DEX in differentiation stage of MSCs to osteoblasts, reflecting the manner of physiological expression.
Conclusion
The OC-Luc/MAC reporter system may contribute not only to monitoring the osteogenic differentiation stage from MSC but also to identify novel osteogenic drugs.
Keywords: mammalian artificial chromosome, mesenchymal stem cells, osteogenic differentiation, osteocalcin, reporter assay
In the human body, the skeletal system is one of the largest organs, which undergoes remodeling throughout a person’s lifespan.1 Bone remodeling and fracture healing processes are thought to be mediated via osteoprogenitors and their ancestors, mesenchymal stem cells (MSCs).2 In in vitro studies, a protocol using dexamethasone (DEX) is generally accepted for induction of osteoblastic differentiation of MSCs.3 In a clinical setting, however, the use of DEX is not practical because of its adverse side effects such as glucocorticoid-induced osteoporosis.4 For successful treatment of bone-loss disorders, chemical compounds and bioactive substances that effectively induce bone formation are required.
In an effort to develop an evaluation system for bone remodeling, we previously generated transgenic mice that express the luciferase (Luc) reporter gene under the control of human osteocalcin enhancer/promoter sequences.5 Osteocalcin (OC) is the main non-collagenous hydroxyapatite-binding protein, which is synthesized by osteoblasts, odontoblasts and hypertrophic chondrocytes.6 It was recognized as a marker of bone formation6 and has been used for detection of osteoblast maturation and osteogenic activation. 7 Using in vivo bioluminescence imaging in the OC-Luc transgenic mice, Luc activity was induced throughout their skeletons by administration of 1,25-dihydroxyvitamin D3 (VD3), which regulates the expression of a number of genes including OC in bone cells. 8 Furthermore, enhanced bioluminescence was observed at the forced radius fracture site reflecting progression of the fracture repair. Although the OC-Luc transgenic mice would contribute to the final confirmation of drug efficacy selected from hundreds of candidates, an in vitro cell based-assay system is preferable for the first screening of high content candidate compounds.
Mammalian artificial chromosome vectors have been utilized for introduction of genes to recipient cells.9 As for the origin of their chromosomes, there are two kinds of artificial chromosomes, i.e. human artificial chromosome (HAC) and mouse artificial chromosome (MAC) that have been constructed by chromosome engineering. Since HAC or MAC is maintained as an independent chromosome in recipient cells, physiological regulation of the loaded gene is expected.10 Previously, feasibility of cell lineage-specific expression of a transgene by a HAC vector was demonstrated in a human MSC line.11 The GFP reporter gene under the regulation of human osteopontin (OPN) promoter loaded on a HAC vector was transferred to a human MSC line. OPN is another marker of bone formation, whose expression is upregulated during maturation of osteoblasts.7 The reporter GFP gene in the MSCs was specifically expressed in response to osteogenic differentiation induction in coordination with the transcription of endogenous OPN. In order to distinguish the osteoblast differentiation process from the subsequently occurred maturation processes accurately, however, reference reporter system, which can trace osteoblast differentiation steps, is required.
Our aim is setting up a screening platform to identify compounds that promote or induce differentiation of MSCs towards osteoblasts. To this end, we made a reporter construct composed of the Luc gene under the regulation of OC enhancer/promoter in a MAC vector (OC-Luc/MAC). The MSC lines carrying the OC-Luc/MAC vector were tested for differentiation ability to osteogenic lineage. The induction or restraint of Luc activity responding to the already-known positive stimulus via VD3 or negative stimulus via DEX treatment was also addressed for validation as monitor cells.
