Abstract
Background
Surgery has been reported to suppress cell-mediated immunity; however, the detailed mechanisms responsible for this remain unclear. This study determined the expression of lymphocyte activation gene 3 (LAG-3) and programmed cell death 1 (PD-1) in lymphocytes following surgery for gastric cancer.
Methods
LAG-3 and PD-1 expression on both CD4+ and CD8+ T cells obtained pre- and post-operatively from gastric cancer patients were evaluated by multicolor flow cytometry.
Results
The total lymphocyte count decreased rapidly from preoperative levels, reaching a minimum on postoperative day 1 and remaining significantly decreased on days 3 and 7. PD-1+CD4+ T cells significantly increased, reaching a maximum on postoperative day 1 and remaining significantly elevated on day 3. PD-1+CD8+ T cells significantly increased and reached a maximum on day 7 before returning to the preoperative level on day 30. There were no statistically significant differences in the frequency of LAG-3+CD4+ or LAG-3+CD8+ T cells after surgery. There were significant positive correlations between PD-1 and LAG-3 expression on both CD4+ andCD8+ T cells.
Conclusion
PD-1 and LAG-3 expression on both CD4+ and CD8+ T cells was up-regulated and might be related to impaired cell-mediated immunity after surgery for gastric cancer.
Keywords: gastric cancer, lymphocyte activation gene 3, programmed cell death 1, T-cell
Gastric cancer is one of the most common malignancies and ranks second among all cancer deaths worldwide. 1 Radical (R0) resection offers the best chance for a cure because it involves the complete surgical removal of any residual cancer cells in the tumor bed. However, a substantial number of patients experience recurrence even after R0 resection because of the occurrence of micrometastasis. Therefore, Japanese gastric cancer treatment guidelines recommend adjuvant chemotherapy with TS-1 for stage II/III gastric cancer patients to control micrometastasis and prevent recurrence.2 This recommendation is based on the results of the Adjuvant Chemotherapy Trial of TS-1 for Gastric Cancer study, which showed a survival benefit with adjuvant chemotherapy after D2 gastrectomy compared with surgery alone for stage II/III gastric cancer patients.3
The immune response is also thought to control micrometastasis. Yokohama et al. demonstrated in a mouse model of human gastric cancer micrometastasis that spontaneous regression of isolated tumor cells in the regional lymph nodes occurred following removal of the primary tumor and that this appeared to be primarily through natural killer (NK) cell mediated antitumor activity. 4 However, surgical stress generally induces suppression of cell-mediated immunity that is harmful to patients with cancer because it may diminish their ability to prevent postoperative infections and tumor recurrence.5– 7 T cells are a critical component of cellular immunity that are involved in the recruitment and activation of effector cells and amplification of the specific immune response to pathogens and cancer cells. T cell dysfunctions are characterized by diminished cytokine production, impaired killing and hypoproliferation. Wichmann et al. demonstrated a significant decrease in cell numbers of lymphocyte subpopulations, such as helper T (Th) lymphocytes, cytotoxic T lymphocytes and NK cells. 5 Brune et al. reported that interferon-gamma, tumor necrosis factor-alpha and interleukin-2 production by T cells decreases significantly after conventional cholecystectomy.6 Decker et al. showed that surgical stress induces a shift in the Th1/Th2 balance toward Th2, suggesting that cell-mediated immunity is downregulated and antibody-mediated immunity is upregulated after surgery. 7 However, the detailed mechanisms responsible for T cell dysfunction after surgery remain unclear.
Recently, several intrinsic factors, including lymphocyte-activation gene 3 (LAG-3) and programmed cell death 1 (PD-1), have been recognized as negative regulatory proteins that block the expansion and suppress the effector function of antigen-specific CD8+ T cells. 8–10 PD-1 (CD279) was identified as a gene that was upregulated in a T cell hybridoma undergoing apoptotic cell death. 11 PD-1 is inducibly expressed on CD4+ T cells, CD8+ T cells, natural killer-like T cells, B cells and monocytes upon activation. 11, 12 Recent studies have shown that PD-1 is highly expressed by CD8+ T cells during chronic lymphocytic choriomeningitis virus infection and that the PD-1–PD-1 ligand (PD-L) pathway plays a major role in regulating T cell exhaustion during infection.13 Furthermore, several new studies suggest a role for the PD-1–PD-L pathway in the exhaustion of virus-specific CD8+ T cells during HIV infection. Several reports have shown that PD-1 expression is elevated on HIV-specific CD8+ T cells and that blocking the PD-1–PD-L pathway leads to increased T cell proliferation and effector cytokine production.14– 16 Previous work has shown that PD-L1 is upregulated in HIV infection.17 Collectively, these observations suggest that the PD-1–PD-L pathway may indeed be operating during chronic HIV infection.
