Highlights
-
•
We establish five inducible/repressible promoters for use in Z. tritici.
-
•
All promoters express cytoplasmic GFP when induced by media supplements.
-
•
The tested conditional promoter range from weak to strong expression.
-
•
3 of 5 promoters are tight under repressed conditions, 2 promoters show background expression.
-
•
A conditional α-tubulin mutant dies under repressed conditions, showing proof of principle.
Abbreviations: P, promoter; nar1, nitrate reductase; ex1A, 1,4-β-endoxylanase; laraB, l-arabinofuranosidase B; gal7, galactose-1-phosphate uridylyltransferase 7; icl1, isocitrate lyase; tub2, α-tubulin; sdi1, succinate dehydrogenase 1; aa, amino acid; eGFP, enhanced green fluorescent protein; DNA, deoxyribonucleic acid; PCR, polymerase chain reaction; MT, microtubule; ROI, region of interest
Keywords: Inducible–repressible promoter, Fungal wheat pathogen, Septoria tritici blotch, Mycosphaerella graminicola
Abstract
Development of new fungicides, needed for sustainable control of fungal plant pathogens, requires identification of novel anti-fungal targets. Essential fungal-specific proteins are good candidates, but due to their importance, gene deletion mutants are not viable. Consequently, their cellular role often remains elusive. This hindrance can be overcome by the use of conditional mutants, where expression is controlled by an inducible/repressible promoter. Here, we introduce 5 inducible/repressible promoter systems to study essential genes in the wheat pathogen Zymoseptoria tritici. We fused the gene for enhanced green-fluorescent protein (egfp) to the promoter region of Z. tritici nitrate reductase (Pnar1; induced by nitrogen and repressed by ammonium), 1,4-β-endoxylanase A (Pex1A; induced by xylose and repressed by maltodextrin), l-arabinofuranosidase B (PlaraB; induced by arabinose and repressed by glucose), galactose-1-phosphate uridylyltransferase 7 (Pgal7; induced by galactose and repressed by glucose) and isocitrate lyase (Picl1; induced by sodium acetate and repressed by glucose). This was followed by quantitative analysis of cytoplasmic reporter fluorescence under induced and repressed conditions. We show that Pnar1, PlaraB and Pex1A drive very little or no egfp expression when repressed, but induce moderate protein production when induced. In contrast, Pgal7 and Picl1 show considerable egfp expression when repressed, and were strongly induced in the presence of their inducers. Normalising the expression levels of all promoters to that of the α-tubulin promoter Ptub2 revealed that PlaraB was the weakest promoter (∼20% of Ptub2), whereas Picl1 strongly expressed the reporter (∼250% of Ptub2). The use of these tools promises a better understanding of essential genes, which will help developing novel control strategies that protect wheat from Z. tritici.
1. Introduction
Effective management of fungal pathogens requires in-depth knowledge of their cell biology and host invasion strategies. Sophisticated sequencing methods have provided a large wealth of genes and predicted proteins, which need to be functionally analyzed to gain insight into their cellular roles. The generation of gene mutants, such as knock-out, knock-down and over-expression mutants, has greatly helped understanding protein function in eukaryotic cells (Meyer et al., 2011). Examples are the systematic deletion of genes in the budding yeast Saccharomyces cerevisiae (Giaever et al., 2002) and the genome-wide knock-down of proteins by RNAi silencing methods in the worm Caenorhabditis elegans (Kamath et al., 2003). However, a major limitation of these approaches is that cellular function of essential genes and their protein products cannot be analyzed (Meyer et al., 2011). This caveat can be overcome by the generation of conditional promoter mutants, where the expression of a gene of interest depends on the experimental condition. Such substrate-induced promoter mutants have been used in a wide range of fungi. Examples include galactose-inducible promoters of galactose-1-phosphate uridylyltransferase in Cryptococcus neoformans (Ruff et al., 2009), the sodium acetate inducible isocitrate lyase promoter in Magnaporthe grisea (Wang et al., 2003), or the arabinose-inducible, glucose repressible crg promoter in Ustilago maydis (Bottin et al., 1996). An essential requirement for conditional promoters is that they are tightly repressed in the absence of an inducer (Vu et al., 2013). On the other hand, controlled over-expression of entire genes or DNA encoding domains of interest also provides a useful way of gaining insight into their cellular role (Schuster et al., 2011).
Here, we establish 5 homologous inducible/repressible promoters in the wheat pathogen Zymoseptoria tritici. We identify the promoter region of nitrate reductase homologue (Pnar1) in Z. tritici, which induced by nitrate and repressed by ammonium in U. maydis (Banks et al., 1993). Secondly, we use the promoter of l-arabinofuranosidase B homologue (PlaraB) in Z. tritici that is induced by arabinose and repressed by glucose in Aspergillus niger (v d Veen et al., 1993). We establish use of the promoter of a 1,4-β-endoxylanase A homologue (Pex1A) in Z. tritici, which is induced by xylose and repressed by maltodextrin in Aspergillus awamori (Gouka et al., 1996). Moreover, we express eGFP under the promoter of a galactose-1-phosphate uridylyltransferase homologue (Pgal7) in Z. tritici, which is induced by galactose and repressed by glucose in C. neoformans (Ruff et al., 2009). Finally, we use the promoter of a homologue of isocitrate lyase (Picl1), induced by sodium acetate and repressed by glucose in M. grisea (Wang et al., 2003). Using enhanced green-fluorescent protein (eGFP) as a reporter, we show that all 5 promoter systems derive eGFP expression when induced in liquid culture. Comparison with auto-fluorescence in control strains reveals that Pnar1, PlaraB and Pex1A are “shut down” under OFF conditions, whereas significant background fluorescence of eGFP was found when the reporter was placed behind Pgal7 and Picl1. Comparing the relative expression strength of all promoters with that of the α-tubulin promoter shows that PlaraB, Pnar1 and Pex1A drive relatively weak expression under induced conditions (ON conditions), whereas Pgal7 and, in particular, Picl1 are stronger promoters. When α-tubulin was expressed under the Pex1A promoter, cells grew normally, but growth was significantly impaired when Pex1A was repressed. This demonstrates the use of conditional promoters in functional analysis of essential genes in Z. tritici.