MATERIALS AND METHODS
Cell culture
A Chinese hamster ovary (CHO) hybrid cell line containing the MAC4 vector 10 was maintained in Ham’s F-12 nutrient mixture (Wako, Osaka, Japan) supplemented with 10 % fetal bovine serum (FBS) (Biowest,Kansas, MO). The MAC4 carries an EGFP gene, 5′HPRT-loxP and a hygromycin gene.10 A mouse A9 hybrid cell line carrying the MAC4 vector was maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Wako) supplemented with 10% FBS (Biowest). A human immortalized MSC line (hiMSC) 12 was maintained in DMEM (Wako) supplemented with 10% FBS (Biowest). To induce osteogenic differentiation, hiMSC-derived hybrid cells were fed with osteogenic differentiation medium (LONZA, Basel, Swiss Confederation). Cells were maintained at 37 ˚C with 5% CO2.
Construction of OC-Luc/MAC4 reporter vector
The plasmid pOC-Luc contains firefly Luc gene derived from pGL3 (Promega, Madison, WI) driven by 10 kb enhancer/promoter region of the human OC gene.5 The plasmid v907-3′HPRT/loxP contains the donor loxP site and latter half of the HPRT gene. The 14.4 kb AseI/SalI fragment containing the Luc gene expression cassette was excised from the pOC-Luc plasmid and ligated to the compatible AseI/SalI site of the v907-3′HPRT/loxP plasmid, yielding the v907 OC-Luc-ins-3′HPRT/loxP. Plasmid transfection was carried out by lipofection (Lipofectamine 2000, Invitrogen, Carlsbad, CA). Drug resistant colonies obtained by selection with HAT (Sigma-Aldrich, St.Louis, MO) were picked up and expanded. Presence of the sequence on OC-Luc/MAC4 was tested by PCR amplification.
Assessment of the hOC/Luc transcription activity in CHO/MAC4 cells
The HAT resistant CHO/MAC4 transfectants were plated (1 × 106 cells in 6 well cluster). The next day, cells were harvested and lysed in 100 mL lysis buffer (Reporter Lysis Buffer, Promega). An aliquot (20 mL) of lysate was mixed with 50 mL of Luc solution and measured with a luminometer (ALVO MX, Perkin Elmer, Waltham, MA). For OC-Luc stimulation study, the HAT resistant CHO/MAC4 transfectants were plated (1 × 106 cells in 35 mm dish). After treatment with 100 nM VD3 or vehicle (DMSO) for 6 h, cells were washed twice with phosphate-buffered saline, harvested and lysed in 100 mL lysis buffer. Lysate (20 mL) was mixed with 50 mL of Luc substrate. Luc activity was measured by a luminometer (ALVO MX, Perkin Elmer). Statistical analysis was performed using unpaired Student’s t-test (two-tailed).
Microcell mediated chromosome transfer
The OC-Luc/MAC4 vector was introduced into hiMSCs by microcell-mediated chromosome transfer (MMCT) as described previously.13, 14 While microcells prepared from the A9 donor cells were fused with hiMSCs using 47% polyethylene glycol (molecular weight 1000) (Wako) and 10% DMSO (Sigma-Aldrich), microcells from the CHO donor cells were fused by fusogenic envelope proteins derived from measles virus.14 Microcell hybrids were selected with HAT supplement (Sigma-Aldrich) for 2 weeks.
FISH
Preparation of metaphase chromosome spreads from the hybrid cells and fluorescence in situ hybridization (FISH) analyses were carried out with standard protocols. 13 Digoxigenin-labelled (Roche, Tokyo, Japan) mouse Cot-1 DNA (Invitrogen) and biotin-labelled (Roche) hOC-Luc DNA were used as probes. Chromosomal DNA of host cells was counterstained with DAPI (Sigma-Aldrich). Microscopic images were captured using the NIS-Elements system (Nikon, Tokyo). Thirty metaphase spreads were observed for each analysis.
Induction of osteogenic differentiation
The hiMSC cells (3 × 104 cells per well) were seeded in 6-well plates with growth medium. The next day, medium was changed to differentiation medium (LONZA) with or without 10 nM VD3 (Calbiochem, Tokyo). After 1 week of osteogenesis culture, alkaline phosphatase activity (ALP) and luciferase activity were tested.
