Abstract
Background
The etiology of many cases of childhood-onset chorea remains undetermined, although advances in genomics are revealing both new disease-associated genes and variant phenotypes associated with known genes.
Methods
We report a Saudi family with a neurodegenerative course dominated by progressive chorea and dementia in whom we performed homozygosity mapping and whole exome sequencing.
Results
We identified a homozygous missense mutation in GM2A within a prominent block of homozygosity. This mutation is predicted to impair protein function.
Discussion
Although discovered more than two decades ago, to date, only five patients with this rare form of GM2 gangliosidosis have been reported. The phenotype of previously described GM2A patients has been typified by onset in infancy, profound hypotonia and impaired volitional movement, intractable seizures, hyperacusis, and a macular cherry red spot. Our findings expand the phenotypic spectrum of GM2A mutation-positive gangliosidosis to include generalized chorea without macular findings or hyperacusis and highlight how mutations in neurodegenerative disease genes may present in unexpected ways.
Keywords: Chorea, dementia, neurodegeneration, GM2 gangliosidosis
Introduction
As part of an ongoing effort to elucidate the genetic basis of HTT repeat expansion-negative Huntington-like syndromes, we characterized a multiplex, consanguineous Saudi family with chorea, dementia, and a slowly progressive course. We detected a novel homozygous mutation in the GM2 activator protein, GM2A, that segregated with affected status in the family. This familial mutation is associated with a phenotype distinct from prior reports, which featured earlier-onset, hyperacusis and cherry red spots and a rapid degenerative course.
Methods
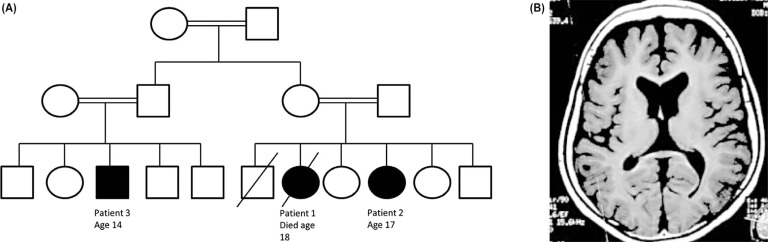
We enrolled the patients in our ongoing ethics and institutional review board-approved research studies after written informed consent had been obtained. The patients were independently ascertained and reported in table form recently.1 Patient 1, a girl, was born at term after an uncomplicated pregnancy (Figure 1A). Her early development was normal until age 7 years, when she was held back due to severe anxiety related to the prospect of moving on to the second grade. Cognitive decline ensued. By age 9 years the patient was only able to communicate using telegraphic sentences. She developed a pseudobulbar affect. Examinations at school age showed height and weight below the third centiles with head circumference at the 50th centile. Neurological examination disclosed mild spastic quadriparesis, limb dystonia, pyramidal tract signs, and generalized chorea. The patient exhibited a positive glabellar reflex. Ophthalmological examination showed no evidence of a cherry red spot. Her startle reactions were normal. Expressive speech was absent by age 11 years. Her gait progressively deteriorated and she lost the ability to ambulate at age 12 years as her hyperkinetic disorder gave way to a rigid, akinetic state. All laboratory investigations were unrevealing. Electroencephalogram (EEG) was normal. Her magnetic resonance imaging (MRI) showed mild generalized cerebral atrophy. A muscle biopsy revealed only non-specific findings. The patient died at age 18 years related to respiratory tract infection. No post-mortem evaluation was performed.
Figure 1. Features of Index Family. (A) Pedigree depicting family structure: Circle represent females, squares depict males. Consanguinity is shown via double lines linking parents. Affected status is denoted by a filled shape. Death is indicated by a slash. (B) Brain magnetic resonance imaging demonstrating generalized cortical atrophy in patient 3.
The patient’s affected sister (patient 2) had a very similar disease course. Pregnancy, delivery, and early neurodevelopment were normal. She developed phobias, school avoidance, and intellectual decline beginning at age 8 years. Height and weight were <3% but head circumference was normal. A cherry red spot was conspicuously absent upon initial funduscopic examination and on repeat examination at age 18 years. Neurological examination demonstrated mild scoliosis, a positive glabellar response, and a normoactive startle. Generalized chorea was present and she developed mild spastic quadriparesis with limb dystonia; she became progressively hypokinetic but retained the ability to ambulate until age 17 years. Electromyogram and EEG were normal, and MRI showed generalized cerebral atrophy.
A male cousin (patient 3) was similarly affected. His birth and development were normal until age 8 years, when he too demonstrated multiple phobias. He then developed dysarthria, spasticity affecting his gait, dystonia, and generalized chorea. He did not show hyperacusis and no cherry red spot was detected. He lost all expressive speech by his early teens. Locomotion was preserved until 13 years of age, when he became dependent on a wheelchair and hypokinetic features began to dominate his clinical picture (Video 1). MRI demonstrated generalized cerebral atrophy (Figure 1B). Investigations showed normal complete blood count with no acanthocytosis and normal liver and kidney functions.