2. Materials and methods
2.1. Bacterial and fungal strains and growth conditions
Escherichia coli strain DH5α was used for the maintenance of plasmids. Agrobacterium tumefaciens strain EHA105 (Hood et al., 1993) was used for maintenance of plasmids and subsequently for A. tumefaciens-mediated transformation of Z. tritici. E. coli and A. tumefaciens were grown in DYT broth or DYT agar at 37 °C and 28 °C respectively. The fully sequenced Z. tritici wild-type isolate IPO323 (Kema and van Silfhout, 1997) was used as recipient strain for the genetic transformation experiments. The isolate was inoculated from stocks stored in glycerol at −80 °C onto solid YPD agar (yeast extract, 10 g/l; peptone, 20 g/l; glucose, 20 g/l; agar, 20 g/l) and grown at 18 °C for 4–5 days.
2.2. Identification of enzymes in Z. tritici and bioinformatics
To identify conditional promoters, we obtained the sequences of U. maydis Nar1 sequence (XP_759994.1), Aspergillus fumigatus LaraB sequence (KEY83958.1), A. awamori Ex1A sequence (CAA55005.1), C. neoformans Gal7 sequence (XP_568349.1) and M. oryzae Icl1 sequence (XP_003712381.1) from the NCBI server (http://www.ncbi.nlm.nih.gov/pubmed). We used these to screen the published sequence of Z. tritici (http://genome.jgi.doe.gov/Mycgr3/Mycgr3.home.html) using the provided BLASTP function and compared the best hits in EMBOSS Needle (http://www.ebi.ac.uk/Tools/psa/emboss_needle/). The domain structure was analyzed in PFAM (http://pfam.xfam.org/search/sequence). The promoter sequences upstream of the open reading frame were obtained from the JIG server (http://genome.jgi.doe.gov/Mycgr3/Mycgr3.home.html).
2.3. Molecular cloning
All vectors in this study were generated by in vivo recombination in the yeast S. cerevisiae DS94 (MATα, ura3-52, trp1-1, leu2-3, his3-111, and lys2-801 (Tang et al., 1996) following published procedures (Raymond et al., 1999; Kilaru and Steinberg, 2015). For all the recombination events, the fragments were amplified with 30 bp homologous sequences to the upstream and downstream of the fragments to be cloned (see Table 1 for primer details). PCR reactions and other molecular techniques followed standard protocols (Sambrook and Russell, 2001). All restriction enzymes and reagents were obtained from New England Biolabs Inc (NEB, Herts, UK).
Table 1.
Primers used in this study.
| Primer name | Direction | Sequence (5′ to 3′)a |
|---|---|---|
| SK-Sep-10 | Sense | TGGCAGGATATATTGTGGTGTAAACAAATTGACCTTCCACATCTACCGATGG |
| SK-Sep-13 | Antisense | CTTCCGTCGATTTCGAGACAGC |
| SK-Sep-14 | Sense | CATTTGCGGCTGTCTCGAAATCGACGGAAGGCAGTCGACGCCAGATGATGG |
| SK-Sep-15 | Antisense | GGTGAACAGCTCCTCGCCCTTGCTCACCATGGCGATGGTGGTATGCGGATG |
| SK-Sep-112 | Sense | CATTTGCGGCTGTCTCGAAATCGACGGAAGATAGTTGCTCTACGACCAATGCC |
| SK-Sep-113 | Antisense | GGTGAACAGCTCCTCGCCCTTGCTCACCATTGCGGGAGAGGACATAGTAACG |
| SK-Sep-114 | Sense | CATTTGCGGCTGTCTCGAAATCGACGGAAGGGGAGAGCTTGCCCGCTGGC |
| SK-Sep-115 | Antisense | GGTGAACAGCTCCTCGCCCTTGCTCACCATGACTTTGTTTGATCTCGAAGCTGA |
| SK-Sep-118 | Sense | CATTTGCGGCTGTCTCGAAATCGACGGAAGGCTTCTACACCGACTCTGAAGAC |
| SK-Sep-119 | Antisense | GGTGAACAGCTCCTCGCCCTTGCTCACCATGTTGAAGGTAATGAGGTGGTAAAG |
| SK-Sep-120 | Antisense | CATTTGCGGCTGTCTCGAAATCGACGGAAGATGACAGCTAAACCTTGGGACAG |
| SK-Sep-121 | Antisense | GGTGAACAGCTCCTCGCCCTTGCTCACCATGCTGGGCGTGTGTTGATGGAC |
| SK-Sep-126 | Antisense | CATTTGCGGCTGTCTCGAAATCGACGGAAGGTCACCAAGTATGCCGATGACAAC |
| SK-Sep-127 | Antisense | GGTGAACAGCTCCTCGCCCTTGCTCACCATGTCGGGCTTGTGACAGCAGGTG |
| SK-Sep-136 | Sense | CCCAACTGATATTGAAGGAGCATT |
| SK-Sep-137 | Antisense | CCCGATCTAGTAACATAGATGACA |
| SK-Sep-217 | Sense | TGGCAGGATATATTGTGGTGTAAACAAATTGCAGTCGACGCCAGATGATGG |
| SK-Sep-218 | Antisense | CCAAAAAATGCTCCTTCAATATCAGTTGGGGGCGATGGTGGTATGCGGATG |
| SK-Sep-221 | Sense | ATGCGTGAAGTCATCTCCTTGAAC |
| SK-Sep-222 | Antisense | TAAACGCTCTTTTCTCTTAGGTTTACCCGCTGGGACGCTCGATGCCAAGGTT |
| SK-Sep-225 | Sense | GCGCGGTGTCATCTATGTTACTAGATCGGGGGGAGAGCTTGCCCGCTGGC |
| SK-Sep-226 | Antisense | CGTACCGTTCAAGGAGATGACTTCACGCATGACTTTGTTTGATCTCGAAGCTGA |
Italics indicate part of the primer that is complementary with another DNA fragment, to be ligated by homologous recombination in S. cerevisiae.