Detection of ALP activity
Histochemical staining for ALP activity was carried out using a commercially available kit based on naphthol AS-MX phosphate and fast red violet B salt (Sigma-Aldrich). The cultured cells were fixed with citrate fixative containing 60% acetone for 30 s at room temperature, rinsed twice with deionized water, treated with ALP staining solution for 1 h, rinsed twice with deionized water and then photographed.
Luciferase assay
Cells were lysed by the addition of 100 µL Reporter Lysis Buffer (Promega) in culture vessels, followed by a freeze-thaw cycle. Protein concentration of the cell lysate was determined by ultraviolet absorption spectrophotometry. Luc activity was measured using the Pikkagene Luciferase Assay System (TOYO B-Net, Tokyo). Luc activity was normalized to the protein concentration of the cell lysate.
RESULTS
Preparation of the OC-Luc/MAC reporter vector in donor CHO cells
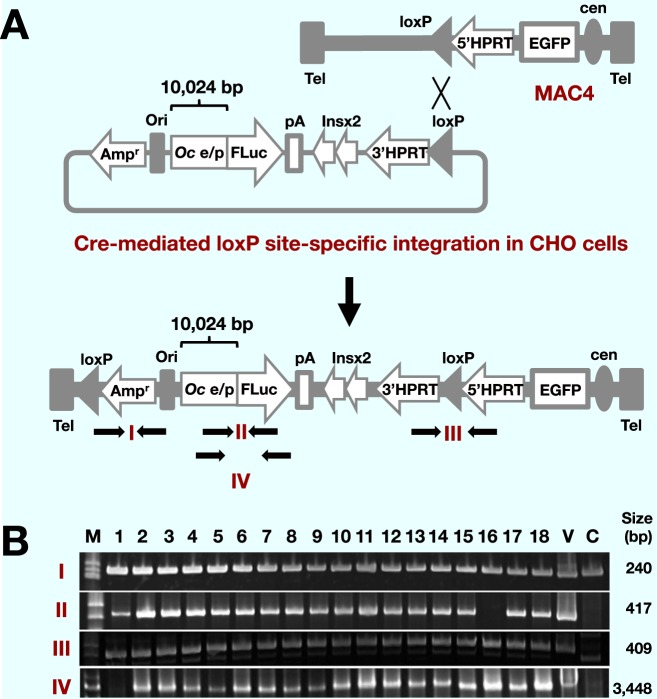
Stable maintenance of vectors in host cells is crucial for gene delivery studies. We chose the MAC4 vector as a platform for the OC-Luc reporter construct, because of its stable maintenance in cultured human cells in addition to mouse cells and transchromosomic mice.15 It carries the GFP gene driven by CAG promoter, which allows constitutive expression in various types of cells. As a regulatory sequence for the monitoring of OC gene expression, an approximately 10 kb genomic DNA fragment carrying upstream from the human OC gene was chosen ( Fig. 1A). The fragment contains a VD3-responsive element (VDRE), a GAGA motif and a TATA box.5 Co-transfection of the OC-Luc reporter construct and the Cre recombinase expression vector pBS185 into the CHO cells carrying the MAC4 vector yielded HAT resistant colonies after selection culture. Presence of the OC-Luc sequence in the HATr cell clones was first tested by PCR amplification of the OC-Luc and MAC4 specific sequences in genomic DNA of the candidate CHO clones (Fig. 1B). The junction sequence between the OC promoter and Luc gene was detected in almost all clones tested. The sequence spanning the 5′half and 3′half of the HPRT gene was also detected, indicating the correct site-specific insertion of the OC-Luc construct at the loxP site on the MAC4 vector.
Fig. 1.
Construction of the OC-Luc/MAC4 reporter vector in donor CHO cells.
A: A scheme of loading human osteocalcin luciferase (hOC-Luc) into MAC4. MAC4 has an EGFP gene driven by CAG promoter, 5′HPRT and acceptor loxP site. The OC-Luc construct is composed of 10 kb human osteocalcin enhancer/promoter sequences with 60 bp of the 5′-untranslated sequences (Oc e/p), a luciferase gene (FLuc), the SV40 late polyadenylation signal (pA), the two copies of insulator sequences (Ins), 3′HPRT and donor loxP site. The loxP site-specific integration reconstructs the HPRT gene that confers resistance to HAT selection. The predicted composition after the loxP site-specific integration is presented at the bottom. The arrows indicate PCR primers for detection of the OC-Luc in MAC4.