We initially tested the affected children for HTT CAG numbers by repeat expansion analysis. Given the family structure (Figure 1A), we suspected an autosomal recessive disorder with identity by descent. Homozygosity mapping was performed on patients 1 and 2 and an unaffected older sister using Affymetrix Whole-Genome 2.7M single nucleotide polymorphism arrays according to the manufacturer’s instructions. Whole exome sequencing was performed on patients 1, 2, and 3 using the Roche/NimbleGen SeqCap EZ V2 capture product followed by paired-end sequencing using the Illumina HiSeq2000 generating paired-end reads with mean length of 74 bp. Average coverage depth was >70× with >93% mapped reads, and 12,090, 11,900, and 11,875 homozygous variants detected in patients 1, 2, and 3, respectively.
Results
CAG analysis did not disclose any HTT repeat expansions. Homozygosity mapping identified two regions of homozygosity >3 Mb in size. Within the larger region (chr5:125,904,993-169,353,466; 43.5 Mb), whole exome sequencing identified a homozygous c.164C>T (p.P55L) sequence variant in GM2A (ENST00000357164) with an allele frequency of 0.000825 reported in the ExAc browser. This change is predicted to be deleterious using SIFT, PolyPhen-2, and Mutation Taster. This mutation affects a highly conserved proline within the MD-2-related lipid-recognition domain of GM2A (Figure 2). The only other variant within the chromosome 5 region of identity by descent was a homozygous substitution of aspartate for alanine at position 1079 within FAT2. As mutations in this gene have never before been associated with disease and transcript has been detected only in the cerebellum,2 this candidate was felt to be unlikely to explain the observed phenotype, although this possibility cannot be completely excluded. Mutations in c9orf72, SCA17, VPS13A, HDL2, FXN, RNF216, and PRNP were not detected, although repeat expansions may evade detection by short read platforms such as the HiSeq.
Figure 2. Clustal Omega cross-species alignment of amino acid residues. The proline at position 55 is conserved throughout eukaryotes.
Video 1. Patient 3. The patient is shown at age 15 years, with mild residual chorea, dystonia, and masked facies.
Discussion
GM2A encodes for the GM2 activator protein required for the extraction of lipids from bilayers, lipid solubilization, and presentation for degradation by hexosaminidase A.3 The mutation we identified is expected to affect a highly conserved proline that represents the tip of the hydrophobic region that surrounds the lipid-interacting cup of GM2A. To date, only five patients with GM2A mutations (GM2 AB variant; OMIM #272750) have previously been reported.3–7
To our knowledge, this represents the first report of a movement disorder associated with GM2A mutations, although patients with other forms of GM2 gangliosidoses (Tay–Sachs or Sandhoff disease) may present with prominent ataxia,8 dystonia,9 or parkinsonism.10 Hyperkinetic movement disorders are not uncommon in GM2 disease, although our patients’ prominent chorea was unusual.11 In addition, our patients lacked the hyperactive startle and macular cherry red spots that typify early-onset forms of GM2 gangliosidoses, including those with GM2A mutations (Table 1). Previously reported GM2A patients demonstrated infantile onset, profound hypotonia, intractable seizures, and diminished volitional movement. In contrast, our patients presented at a later age with features that led to a clinical diagnosis of an HTT-negative Huntington-like syndrome.
Table 1. Clinical and genetic features of GM2A-associated disease.
| Source | Mutation | Ethnicity | Age of Onset | Symptoms | Examination | Other |
|---|---|---|---|---|---|---|
| de Baecque, et al.12 | p.C107R homozygous | African American | 9 months | Decline in mobility; hyperacusis; regression | Hypotonia; increased DTRs; cherry red spots | Normal HexA and HexB activities; brain biopsy showed Zebra bodies and membranous cytoplasmic bodies; pleomorphic intracytoplasmic astrocytic inclusions; reported by Schroder et al.3 and Xie et al.4 |
| Schroder et al.13 | p.R169P homozygous | Indian | 5 months | Nystagmus, motor delay | Hyperacusis; juvenile spasms; cherry red spot | Normal HexA and HexB activities; rectal biopsy showed storage material; died at age 5 |
| Schepers et al.6 | p.H137fsX33 homozygous | Spanish | 7 months | Developmental delay | Hypotonia, limb hypertonia; hyperacusis; cherry red spots | Normal HexA and HexB activities; MRI showed ↑ cerebral and cerebellar white matter signal |
| Schepers et al.6 | p.88Ldel homozygous | Saudi | 8 months | Weakness; head lag; infantile spasms; tonic–clonic seizures | Hypotonia; ↓ visual attention; hyperacusis; cherry red spots | Normal HexA and HexB activities; diffuse brain atrophy on MRI; rapid progression after 24 months |
| Chen et al.7 | p.E54X homozygous | Laotian Hmong | 5 months | Developmental delay; weakness; extreme hyperacusis; generalized tonic–clonic and myoclonic seizures | Severe hypotonia; dysarthria, dysphagia; ↓ volitional movement; ↓ response to environment; roving eye movements; cherry red spots | Normal HexA enzyme activity; ↑ CSF gangliosides; abnormal basal ganglia and white matter signal |
| Present report (3 patients) | p.P55L homozygous | Saudi | 7 or 8 years | Anxiety, intellectual regression, chorea | Spastic quadriparesis, limb dystonia, pyramidal tract signs, chorea | Diffuse cortical atrophy |
Hex A, Hexosaminidase A; Hex B, Hexosaminidase B; MRI, Magnetic Resonance Imaging.