2.4. Construction of vectors pCPnar1eGFP, pCPex1eGFP, pCPlaraB-eGFP, pCPgal7-eGFP, pCPicl1-eGFP and pHex1A-tub2
Vector pCPPnar1eGFP contains egfp under the control of Z. tritici nar1 promoter for integration into the sdi1 locus by using carboxin as a selectable marker. A 13,083 bp fragment of pCeGFPTub2 (digested with PmlI; Schuster et al., 2015) and 1000 bp Z. tritici nar1 promoter (amplified with SK-Sep-112 and SK-Sep-113; Table 1) were recombined in S. cerevisiae to obtain the vector pCPnar1eGFP. Vector pCPex1eGFP contains egfp under the control of Z. tritici ex1A promoter for integration into the sdi1 locus by using carboxin as a selectable marker. A 13,083 bp fragment of pCeGFPTub2 (digested with PmlI) and 1000 bp Z. tritici ex1A promoter (amplified with SK-Sep-114 and SK-Sep-115; Table 1) were recombined in S. cerevisiae to obtain the vector pCPex1eGFP. Vector pCPlaraBeGFP contains egfp under the control of Z. tritici laraB promoter for integration into the sdi1 locus by using carboxin as a selectable marker. A 13,083 bp fragment of pCeGFPTub2 (digested with PmlI) and 1,000 bp laraB promoter (amplified with SK-Sep-118 and SK-Sep-119; Table 1) were recombined in S. cerevisiae to obtain the vector pCPlaraBeGFP. Vector pCPgal7-eGFP contains egfp under the control of Z. tritici gal7 promoter for integration into the sdi1 locus by using carboxin as a selectable marker. A 13,083 bp fragment of pCeGFPTub2 (digested with PmlI) and 1000 bp Z. tritici gal7 promoter (amplified with SK-Sep-126 and SK-Sep-127; Table 1) were recombined in S. cerevisiae to obtain the vector pCPgal7-eGFP. Vector pCPicl1-eGFP contains egfp under the control of Z. tritici icl1 promoter for integration into the sdi1 locus by using carboxin as a selectable marker. A 13,083 bp fragment of pCeGFPTub2 (digested with PmlI) and 1000 bp Z. tritici icl1 promoter (amplified with SK-Sep-120 and SK-Sep-121; Table 1) were recombined in S. cerevisiae to obtain the vector pCPicl1-eGFP. Vector pHPex1ATub2 contains the Z. tritici ex1A promoter (Pex1A) fused to tub2 gene for replacement of endogenous tub2 promoter in Z. tritici with ex1A promoter using hygromycin as a selectable marker. A 9533 bp fragment of pCeGFPTub2 (digested with BamHI and HindIII), 1149 bp tub2 promoter (amplified with SK-Sep-217 and SK-Sep-218; Table 1), 1806 bp hygromycin resistance cassette (amplified with SK-Sep-136 and SK-Sep-137; Table 1), 1000 bp ex1A promoter (amplified with SK-Sep-225 and SK-Sep-226; Table 1), 5′ end of 1002 bp tub2 gene (amplified with SK-Sep-221 and SK-Sep-222; Table 1) were recombined in S. cerevisiae to obtain the vector pHPex1Atub2.
2.5. Z. tritici transformation
The vectors pCPnar1eGFP, pCPex1eGFP and pCPlaraBeGFP, pCPgal7eGFP and pCPicl1eGFP were transformed into A. tumefaciens strain EHA105 by heat shock method (Holsters et al., 1978), following previously published protocols (Zwiers and De Waard, 2001). Further details on this method are provided in Kilaru et al. (2015a).
2.6. Z. tritici transformation of vector pHPex1ATub2
The plasmid pHPex1ATub2 was transformed to Z. tritici isolate IPO323 as described in Kilaru et al. (2015a) with few modifications resulting in strain IPO323_Pex1ATub2. In order to ensure that ex1A promoter is ON, 5% xylose (Sigma–Aldrich, Gillingham, UK) was added to Agrobacterium induction medium agar plates. For selection of transformants, 200 μg/ml hygromycin (Roche, Burgess Hill, UK) was added to the Czapekdox agar containing 5% xylose, along with100 μg/ml cefotaxime and 100 μg/ml timentin. The individual transformants were transferred to complete medium CM (casaminoacids, 2.5 g/l; yeast extract. 1.0 g/l; NH4NO3, 1.5 g/l; DNA from herring sperm, 0.5 g/l; salt solution, 62.5 ml/l; vitamin solution 10.0 ml/l; pH-7.0; Holliday, 1974) with 5% xylose, 200 μg/ml hygromycin, 100 μg/ml cefotaxime and 100 μg/ml timentin and grown at 18 °C for 3–4 days.
2.7. Molecular analysis of transformants
To confirm the integration of vectors pCPnar1eGFP, pCPex1eGFP, pCPlaraBeGFP, pCPgal7eGFP and pCPicl1eGFP into the sdi1 locus of Z. tritici, Southern blot hybridizations were performed by using the standard procedures (Sambrook and Russell, 2001). Z. tritici wild-type IPO323 and transformants obtained with vectors pCPnar1eGFP, pCPex1eGFP, pCPlaraBeGFP, pCPgal7eGFP and pCPicl1eGFP were grown in YG broth (yeast extract, 10 g/l; glucose 30 g/l) for 3 days at 18 °C with 200 rpm. 3 ml of yeast-like cells were used for fungal genomic DNA extraction as described (Kilaru et al., 2015a). Approximately 3 μg of genomic DNA of IPO323 and transformants obtained with vectors pCPnar1eGFP, pCPex1eGFP, pCPlaraBeGFP, pCPgal7eGFP and pCPicl1eGFP were digested with BglII and separated on a 1.0% agarose gel and capillary transferred to a Hybond-N membrane (GE healthcare, Little Chalfont, United Kingdom). 1014 bp sdi1 probe (3′ end of the sdi1R gene and sdi1 terminator) was generated by using DIG labeling PCR mix (Life Science Technologies, Paisley, UK) with primers SK-Sep-10 and SK-Sep-13; Table 1. Hybridizations were performed at 62 °C for overnight autoradiographs were developed after an appropriate time period.