B: Presence of the OC-Luc sequences in HAT resistant CHO transfectants was detected by PCR amplification with the primer pairs indicated in A. Size of each amplicon is shown on the right side of the panel. C, CHO/MAC4 genomic DNA; M, size marker; V, vector DNA (OC/Luc) used for transfection.
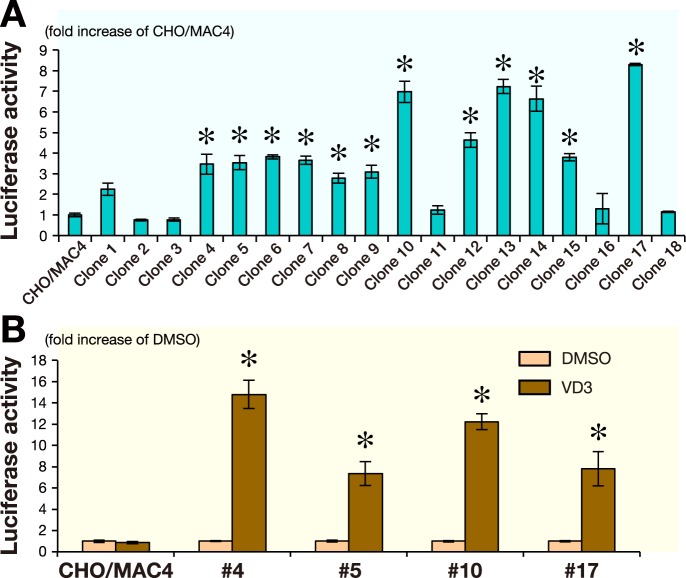
Since the 560 bp minimum promoter of the OC gene was previously found to exhibit promoter activity in CHO cells,16 we next addressed the functional maintenance of the OC-Luc reporter activity in CHO cells by Luc assay ( Fig. 2A ). Luc activity was substantially increased in CHO (OC-Luc/MAC4) cells, compared to the CHO (MAC4) cells in which the OC-Luc construct is absent. As the measured Luc activity varied among CHO clones, we arbitrarily chose the 5 clones showing relatively high Luc activity, and performed FISH analysis to examine the status of the MAC (Table 1). In each clone, among 20 metaphase spreads tested, over 60% showed diploid CHO karyotype plus one copy of the MAC. The retention rate of the MAC in CHO cells was high enough for use as an MMCT donor.
Fig. 2.
Detection of Luc activity in CHO cells.
A: Luciferase activity was assessed in the HAT resistant CHO transfectants. Measured activities were normalized to those of parental CHO/MAC4 cells. *P < 0.01: compared to the CHO/MAC4 control value.
B: Response of the OC/Luc to VD3 was tested in the HAT resisitant CHO transfectants. Luciferase activity was measured after 6 h incubation with or without 100 nM VD3. The measurements were normalized to those of cells treated with DMSO. *P < 0.01: compared to the DMSO control value.
The bar above each column represents SD determined from three independent measurements (A and B).
Table 1. Summary of FISH analyses in CHO (MAC4- hOC/Luc) cells.
| Clone# | MAC# | Others† | ||
| 0 | 1 | Integration* | ||
| 4 | 3 | 13 | 1 | 3 |
| 10 | 1 | 13 | 3 | 3 |
| 13 | 0 | 17 | 1 | 2 |
| 14 | 2 | 13 | 2 | 3 |
| 17 | 1 | 12 | 3 | 4 |
*MAC was integrated into a host chromosome(s).
†Ploidy of the host cells was elevated to tetraploid range.