LAG-3, a CD4+ homolog that binds to major histocompatibility complex class II, 18 is expressed on activated T cells, NK cells, B cells and dendritic cells, plays an important but incompletely understood role in the function of these lymphocyte subsets.19– 21 Recent studies have documented a role for LAG-3 in CD8+ T cell exhaustion during chronic viral infection22 and LAG-3 negatively regulated the tumor-infiltrating NY-ESO-1-specific CD8+ T cells in human ovarian cancer.23 Demeure et al. reported that increased expression of LAG-3 was found on tumor-infiltrating lymphocytes (TILs) isolated from human renal cell carcinomas and other tumors, such as melanomas and lymphomas. 24 Importantly, LAG-3 is closely correlated with the dysfunction of T cells in patients with chronic viral infection22 and cancer.23
Given the association between PD-1 and LAG-3 expression on CD4+ and CD8+ T cells and T cell dysfunction in HIV, HCV and cancer patients, it is likely that these might be related to suppression of cell-mediated immunity after surgery. The purpose of the current study was therefore to examine the expression of PD-1 and LAG-3 on lymphocytes to determine the role of these molecules in the immune suppression that occurs after surgery for gastric cancer.
MATERIALS AND METHODS
Patients
This study enrolled 33 patients treated at Tottori University Hospital who had a pathological diagnosis of gastric adenocarcinoma. None of the patients received radiotherapy, chemotherapy or other medical interventions before surgery. The clinicopathological characteristics of these patients, according to the Japanese Classification of Gastric Carcinoma 25 are shown in Table 1.
Table 1. Clinicopathological characteristics of the patients included in the current study.
| Age, mean (SD) [range], yr | 66.7 (11.8) [55–79] | |
| Gender | Male | 26 |
| Female | 7 | |
| Histopathology* | Differentiated | 15 |
| Undifferentiated | 18 | |
| Depth of invasion† | T1 (early) | 19 |
| T2/T3/T4 (advanced) | 14 | |
| Lymph node metastasis | Absent | 22 |
| Present | 11 | |
| Stage | Stage I/II | 25 |
| Stage III/IV | 8 | |
*Differentiated, papillary or tubular adenocarcinoma; undifferentiated, poorly differentiated or mucinous adenocarcinoma, or signet-ring cell carcinoma.
†T1, tumor has invaded lamina propria or submucosa; T2, tumor has invaded the muscularis propria or the subserosa; T3, penetrating the serosa; T4, invading adjacent organs.
The study protocol was approved by the Institutional Review Board at Tottori University Hospital. Informed consent for drawing of blood samples was obtained from all individuals.
Assessments
Leukocyte and lymphocyte counts, C-reactive protein (CRP) levels and PD-1 and LAG-3 expression on CD4+ and CD8+ T cells were determined before surgery and on postoperative days 1, 3, 7 and 30.
Preparation of peripheral blood mononuclear cells
After informed consent was obtained, approximately 40 mL of peripheral blood was drawn from the patients before surgery and on postoperative days 1, 3, 7 and 30. Blood samples were prepared by centrifugation over a Ficoll-Paque (Pharmacia, Uppsala, Sweden) density gradient.
Flow cytometry analysis
Fluorescence-activated cell sorting (FACS) analysis was performed using a FACS Calibur (Becton Dickinson, Franklin Lakes, NJ). The following antibodies were used to determine cell phenotype: anti-CD4-FITC (RPA-T4), anti-CD8-FITC (HIT8a), anti-PD-1-PE (EH12.1) (BD Pharmingen, Franklin Lakes, NJ), anti-LAG-3-PE (R&D Systems, Minneapolis, MN) and anti-CD3-PE-Cy5 (UCHT1) (BD Pharmingen, Franklin Lakes, NJ). Furthermore, the following antibodies were used for negative control: FITC mouse immunoglobulin G1 (IgG1), PE mouse IgG1, PE-Cy5 mouse IgG1 (clone MOPC-21; BD Pharmingen, Franklin Lakes, NJ) and goat IgG PE-conjugated antibody (R&D Systems).