Integration of vector pHPex1ATub2 into the tub2 locus of Z. tritici was confirmed by Southern blot. Z. tritici transformants obtained with plasmid pHPex1ATub2 were grown in complete medium CM with 5% xylose for 3 days at 18 °C with 200 rpm. Approximately 3 μg of genomic DNA of IPO323 and transformants obtained with vector pHPex1ATub2 were digested with SacII, and separated on a 1.0% agarose gel and capillary transferred to a Hybond-N membrane (GE healthcare, Little Chalfont, United Kingdom). 1149 bp tub2 probe (α-tubulin promoter) was generated by using DIG labeling PCR mix (Life Science Technologies, Paisley, UK) with primers SK-Sep-217 and SK-Sep-218; Table 1. Hybridizations were performed at 62 °C for overnight autoradiographs were developed after an appropriate time period.
2.8. Investigation of eGFP expression under ON- and OF conditions
IPO323_CPnar1eGFP cells were grown in Nitrate minimal medium NM (KNO3, 3.0 g/l; salt solution, 62.5 ml/l; pH-7.0; Holliday, 1974) with 1% glucose (ON) and Complete medium (CM) with 1% glucose (OFF); the IPO323_CPex1AeGFP cells were grown in Aspergillus minimal medium (NaNO3, 6 g/l; KCl, 0.52 g/l; KH2PO4, 1.52 g/l; MgSO4·7H2O, 0.52 g/l and trace elements solution 1 ml; pH-6.5, Kafer, 1977) with 5% xylose (ON) and Aspergillus minimal medium with 5% maltodextrin (OFF); the IPO323_CPlaraBeGFP cells were grown in Aspergillus minimal medium with 2% arabinose (ON) and Aspergillus minimal medium with 1% glucose (OFF); the IPO323_CPgal7eGFP cells were grown in Aspergillus minimal medium with 0.5% galactose (ON) and Aspergillus minimal medium with 2% glucose (OFF); the IPO323_CPicl1eGFP cells were grown in Aspergillus minimal medium with 20 mM sodium acetate (ON) and Aspergillus minimal medium with 2% glucose (OFF) at 18 °C with 200 rpm for 24 h. In order to compare the expression levels of eGFP in strains IPO323_CPnar1eGFP, IPO323_CPex1AeGFP, IPO323_CPlaraBeGFP, IPO323_CPgal7eGFP and IPO323_CPicl1eGFP with eGFP expression under the tub2 promoter in strain IPO323_CeGFP (Kilaru et al., 2015a). Strain IPO323_CeGFP was used in all corresponding control experiments.
Fluorescence microscopy was performed as previously described (Schuster et al., 2011). In brief, cells were placed onto a 2% agar cushion for direct observation using a motorized inverted microscope (IX81; Olympus, Hamburg, Germany), equipped with a PlanApo 100×/1.45 Oil TIRF (Olympus, Hamburg, Germany). The expression of eGFP under the different inducible/repressible promoters and tub2 promoter was analyzed by capturing single images with a 150 ms exposure time using a CoolSNAP HQ2 camera (Photometrics/Roper Scientific, Tucson, USA). The fluorescent tags of all strains, with the exception of IPO323_Picl1eGFP, were excited using a VS-LMS4 Laser Merge System with a 488 nm solid-state laser (75 mW; Visitron Systems, Puchheim, Germany) at 20%. IPO323_Picl1eGFP was excited with 10% of the 488 nm solid-state laser to avoid camera saturation.
Average intensity was analyzed in those images by creating a region of interest (ROI) within a cell covering only a part of the cytoplasm but excluding the nucleus or vacuoles. A copy of the same ROI was placed next to the cell to get the average intensity of the neighbouring background. Both values were transferred to Excel (Microsoft, Redmond, WA, USA) and the value of the background was subtracted from the value of the cell. Those corrected values were transferred to Prism 4.03 (GraphPad Software, La Jolla, CA, USA) to perform all statistical analysis. All parts of the system were under the control of the software package MetaMorph (Molecular Devices, Wokingham, UK).
2.9. Plate growth assay
The complete medium CM agar either with 5% xylose (Sigma–Aldrich, Gillingham, UK) or 5% maltodextrin (Sigma–Aldrich, Gillingham, UK) was used to examine the growth habit of IPO323 and IPO323_HPex1ATub2. For better visualisation of the colonies, 1% activated charcoal was added to the media. The IPO323 and IPO323_HPex1ATub2 cells were grown in CM with 5% xylose at 18 °C with 200 rpm for 2 days. The cell density was adjusted to an optical density of 0.6 at 660 nm. 1,000 μl of cell culture was transferred to 1.5 ml Eppendorf tube and centrifuged at 3000 rpm for 3 min. The supernatant was discarded and the cell pellet was resuspended either with CM containing 5% xylose (ON condition) or 5% maltodextrin (OFF condition). The cell cultures were serially diluted (10 times each) with the corresponding media. The serial diluted cultures were then applied as 5 μl droplets on either CM agar with 5% xylose and 1% charcoal or CM agar with 5% maltodextrin and 1% charcoal and grown at 18 °C for 6 days. Photographs of the relative colony densities were taken using a canon digital IXUS 80 IS camera (Canon, Surrey, UK).