Since CHO cells were reported to respond to VD3 stimulation through VD3 receptor expression,17 we next tested the OC-Luc reporter activity in the CHO cells (Fig.2B ). VD3 treatment induced Luc activity that confirmed the responsiveness of the OC regulatory sequence in the OC-Luc/Mac4 vector in the CHO background. Two CHO clones (#4 and #17) were arbitrarily chosen as the donor cells for the transfer of the OC-Luc/MAC4 vector to the human MSC cells. From CHO clone #4, the OCLuc/MAC4 vector was once transferred by MMCT to mouse A9 cells that were used as intermediate host cells because of being highly competent to produce micronuclei.
Transfer of the OC-Luc/MAC4 reporter vector to a mesenchymal stem cell line
A mesenchymal stem cell line hiMSC, which retains differentiation potential to osteogenic lineages12 provided recipient cells for the assessment of the OC-Luc/MAC4 reporter vector. To utilize the reconstructed HPRT gene as a selection marker for MMCT, an HPRT–subline established by 6-thioguanine selection was used. From the donor cells (A9#4 and CHO#17), MMCT was carried out to the hiMSC (HPRT–) cells. As a result of HAT selection culture, five and one HPRTr hybrids were obtained from the two OC-Luc/MAC4 donor clones #4 and #17, respectively. Retention of the OC-Luc/MAC4 was addressed by FISH analysis ( Fig. 3A). Karyotypes of the metaphase spreads were not necessarily uniform and a few variations were observed (data not shown). Since the extent of the variation differed among the clones, we chose the two hiMSC clones (#4 and #17) showing the highest retention score (over 40%) of a single copy MAC without host chromosome integration.
Fig. 3.
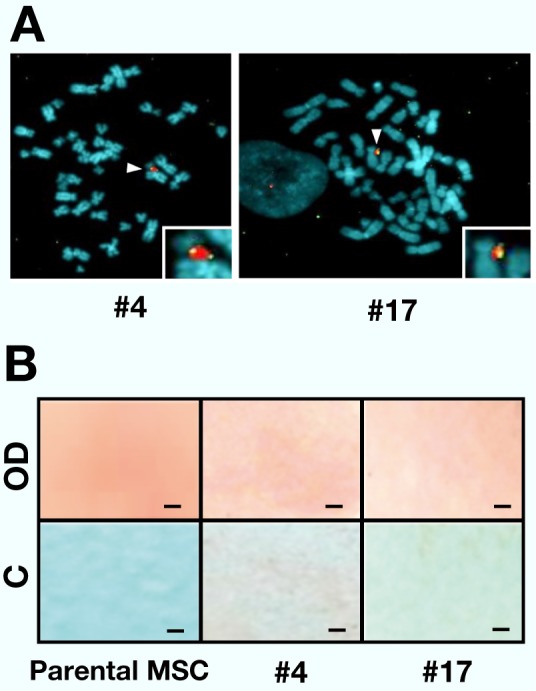
Detection and characterization of the OC-Luc/MAC4 vector in hiMSC hybrid cells.
A: Representative metaphase spreads of FISH of the hiMSC hybrid cells. Digoxigenin-labelled mouse Cot-1 DNA (red) was used for detection of the MAC4. Biotin-labelled OC-Luc DNA (green) was used for detection of the OC-Luc cassette in the MAC4. Chromosomal DNA in the host hiMSC cell was counterstained with DAPI (blue). White arrowheads indicate the OC-Luc/MAC4 vectors and the insets show enlarged images of the OC-Luc/MAC4.
B: ALP staining of the hiMSC hybrid and parental cells. Photographs of the fixed and stained cells on the culture vessel were presented. Upper panels show the cells cultured for 7 days in the differentiation induction medium (OD), and lower panels show the cells cultured for 7 days in the growth medium (C). Red dye staining indicates ALP activity. Scale bar shows 1 mm.