Statistical analysis
Statistical differences between two groups were determinedby paired t-test. The accepted level of significance was P < 0.05. The GraphPad Prism software (GraphPadSoftware, La Jolla, CA) was used for all statistical analyses.
RESULTS
Absolute leukocyte and lymphocyte counts and CRP levels
The total leukocyte count increased postoperatively and reached a maximum on day 1. Although it then decreased, it was still significantly elevated on day 3 ( Fig. 1a). A significant increase in CRP levels was also observed on postoperative days 1, 3 and 7, compared with preoperative values (Fig. 1b). In contrast, a rapid and significant decrease in the total lymphocyte count was observed on day 1 after surgery (Fig. 1c). The lymphocyte count reached a minimum on day 1 and then increased, but was still significantly lower than before surgery on days 3 and 7.
Fig. 1.
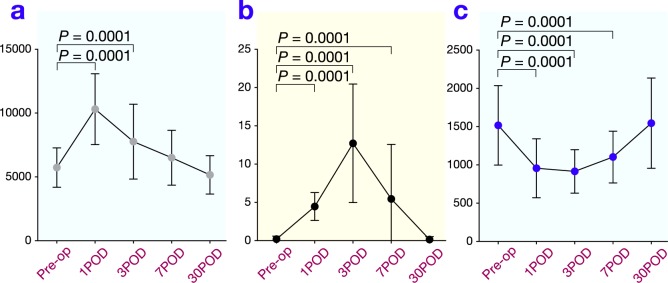
Changes in total leukocyte count (a), serum concentration of C-reactive protein (CRP) (b) and total lymphocyte count (c) after surgery. POD, postoperative day; Pre-op, preoperation.
PD-1 and LAG-3 expression on CD4+ and CD8+ T cells
The frequency of PD-1+CD4+ T cells significantly increased postoperatively and reached a maximum on day 1. It then decreased, but remained significantly elevated on day 3 (Fig. 2). The frequency of LAG-3+CD4+ T cells also increased and was slightly higher than the preoperative level on day 3 (Fig. 3; P = 0.098). The frequency of PD-1+CD8+ T cells significantly increased and reached a maximum on day 7 after surgery before returning to the preoperative level on day 30 (Fig. 4 ). The frequency of LAG-3+CD8+ T cells was slightly higher on day 7 than the preoperative level (Fig. 5; P = 0.077).
Fig. 2.
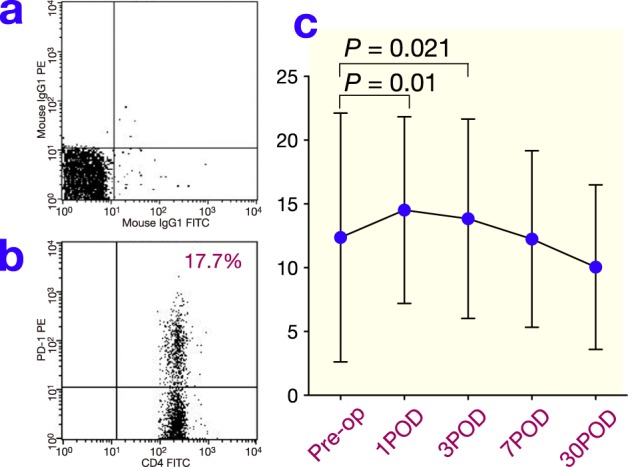
(a) The reaction of isotypic control to CD4+ T cells after surgery by FACS. (b) Representative results for PD-1 on CD4+ T cells in peripheral blood obtained from gastric cancer patients after surgery by FACS. (c) Changes in PD-1 expression on CD4+ T cells after surgery. POD, postoperative day; Pre-op, preoperation.
Fig. 3.

(a) The reaction of isotypic control to CD4+ T cells after surgery by FACS. (b) Representative results for LAG-3 on CD4+ T cells in peripheral blood obtained from gastric cancer patients after surgery by FACS. (c) Changes in LAG-3 expression on CD4+ T cells after surgery. POD, postoperative day; Pre-op, preoperation.
Fig. 4.