3. Results and discussion
3.1. Identification of homologues of nitrate reductase, of l-arabinofuranosidase B, 1,4-β-endoxylanase A, galactose-1-phosphate uridylyltransferase and isocitrate lyase in Z. tritici
The Z. tritici homologues of the U. maydis nitrate reductase (Nar1), A. fumigatus l-arabinofuranosidase B (LaraB) and A. awamori 1,4-β-endoxylanase A (Ex1A), C. neoformans galactose-1-phosphate uridylyltransferase (Gal7) and M. oryzae isocitrate lyase (Icl1) were identified by BLASTP search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) with the U. maydis Nar1 sequence (XP_759994.1), A. fumigatus LaraB sequence (KEY83958.1), A. awamori Ex1A sequence (CAA55005.1), C. neoformans Gal7 sequence (XP_568349.1) and M. oryzae Icl1 sequence (XP_003712381.1) against the Z. tritici published genome sequence of IPO323 (Kema and van Silfhout, 1997) at the Joint Genome Institute (http://genome.jgi.doe.gov/Mycgr3/Mycgr3.home.html). Most similar to U. maydis Nar1 in Z. tritici was protein 111003 (NCBI accession number: XP_003849160.1), to A. fumigatus LaraB in Z. tritici was protein 70396 (NCBI accession number: XP_003854224.1), to A. awamori Ex1A in Z. tritici was protein 60105 (NCBI accession number: XP XP_003851797.1), to C. neoformans Gal7 in Z. tritici was protein 72281 (NCBI accession number: XP_003852576.1) and to M. oryzae Icl1 in Z. tritici was protein 102083 (XP_003855965.1), respectively. The predicted proteins ZtNar1, ZtLaraB, ZtEx1A, ZtGal7 and ZtIcl1 shared 39.6%, 45.3%, 40.4%, 42.0% and 71.7% aa identity with their homologues and have a similar domain structure. ZtNar1 shares with Nar1 from U. maydis an oxidoreductase molybdopterin binding domain (P = 8.6e−49), a molybdopterin cofactor oxidoreductase dimerisation domain (P = 8.6e−4), a cytochrome b5-like heme/steroid binding domain (P = 3.8e-23), an oxidoreductase FAD-binding domain (P = 3.5e−31) and an oxidoreductase NAD-binding domain (P = 6.4e−05). ZtLaraB shares with LaraB from A. fumigatus an alpha-l-arabinofuranosidase B domain (P = 2.1e−132), and ZtEx1A shares with Ex1A from A. awamori a glycosyl hydrolases family 11 domain (P = 4.6e−83). ZtGal7 shares with Gal7 from C. neoformans two Galactose-1-phosphate uridyl transferase domains (P = 2.6e−68, 1.6e−55) and ZtIcl1 shares an isocitrate lyase family domain with Icl1 from M. oryzae (P = 1.7e−278).
3.2. Vectors for targeted ectopic integration of eGFP, expressed under the promoters Pnar1, Pex1A, PlaraB, Pgal7 and Picl1
We next defined the promoter region upstream of each open reading frame (Pnar1, Pex1A, PlaraB, Pgal7 and Picl1). We choose 1000 bp, which includes the majority of putative promoter sequences in the yeast S. cerevisiae (Kristiansson et al., 2009). To test if these putative promoter regions are inducible/repressible, we generated 5 vectors (pCPnar1eGFP, pCPlaraBeGFP, pCPex1AeGFP, pCPgal7eGFP and pCPicl1eGFP) that drive expression of eGFPs in Z. tritici. All vectors were built on the Agrobacterium binary vector pCAMBIA0380 (CAMBIA, Canberra, Australia) to enable A. tumefaciens-based transformation into Z. tritici, which is based on the 25 bp imperfect directional repeat sequences of the T-DNA borders (right and left border, RB and LB; Fig. 1A). In addition, all three vectors comprise a “yeast recombination cassette”, allowing yeast recombination-based cloning (for more details see Kilaru and Steinberg, 2015). Finally, we designed all vectors for targeted integration into the sdi1 locus of Z. tritici genome, by using a mutated downstream stretch of the sdi1 sequence, carrying a carboxin resistance conferring point mutation (H267L; Fig. 1A, left flank), and a sequence stretch downstream of sdi1 (Fig. 1A, right flank of sdi1). Incorporation by homologous recombination mutates the sdi1 gene and integrates the eGFP constructs into the sdi1 locus (for details see Kilaru et al., 2015a). Thus, all 5 constructs allow eGFP expression in an identical genomic environment and single integration of each construct, which is essential for quantitative analysis of fluorescent intensities.
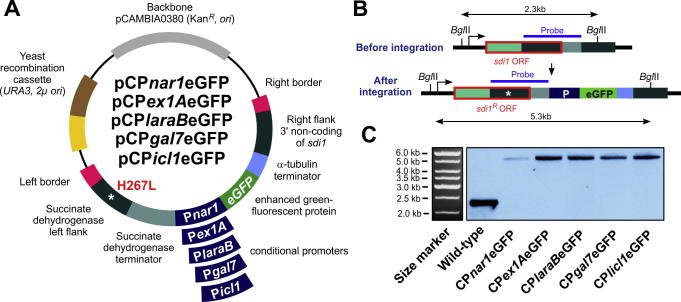
Fig. 1.