Evaluation of the OC-Luc/MAC4 reporter vector in the mesenchymal stem cell hybrids
As differentiation potential is a prerequisite for monitoring the transcriptional regulation of the OC-Luc reporter gene, the hiMSC hybrids were cultured in osteogenic differentiation medium, which contains DEX, ascorbic acid and beta-glycerophosphate.3 During osteogenic differentiation of MSCs, ALP activity is upregulated in the early stage.7 ALP activity was addressed by histochemical staining assay at day 7 (Fig. 3B). In the hiMSC hybrid cells, qualitative difference in ALP activity between DEX treated and untreated culture was detected, indicating that the hiMSC hybrid cells retain osteogenic differentiation potential.
Next, the hiMSC hybrid cells were tested for their OC-Luc activity in response to a given stimulation. As agonistic and antagonistic compounds for the OC transcription, VD3 and DEX were used, respectively (Fig. 4). While culture for 7 days in differentiation medium with DEX scarcely induced luciferase activity, addition of VD3 in either growth medium or differentiation medium affected the induction of Luc activity. These results support the previously reported effects of VD3 and DEX for OC transcriptional regulation in the early stage of osteogenic differentiation of human MSCs.3, 18 It was noted that combined application of VD3 to the DEX medium exceeded the extent of activation by VD3 treatment alone, suggesting a synergistic effect of differentiation induction and VD3.
Fig. 4.
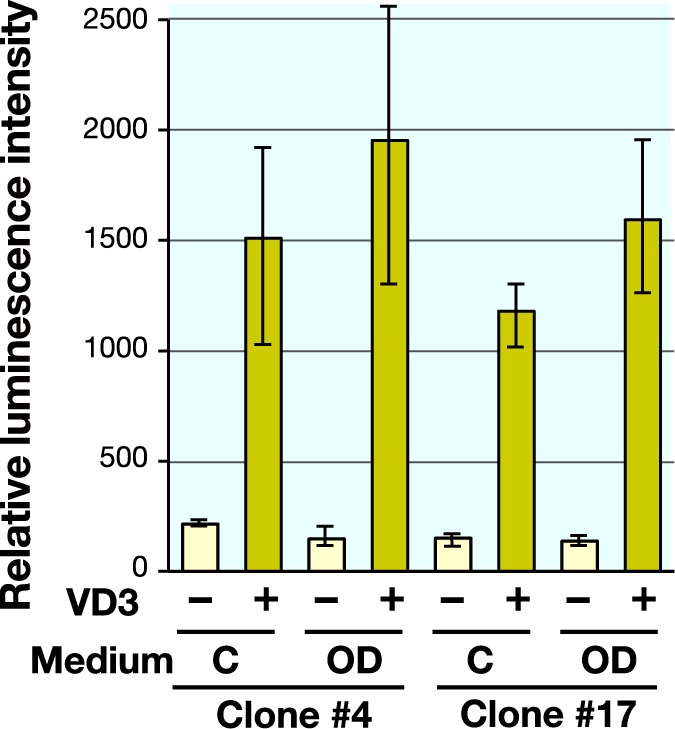
Responsiveness of the OC-Luc activity was tested against VD3 treatment and osteogenic induction medium containing DEX. Effect of VD3 addition (10 nM) was tested in culture medium (C) and osteogenic induction medium (OD) at day 7. Data are expressed by mean values and distribution range from duplicate experiment.
The MAC4 vector is equipped with the EGFP gene under the regulation of CAG promoter (Fig. 1A). GFP fluorescence was consistently observed regardless of culture medium compositions and added supplements (data not shown), indicating the utility of GFP as an internal control for the evaluation by the OC-Luc reporter system.
DISCUSSION
In addition to its role in systemic calcium homeostasis, VD3 affects cell proliferation, differentiation and function of cells expressing the vitamin D receptor. 19 VD3 was reported to enhance the differentiation of hMSCs to osteoblasts, suggesting a potential autocrine/paracrine role of VD3 in osteoblast differentiation. 20 On the contrary, DEX contained in the osteogenic differentiation medium3 negatively regulates OC transcription through glucocorticoid receptor during osteogenic lineage commitment of MSCs. 21 It was also reported that glucocorticoid receptor binding interfered with the OC basal promoter.22 In the present study, we showed that OC-Luc activity in the MSC hybrid cells was stimulated by VD3 and restrained by DEX. These results suggest that the OC-Luc reporter construct is faithfully regulated by positive and negative stimulation during the differentiation of MSCs.