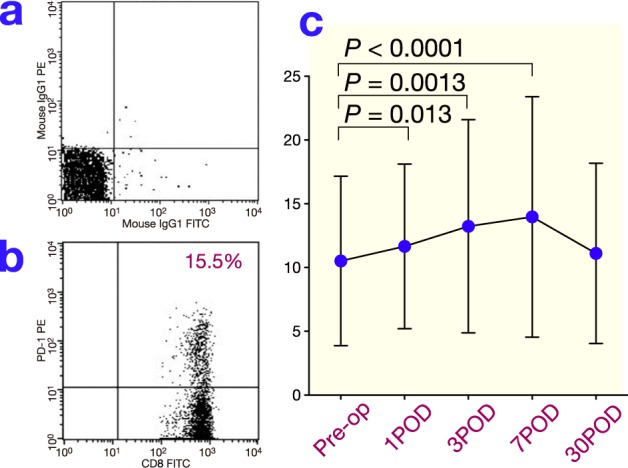
(a) The reaction of isotypic control to CD8+ T cells after surgery by FACS. (b) Representative results for PD-1 on CD8+ T cells in peripheral blood obtained from gastric cancer patients after surgery by FACS. (c) Changes in PD-1 expression on CD8+ T cells after surgery. POD, postoperative day; Pre-op, preoperation.
Fig. 5.
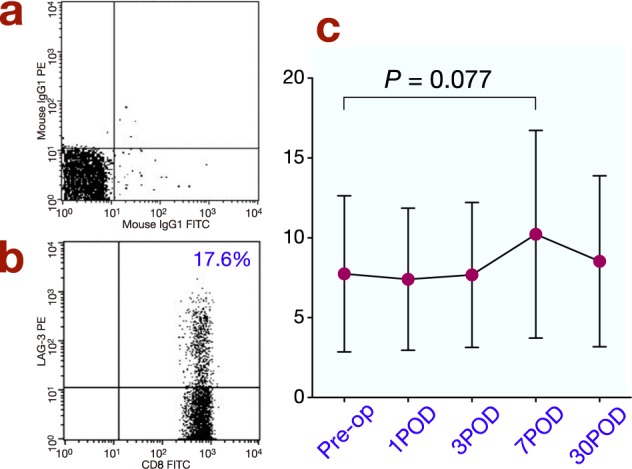
(a) The reaction of isotypic control to CD8+ T cells after surgery by FACS. (b) Representative results for LAG-3 on CD8+ T cells in peripheral blood obtained from gastric cancer patients after surgery by FACS. (c) Changes in LAG-3 expression on CD8+ T cells after surgery. POD, postoperative day; Pre-op, preoperation.
Because increases in both PD-1 and LAG-3 expression on CD4+ T cells were observed on day 3, the correlation between PD-1 and LAG-3 expression on CD4+ T cells on day 3 was determined; a significant positive correlation was found (Fig. 6a; r = 0.39, P = 0.024). Increases in PD-1 and LAG-3 expression on CD8+ T cells were observed on day 7 and a significant positive correlation was found on day 7 (Fig. 6b; r = 0.37, P = 0.033).
Fig. 6.

Correlation between PD-1 and LAG-3 expression on CD4+ (a) and CD8+ T cells (b).
DISCUSSION
During the perioperative and postoperative periods, a complex biological response takes place in response to surgical stress. This response is intended to restore homeostasis as one aspect of host defenses against surgical stress and is thus a very important response for the host. However, surgical stress induces suppression of cell-mediated immunity that is harmful to patients with cancer who have undergone surgery because it may diminish their ability to prevent postoperative infections. Importantly, surgical stress-induced suppression of cell-mediated immunity may also place a patient at higher risk for tumor recurrence. T cells are a critical component of cellular immunity and in the current study the absolute number of lymphocytes significantly decreased after surgery in gastric cancer patients. There was evidence of impaired cell-mediated immunity after operation, consistent with a previous report by Wichmann et al.5
Regulatory pathways that limit the immune response of T cells are becoming increasingly well characterized. For instance, cosignaling molecules are cell-surface glycoproteins that can direct, modulate and fine-tune T cell receptor signals. On the basis of their functional outcome, cosignaling molecules can be divided into co-stimulators and coinhibitors, which promote or suppress T cell activation, respectively. Through expression at the appropriate time and location, cosignaling molecules positively and negatively control the priming, growth, differentiation and functional maturation of a T cell response.26 PD-1 and LAG-3 are coinhibitors. In the current study, we determined the PD-1 and LAG-3 expression on both CD4+ and CD8+ T cells to demonstrate the detailed mechanisms responsible for the impaired cell immunity observed after surgery in gastric cancer patients. The expression of both PD-1 and LAG-3 was up-regulated on CD4+ and CD8+ T cells after surgery. We have previously reported that PD-1 expression on CD4+ and CD8+ T cells was increased compared with normal controls in gastric cancer patients. 