Vectors for succinate dehydrogenase (sdi1) locus integration that allow expression of enhanced GFP under inducible/repressible promoters. (A) Organization of three vectors that allow enhanced GFP expression after induction of inducible/repressible promoters Pnar1 (promoter region of a Z. tritici homologue of nitrate reductase; NCBI accession number, XP_003849160.1), Pex1A (promoter region of a Z. tritici homologue of 1,4-b-endoxylanase A), PlaraB (promoter region of a Z. tritici homologue of l-arabinofuranosidase B), Pgal7 (promoter region of a Z. tritici homologue of galactose-1-phosphate uridylyltransferase) and Picl1 (promoter region of a Z. tritici homologue of isocitrate lyase). After integration into the sdi1 locus, the vector confers carboxin resistance due to a point mutation in the succinate dehydrogenase gene sdi1, which changes a histidine to a leucine (H267L). For more details of this integration into the “succinate dehydrogenase locus” see Kilaru et al., 2015a. Left and right border enable A. tumefaciens-based transformation of Z. tritici. (B) Diagram showing the organization of the sdi1 locus before and after integration of the GFP-encoding vectors. Note that integration of the point mutated sdi1 left flank (see Fig. 1A; point mutation indicated by asterisk) replaces a part of the sdi1 open reading frame (sdi1 ORF) and confers carboxin resistance (sdi1R ORF). Successful integration of the vector increases the size of a DNA fragment after digestion with the restriction enzyme BglII and subsequent detection with a labelled DNA probe (blue bar). (C) Southern blot, showing integration of the described vectors into the sdi1 locus. After digestion of the genomic DNA with BglII and subsequent hybridisation with a labelled DNA probe, a shift in the DNA fragment from 2.3 kb to 5.3 kb is detected. The size markers in the corresponding agarose gel are shown to the left.
We next transformed all 5 vectors into Z. tritici wild-type IPO323 (Kema and van Silfhout, 1997), using A. tumefaciens-mediated transformation (Zwiers and De Waard, 2001). In order to confirm the single copy integration into the sdi1 locus, we purified genomic DNA from the transformants and wild-type isolate IPO323, digested with BglII and hybridized this to an sdi1 probe (Fig. 1B). Indeed, integration of the respective vectors resulted in shifting of a single band at the expected sizes (from 2.3 to 5.3 kb, Fig. 1B and C; marker bands in corresponding agarose gel are shown in Fig. 1C). This confirmed that the Pnar1, PlaraB, Pex1A, Pgal7 and Picl1 promoters and the downstream egfp were integrated correctly into the sdi1 locus as single copies. We took single transformants of each strain and named them IPO323_CPnar1eGFP, IPO323_CPlaraBeGFP, IPO323_CPex1AeGFP, IPO323_CPgal7eGFP and IPO323_CPicl1eGFP. All strains contained a single promoter-gfp reporter construct in their sdi1 locus, which allowed quantitative comparison of the cytoplasmic eGFP signals as a reporter for egfp gene expression.
3.3. Pnar1, PlaraB, Pex1A, Pgal7 and Picl1 controlled eGFP expression under induced and repressed conditions
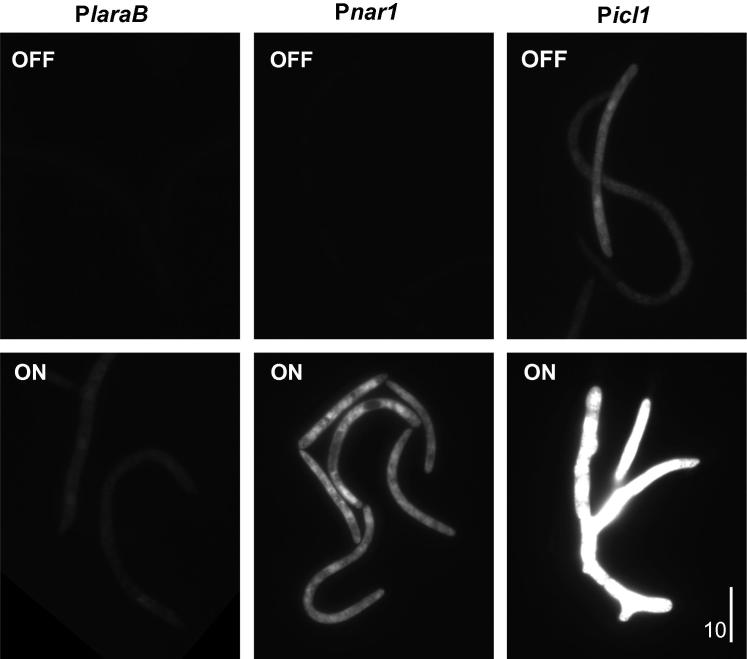
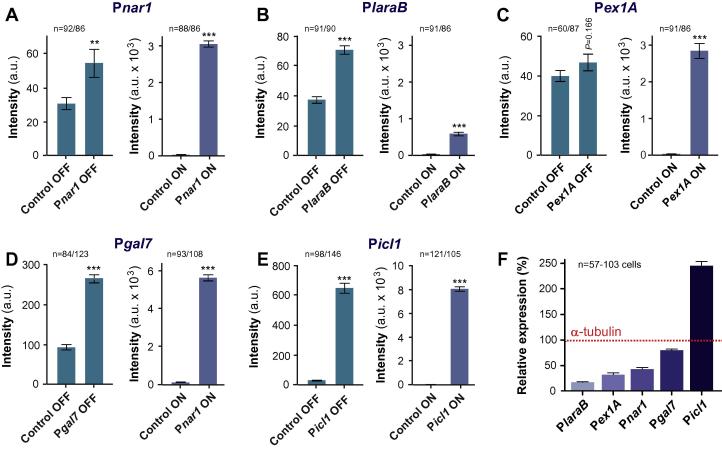
To test if Pnar1, PlaraB, Pex1A, Pgal7 and Picl1 are substrate-repressible, we grew liquid cultures of strains IPO323_CPnar1eGFP, IPO323_CPlaraBeGFP, IPO323_CPex1AeGFP, IPO323_CPgal7eGFP and IPO323_CPicl1eGFP for 24 h in the presence of the respective repressor (Pnar1: 20 mM ammonium; PlaraB: 1% glucose; Pex1A: 5% maltodextrin; Pgal7: 2% glucose; Picl1: 2% glucose). Under these conditions, almost no cytoplasmic fluorescence was observed for Pnar1, PlaraB and Pex1A (Fig. 2, OFF; only PlaraB and Pnar1 shown; 3A–C, OFF). Notably, Pex1A-egfp containing cells showed a signal intensity that was not significantly higher than that of untransformed IPO323 (Control; Fig. 3C; P = 0.166, Student t-test), suggesting that the promoter is “tight” under repressed conditions. In contrast, Pgal7 and Picl1 were not repressed entirely and weak GFP fluorescence was still visible under OFF conditions (Fig. 2, OFF; only Picl1 is shown; Fig. 3D and E, OFF). After 24 h growth of cells in the media supplemented with their respective inducers (Pnar1: 30 mM nitrate; PlaraB: 2% arabinose; Pex1A: 5% xylose; Pgal7: 0.5% galactose; Picl1: 20 mM sodium acetate), all strains showed cytoplasmic fluorescence, indicating eGFP expression from the induced promoters (Figs. 2 and 3A–E, ON). However, induction levels varied between the three promoters. Pnar1 and Pex1A were induced ∼127 and ∼415-times when compared to repressed conditions, respectively, whereas Picl1, PlaraB and Pgal7 expression was only induced ∼13-, ∼17- and ∼31-times, respectively (Fig. 3, ON). These numbers correspond well with published results in other fungi, where the nar1 promoter in U. maydis is induced 90-times (Banks et al., 1993), the laraB promoter in A. fumigatus is induced 13.7-times (v d Veen et al., 1993), the A. awamori 1,4-β-endoxylanase A promoter is induced 1346-times (Gouka et al., 1996), the gal7 promoter in C. neoformans is induced by 21.6-times (Ruff et al., 2009) and the icl1 promoter in M. oryzae is induced by 6-times (Wang et al., 2003).