Comparison of VD3 and DEX effects on OC induction was reported in human marrow stromal cells.18 The report demonstrated no apparent induction of OC mRNA expression by DEX, moderate induction by VD3 and prominent induction by combined application of DEX and VD3. For the screening of substances using our OC-Luc reporter cells system, four culture medium compositions are supposed, i.e. the MSC growth medium with or without VD3 and the osteogenic differentiation medium containing DEX with or without VD3. Detection of OC-Luc activation in the media without VD3 may be read as identifying the substitution for DEX or novel differentiation inducer. In the media with VD3, it is expected that the synergistic enhancer for VD3 has been found.
In addition to optimizing the composition of culture medium, control of stem cell fate has been explored in the aspect of signal transduction by adhesion and mechanosensory stimuli from the extracellular matrix and substratum. Integrin-dependent cellular interactions with the extracellular matrix could control the differentiation of stem cells.23 Another report described that the elasticity of the underlying substratum influences the differentiation of MSCs.24 Moreover, surface characteristics of substratum, like topography, surface chemistry and surface energy, are critical parameters for the adhesion of stem cells to biomaterials and for differentiation into the osteogenic lineage. In the development of new materials for medical implants, these parameters have to be taken into account. 25 The OC-Luc/MAC repoter system may be applicable for evaluation of biophysical signaling from culture substratum.
In this study, we established an evaluation system, which can trace differentiation of MSCs toward osteoblast by monitoring Luc activity under the control of OC gene regulatory sequences. By utilizing this system, the ability of a given chemical and/or physical stimulation to either facilitate or retard osteoblastic differentiation could be monitored in a time-dependent manner. Consequently, our approach could offer a qualified tool for temporal monitoring of osteogenesis in studies dealing with drug screening and tissue engineering.
Acknowledgments
Acknowledgments:We thank Professor Hiroyuki Kugoh for his invaluable advice and critical review of this research.
This study was supported in part by Regional Innovation Strategy Support Program by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (M.O.).
The author declares no conflict of interest.
REFERENCES
- 1. Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003; 423: 349-55. . [DOI] [PubMed] [Google Scholar]
- 2. Wang X, Wang Y, Gou W, Lu Q, Peng J, Lu S. Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. Int Orthop. 2013; 37: 2491-8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997; 64: 295-312. . [PubMed] [Google Scholar]
- 4. Bultink IE, Baden M, Lems WF. Glucocorticoid-induced osteoporosis: an update on current pharmacotherapy and future directions. Expert Opin Pharmacother. 2013; 14: 185-97. . [DOI] [PubMed] [Google Scholar]
- 5. Nakanishi T, Kokubun K, Oda H, Aoki M, Soma A, Taniguchi M, et al. Bioluminescence imaging of bone formation using hairless osteocalcin-luciferase transgenic mice. Bone. 2012; 51: 369-75. . [DOI] [PubMed] [Google Scholar]
- 6. Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989; 69: 990-1047. . [DOI] [PubMed] [Google Scholar]
- 7. Lian JB, Stein GS. Development of the osteoblast phenotype: molecular mechanisms mediating osteoblast growth and differentiation. Iowa Orthop J. 1995; 15: 118-40. . [PMC free article] [PubMed] [Google Scholar]
- 8. Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 2013; 92: 77-98. . [DOI] [PubMed] [Google Scholar]