27 Furthermore, PD-1-positive CD4+ and CD8+ T cells produced significantly less interferon-gamma than PD-1-negative CD4+ and CD8+ T cells, indicating that upregulation of PD-1 on both CD4+ and CD8+ T cells may be, in part, responsible for the immune evasion observed in gastric cancer patients. 27 The current results indicate that surgical stress induces further suppression of cell-mediated immunity in the postoperative period. Although increases in LAG-3 expression observed in the current study were less than those for PD-1, LAG-3 expression tended to be higher than preoperative levels during the postoperative period. Turnis et al. demonstrated the coexpression of LAG-3 and PD-1 by TILs.28, 29 This coexpression is enriched in lymphocytes infiltrating certain human cancers and correlates with impaired CD8+ effector function. Woo et al. demonstrated that LAG-3 and PD-1 synergistically regulate T cell function to promote tumoral immune escape in mice. 30 Furthermore, Okazaki et al. showed that PD-1 and LAG-3 act synergistically to prevent autoimmunity in mice.31 These data show that synergistic action of both LAG-3 and PD-1 plays an extremely important role in regulating T cell function in various situations. In fact, there were significant positive correlations between PD-1 and LAG-3 expression in both CD4+ and CD8+ T cells in the current study.
Recently, regulatory pathways that limit the immune response to cancer have attracted considerable attention as an important target for immunotherapy. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) is one of the representative molecules that downregulates T cell activation pathways. 32 Ipilimumab, a fully human monoclonal antibody that blocks CTLA-4, has recently been shown to promote antitumor immunity and improve overall survival in patients with previously treated metastatic melanoma.33 Furthermore, it has been demonstrated that treatment with the anti-PD-1 antibody pembrolizumab (lambrolizumab) resulted in a high rate of sustained tumor regression, with mainly grade 1 or 2 adverse events, in patients with advanced melanoma, including those who had disease progression during previous treatment with ipilimumab.34 These results clearly indicate that immunotherapy targeting immune checkpoint molecules is effective for cancer therapy. The suppression of cell-medicated immunity induced by gastric cancer surgery puts patients at high risk of recurrence. However, adjuvant chemotherapy cannot be given immediately after surgery to prevent recurrence because it is likely that early initiation of chemotherapy increases the risk of complications. In contrast, immunotherapy targeting immune checkpoint molecules might be well suited to this situation. Because PD-1 and LAG-3 expression on both CD4+ and CD8+ T cells is upregulated after gastric cancer surgery, immunotherapy targeting PD-1 and LAG-3 may be effective in the treatment of gastric cancer. However, the correlation between overexpression of PD-1 and LAG-3 on lymphocytes and dysfunction of cell-mediated immunity in postoperative situation has not been determined in the current study. Further investigations are urgently required to show the possibility of treatment targeting PD-1 and LAG-3 for the treatment of gastric cancer patients after surgery.
In conclusion, PD-1 and LAG-3 expression on both CD4+ and CD8+ T cells was upregulated and might be related to impaired cell-mediated immunity after surgery for gastric cancer.
The author declares no conflict of interest.