Fig. 2.
Expression of cytoplasmic eGFP under inducible/repressible conditions. Images showing eGFP fluorescence under repressive (OFF) and induced conditions (ON). Note that virtually no signal is detected when PlaraB and Pnar repressed, whereas weak fluorescence appears when Picl1 is repressed. All strains show fluorescence after induction, but the intensity of the signal varies remarkably. Note that all images are processed identically. Bar represents 10 μm.
Fig. 3.
Quantitative analysis of eGFP expression under inducible/repressible conditions. (A) Bar charts showing the extent of cytoplasmic fluorescence of eGFP in control cells and IPO323_CPnar1-eGFP in their sdi1 locus, both grown under repressed (OFF) and induced (ON) conditions. Mean ± standard error of the mean is shown, sample size n represents cells and is indicated. Double asterisk indicates significant difference at P = 0.004; triple asterisk indicates significant difference at P < 0.0001, Student t-test. Note that the data set represent measurements from two experiments and a single transformant. (B) Bar charts showing the extent of cytoplasmic fluorescence of eGFP in control cells and IPO323_CPlaraB-eGFP cells, both grown under repressed (OFF) and induced (ON) conditions. Mean ± standard error of the mean is shown, sample size n represents cells and is indicated. Triple asterisk indicates significant difference at P < 0.0001, Student t-test. Note that the data set represent measurements from two experiments and a single transformant. (C) Bar charts showing the extent of cytoplasmic fluorescence of eGFP in control cells and IPO323_CPex1AeGFP cells, both grown under repressed (OFF) and induced (ON) conditions. Mean ± standard error of the mean is shown, sample size n represents cells and is indicated. Triple asterisk indicates significant difference at P < 0.0001, Student t-test. Note that no green fluorescence was detected when expression of eGFP was controlled by the 1,4-β-endoxylanase A promoter (Pex1A; no difference to auto-fluorescence at P = 0.166; Student t-test). Note that the data set represent measurements from two experiments and a single transformant. (D) Bar charts showing the extent of cytoplasmic fluorescence of eGFP in control cells and IPO323_CPgal7eGFP, both grown under repressed (OFF) and induced (ON) conditions. Mean ± standard error of the mean is shown, sample size n represents cells and is indicated. Triple asterisks indicate significant difference at P < 0.0001, Student t-test. Note that the promoter allows weak expression of eGFP under repressed conditions. Note also that the data set represent measurements from two experiments and a single transformant. (E) Bar charts showing the extent of cytoplasmic fluorescence of eGFP in control cells and IPO323_CPicl1eGFP, both grown under repressed (OFF) and induced (ON) conditions. Mean ± standard error of the mean is shown, sample size n represents cells and is indicated. Triple asterisks indicate significant difference at P < 0.0001, Student t-test. Note that the promoter allows considerable expression of eGFP under repressed conditions. Note also that the data set represent measurements from two experiments and a single transformant. (F) Bar chart showing the relative cytoplasmic fluorescence of eGFP in all promoter strains under induced conditions. All intensities were normalised against eGFP fluorescence, driven by the α-tubulin promoter Ptub2.
We noticed that the fluorescent signal intensity of cytoplasmic eGFP varied remarkably when the reporter was expressed under the various promoters (Fig. 2). We tested the “relative expression strength” of all 5 promoters by comparing their eGFP expression with that of the α-tubulin promoter Ptub2, which was also integrated into the sdi1 locus of IPO323 (strain IPO323_CPtub2eGFP; further details on this strain (named as IPO323_CeGFP) in Kilaru et al., 2015b). We found that PlaraB was shows the weakest relative expression level when induced (∼20% of Ptub2, Fig. 3F; Table 2). Pex1A, Pnar1, and Pgal7 were stronger than PlaraB, but weaker than Ptub2 (∼32%, ∼43%, ∼80%, respectively; Fig. 3F, Table 2). Picl1 induced very strong expression of eGFP (∼250% of Ptub2, Fig. 3F; Table 2).
Table 2.