- 9. Oshimura M, Katoh M. Transfer of human artificial chromosome vectors into stem cells. Reprod Biomed Online. 2008; 16: 57-69. . [DOI] [PubMed] [Google Scholar]
- 10. Takiguchi M, Kazuki Y, Hiramatsu K, Abe S, Iida Y, Takehara S. A novel and stable mouse artificial chromosome vector. ACS Synth Biol. Epub 2012 Oct 17. . [DOI] [PubMed] [Google Scholar]
- 11. Ren X, Katoh M, Hoshiya H, Kurimasa A, Inoue T, Ayabe F. A novel human artificial chromosome vector provides effective cell lineage-specific transgene expression in human mesenchymal stem cells. Stem Cells. 2005; 23: 1608-16. . [DOI] [PubMed] [Google Scholar]
- 12. Okamoto T, Aoyama T, Nakayama T, Nakamata T, Hosaka T, Nishijo K. Clonal heterogeneity in differentiation potential of immortalized human mesenchymal stem cells. Biochem Biophys Res Commun. 2002; 295: 354-61.. [DOI] [PubMed] [Google Scholar]
- 13. Kugoh H, Mitsuya K, Meguro M, Shigenami K, Schulz TC, Oshimura M. Mouse A9 cells containing single human chromosomes for analysis of genomic imprinting. DNA Res. 1999; 6: 165-72. . [DOI] [PubMed] [Google Scholar]
- 14. Katoh M, Kazuki Y, Kazuki K, Kajitani N, Takiguchi M, Nakayama Y. Exploitation of the interaction of measles virus fusogenic envelope proteins with the surface receptor CD46 on human cells for microcell-mediated chromosome transfer. BMC Biotechnol. 2010; 10: 37. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kazuki K, Takehara S, Uno N, Imaoka N, Abe S, Takiguchi M. Highly stable maintenance of a mouse artificial chromosome in human cells and mice. Biochem Biophys Res Commun. 2013; 442: 44-50. . [DOI] [PubMed] [Google Scholar]
- 16. Takahashi Y, Tsuji S, Kazuki Y, Noguchi M, Arifuku I, Umebayashi Y. Development of evaluation system for bioactive substances using human artificial chromosome-mediated osteocalcin gene expression. J Biochem. 2010; 148: 29-34. . [DOI] [PubMed] [Google Scholar]
- 17. Dokoh S, Donaldson CA, Marion SL, Pike JW, Haussler MR. The ovary: a target organ for 1,25-dihydroxyvitamin D3. Endocrinology. 1983; 112: 200-6. . [DOI] [PubMed] [Google Scholar]
- 18. Beresford JN, Joyner CJ, Devlin C, Triffitt JT. The effects of dexamethasone and 1,25-dihydroxyvitamin D3 on osteogenic differentiation of human marrow stromal cells in vitro. Arch Oral Biol. 1994; 39: 941-7. . [DOI] [PubMed] [Google Scholar]
- 19. Geng S, Zhou S, Bi Z, Glowacki J. Vitamin D metabolism in human bone marrow stromal (mesenchymal stem) cells. Metabolism. 2013; 62: 768-77. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu P, Oyajobi BO, Russell RG, Scutt A. Regulation of osteogenic differentiation of human bone marrow stromal cells: interaction between transforming growth factor-beta and 1,25(OH)(2) vitamin D(3) In vitro. Calcif Tissue Int. 1999; 65: 173-80. . [DOI] [PubMed] [Google Scholar]
- 21. Leclerc N, Noh T, Khokhar A, Smith E, Frenkel B. Glucocorticoids inhibit osteocalcin transcription in osteoblasts by suppressing Egr2/Krox20-binding enhancer. Arthritis Rheum. 2005; 52: 929-39. . [DOI] [PubMed] [Google Scholar]
- 22. Meyer T, Gustafsson JA, Carlstedt-Duke J. Glucocorticoiddependent transcriptional repression of the osteocalcin gene by competitive binding at the TATA box. DNA Cell Biol. 1997; 16: 919-27. . [DOI] [PubMed] [Google Scholar]
- 23. Martino MM, Mochizuki M, Rothenfluh DA, Rempel SA, Hubbell JA, Barker TH. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials. 2009; 30: 1089-97. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006; 126: 677-89. . [DOI] [PubMed] [Google Scholar]
- 25. Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000; 21: 667-81. . [DOI] [PubMed] [Google Scholar]