REFERENCES
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Canaer J Clin. 2011; 61: 69-90. . [DOI] [PubMed] [Google Scholar]
- 2. Japanese Gastric Cancer Association. [Japanese gastric cancer treatment guidelines 2014 (ver. 4)]. Tokyo: Kanehara-shuppan; 2014. Japanese. [Google Scholar]
- 3. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007; 357: 1810-20. . [DOI] [PubMed] [Google Scholar]
- 4. Yokoyama H, Nakanishi H, Kodera Y, Ikehara Y, Ohashi N, Ito Y. Biological significance of isolated tumor cells and micrometastasis in lymph nodes evaluated using a green fluorescent protein-tagged human gastric cancer cell line. Clin Cancer Res. 2006; 12: 361-8. . [DOI] [PubMed] [Google Scholar]
- 5. Wichmann MW, Huttl TP, Winter H, Spelsberg F, Angele MK, Heiss MM. Immunological effects of laparoscopic vs open colorectal surgery: a prospective clinical study. Arch Surg. 2005; 140: 692-7. . [DOI] [PubMed] [Google Scholar]
- 6. Brune IB, Wilke W, Hensler T, Holzmann B, Siewert JR. Downregulation of T helper type 1 immune response and altered pro-inflammatory and anti-inflammatory T cell cytokine balance following conventional but not laparoscopic surgery. Am J Surg. 1999; 177: 55-60. . [DOI] [PubMed] [Google Scholar]
- 7. Decker D, Schondorf M, Bidlingmaier F, Hirner A, von Ruecker AA. Surgical stress induces a shift in the type-1/ type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up-regulation of antibody-mediated immunity commensurate to the trauma. Surgery. 1996; 119: 316-25. . [DOI] [PubMed] [Google Scholar]
- 8. Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223). J Immunol. 2005; 174: 688-95. . [DOI] [PubMed] [Google Scholar]
- 9. Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003; 33: 3117-26. . [DOI] [PubMed] [Google Scholar]
- 10. Gajewski TF, Fallarino F, Fields PE, Rivas F, Alegre ML. Absence of CTLA-4 lowers the activation threshold of primed CD8+ TCR-transgenic T cells: lack of correlation with Src homology domain 2-containing protein tyrosine phosphatase. J Immunol. 2001; 166: 3900-7. . [DOI] [PubMed] [Google Scholar]
- 11. Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006; 27: 195-201. . [DOI] [PubMed] [Google Scholar]
- 12. Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005; 23: 515-48. . [DOI] [PubMed] [Google Scholar]
- 13. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006; 439: 682-7. . [DOI] [PubMed] [Google Scholar]
- 14. Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006; 203: 2281-92. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006; 443: 350-4. . [DOI] [PubMed] [Google Scholar]
- 16. Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B. Upregulation of PD-1 expression on HIVspecific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006; 12: 1198-202. . [DOI] [PubMed] [Google Scholar]
- 17. Trabattoni D, Saresella M, Biasin M, Boasso A, Piacentini L, Ferrante P. B7-H1 is up-regulated in HIV infection and is a novel surrogate marker of disease progression. Psychiatr Serv. 2003; 101: 2514-20. . [DOI] [PubMed] [Google Scholar]
- 18. Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E. LAG-3, a novel lymphocyte activation gene closely related to CD4 J Exp Med. 1990; 171: 1393-405. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huard B, Gaulard P, Faure F, Hercend T, Triebel F. Cellular expression and tissue distribution of the human LAG-3-encoded protein, an MHC class II ligand. Immunogenetics. 1994; 39: 213-7. . [DOI] [PubMed] [Google Scholar]
- 20. Kisielow M, Kisielow J, Capoferri-Sollami G, Karjalainen K. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur J Immunol. 2005; 35: 2081-8. . [DOI] [PubMed] [Google Scholar]
- 21. Workman CJ, Wang Y, El Kasmi KC, Pardoll DM, Murray PJ, Drake CG. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol. 2009; 182: 1885-91. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009; 10: 29-37. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010; 107: 7875-80. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Demeure CE, Wolfers J, Martin-Garcia N, Gaulard P, Triebel F. T Lymphocytes infiltrating various tumour types express the MHC class II ligand lymphocyte activation gene-3 (LAG-3): role of LAG-3/MHC class II interactions in cell-cell contacts. Eur J Cancer. 2001; 37: 1709-18. . [DOI] [PubMed] [Google Scholar]
- 25. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14: 101-12. 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 26. Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004; 4: 336-47. . [DOI] [PubMed] [Google Scholar]
- 27. Saito H, Kuroda H, Matsunaga T, Osaki T, Ikeguchi M. Increased PD-1 expression on CD4+ and CD8+ T cells is involved in immune evasion in gastric cancer. J Surg Oncol. 2013; 107: 517-22. . [DOI] [PubMed] [Google Scholar]
- 28.Grosso JF, Goldberg MV, Getnet D, Bruno TC, Yen HR, Pyle KJ. Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J Immunol. 2009; 182: 6659-69. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baitsch L, Legat A, Barba L, Fuertes Marraco SA, Rivals JP, Baumgaertner P. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PloS One. 2012; 7: e30852.-. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012; 72: 917-27. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okazaki T, Okazaki IM, Wang J, Sugiura D, Nakaki F, Yoshida T. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Exp Med. 2011; 208: 395-407.. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007; 7: 95-106. . [DOI] [PubMed] [Google Scholar]
- 33. Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010; 363: 711-23. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013; 369: 134-44. . [DOI] [PMC free article] [PubMed] [Google Scholar]