Our findings showcase the diverse phenotypes that may be associated with mutations in neurodegenerative disease genes. As loss of GM2A function does not affect hexosaminidase A or B activity, mutations in GM2A may be missed by enzymatic screening methods. Our results also highlight links between movement disorders and lysosomal storage diseases as well as the importance of an unbiased approach to the diagnosis of childhood neurodegenerative syndromes.
Acknowledgments
We thank the patients described in this study; without their participation, this work would not have been possible.
Footnotes
Funding: This work was supported by a Sanford Seed Grant to A.M., P.L.C., and M.C.K., the NIH Centers for Mendelian Genomics (5U54HG006504), MAS was supported by the Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia via research group project number RGP-VPP-301, and by a Clinician Scientist Development Award from the Doris Duke Foundation to M.C.K.
Financial Disclosures: None.
Conflict of Interest: M.C.K. receives research support from Retrophin, Inc.
References
- 1.Alazami AM, Patel N, Shamseldin HE, et al. Accelerating novel candidate gene discovery in neurogenetic disorders via whole-exome sequencing of prescreened multiplex consanguineous families. Cell Rep. 2015;10:148–161. doi: 10.1016/j.celrep.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Nakayama M, Nakajima D, Nagase T, Nomura N, Seki N, Ohara O. Identification of high-molecular-weight proteins with multiple EGF-like motifs by motif-trap screening. Genomics. 1998;51:27–34. doi: 10.1006/geno.1998.5341. [DOI] [PubMed] [Google Scholar]
- 3.Schroder M, Schnabel D, Suzuki K, Sandhoff K. A mutation in the gene of a glycolipid-binding protein (GM2 activator) that causes GM2-gangliosidosis variant AB. FEBS Lett. 1991;290:1–3. doi: 10.1016/0014-5793(91)81211-P. [DOI] [PubMed] [Google Scholar]
- 4.Xie B, Wang W, Mahuran DJ. A Cys138-to-Arg substitution in the GM2 activator protein is associated with the AB variant form of GM2 gangliosidosis. Am J Human Gen. 1992;50:1046–1052. [PMC free article] [PubMed] [Google Scholar]
- 5.Schepers U, Glombitza G, Lemm T, et al. Molecular genetics of GM2-gangliosidosis AB variant: A novel mutation and expression in BHK cells. Human Gen. 1993;92:437–440. doi: 10.1007/BF00216446. [DOI] [PubMed] [Google Scholar]
- 6.Schepers U, Glombitza G, Lemm T, et al. Molecular analysis of a GM2-activator deficiency in two patients with GM2-gangliosidosis AB variant. Am J Human Gen. 1996;59:1048–1056. [PMC free article] [PubMed] [Google Scholar]
- 7.Chen B, Rigat B, Curry C, Mahuran DJ. Structure of the GM2A gene: Identification of an exon 2 nonsense mutation and a naturally occurring transcript with an in-frame deletion of exon 2. Am J Human Gen. 1999;65:77–87. doi: 10.1086/302463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Federico A, Palmeri S, Malandrini A, Fabrizi G, Mondelli M, Guazzi GC. The clinical aspects of adult hexosaminidase deficiencies. Development Neurosci. 1991;13:280–287. doi: 10.1159/000112174. [DOI] [PubMed] [Google Scholar]
- 9.Nardocci N, Bertagnolio B, Rumi V, Angelini L. Progressive dystonia symptomatic of juvenile GM2 gangliosidosis. Movement Disord. 1992;7:64–67. doi: 10.1002/mds.870070113. [DOI] [PubMed] [Google Scholar]
- 10.Inzelberg R, Korczyn AD. Parkinsonism in adult-onset GM2 gangliosidosis. Movement Disord. 1994;9:375–377. doi: 10.1002/mds.870090325. [DOI] [PubMed] [Google Scholar]
- 11.Oates CE, Bosch EP, Hart MN. Movement disorders associated with chronic GM2 gangliosidosis. Case report and review of the literature. European Neurol. 1986;25:154–159. doi: 10.1159/000116100. [DOI] [PubMed] [Google Scholar]
- 12.de Baecque CM, Suzuki K, Rapin I, Johnson AB, Whethers DL. GM2-gangliosidosis, AB variant: clinico-pathological study of a case. Acta Neuropathol. 1975;33(3):207–226. doi: 10.1007/BF00688395. [DOI] [PubMed] [Google Scholar]
- 13.Schröder M, Schnabel D, Hurwitz R, Young E, Suzuki K, Sandhoff K. Molecular genetics of GM2-gangliosidosis AB variant: a novel mutation and expression in BHK cells. Hum Genet. 1993;92(5):437–440. doi: 10.1007/BF00216446. [DOI] [PubMed] [Google Scholar]