Overview of the conditional Z. tritici promoters presented in this study.
| Expression under ON conditionsa | Leakiness under OFF conditionsb | Induced by | Repressed by | |
|---|---|---|---|---|
| Pnar1 | 2 | 4 | Nitrate | Ammonium |
| PlaraB | 1 | 3 | Arabinose | Glucose |
| Pex1A | 2 | 5 | Xylose | Maltodextrin |
| Pgal7 | 3 | 2 | Galactose | Glucose |
| Picl1 | 5 | 1 | Sodium acetate | Glucose |
5 = very strong, 4 = strong, 3 = medium, 2 = weak, 1 = very weak.
5 = very tight, 4 = low expression, 3 = considerable expression, 2 = strong background expression, 1 = very strong background expression.
3.4. A conditional α-tubulin mutant in Z. tritici
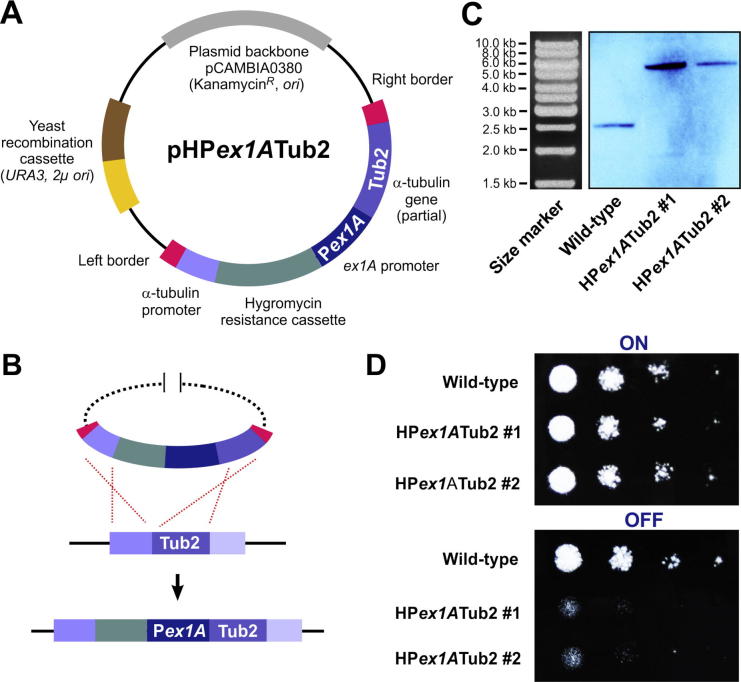
Pex1A was most “tight” under repressed condition, suggesting that it is best suited to investigate the effect of repressing expression of essential genes. We set out to provide “proof of principle”, by expressing the α-tubulin gene tub2 und the control of Pex1A. The predicted gene product ZtTub2 shows 77.4%, aa sequence identity with Tub1 from U. maydis (see Schuster et al., 2015, for more details). We generated plasmid pHPex1ATub2, which carries the 5′ end of tub2 fused to Pex1A (Fig. 4A) and was designed to introduce the conditional promoter into the native tub2 locus of Z. tritici (Fig. 4B shows integration of Pex1ATub2 into tub2 locus). Similar to the plasmids described above, pHPex1ATub2 was built into the Agrobacterium binary vector pCAMBIA0380 (CAMBIA, Canberra, Australia) for A. tumefaciens-based transformation and is suited for yeast recombination-based cloning (for more details on this method see Kilaru and Steinberg, 2015). We transformed vector pHPex1ATub2 into Z. tritici wild-type strain IPO323 and confirmed integration into the tub2 locus by digestion of genomic DNA with SacII, which shifted a fragment, detected by hybridization to a tub2 probe, from 2.6 kb to 5.4 kb (Fig. 4C; 2 transformants shown). This confirmed that the endogenous tub2 promoter in Z. tritici was replaced with ex1A promoter, resulting in strain IPO323_HPex1ATub2. α-Tubulin is an essential protein, required to form microtubules and the mitotic spindle (Schatz et al., 1988). We therefore expected that repression of Pex1A would inhibit growth. We tested this by growing IPO323 (Fig. 4D, Wild-type) and two mutant strains (Fig. 4D, IPO323_HPex1ATub2#1, IPO323_HPex1ATub2#2) on agar medium containing 5% xylose. Under these conditions, tub2 expression was induced and cells grew normally. However, when colonies were cultivated on 5% maltodextrin-containing agar medium, expression of tub2 was repressed in both mutants and cells did not grow, whereas IPO323 was not affected (Fig. 4D). It is worth noting that while Pex1A repression appears to be relatively “tight” and repressing α-tubulin under this promoter impairs growth, other genes of interest that may have low native expression levels may not be sufficiently repressed under these conditions. However, these results show that conditional promoters can be useful tools to study the importance of essential genes in Z. tritici.
Fig. 4.
A conditional α-tubulin mutant in Z. tritici. (A) Vector for homologous integration of the inducible/repressible promoter Pex1A in front of the open reading frame of the Z. tritici α-tubulin gene tub2 (see Schuster et al., 2015, for more information on tub2). Note that fragments are not drawn to scale. See main text for further information. (B) Image illustrates the integration event of vector pHPex1ATub2 into the native tub2 locus of Z. titici. After integration, expression of the endogenous α-tubulin gene is under the control of inducible/repressible promoter. (C) Southern blot, showing integration of vector into the tub2 locus in two transformants. After digestion of the genomic DNA with SacII and subsequent hybridisation with a DIG labelled DNA probe, a shift in the DNA fragment from 2.6 kb to 5.4 kb is detected. The size markers in the corresponding agarose gel are shown to the left. (D) Growth of conditional tub2 mutants on agar plates supplemented with 5% xylose (induces expression, ON) and 5% maltodextrin (represses expression, OFF). α-tubulin is an essential gene and, consequently, colonies cannot be formed when tub2 expression is repressed. Dilution steps are 10 times starting with cell density of optical density of 0.6.
4. Conclusion
In this study we established 5 conditional promoters for use in Z. tritici. This adds a repertoire of homologous promoters to the established heterologous isocitrate lyase promoter derived from N. crassa (Rohel et al., 2001). Using eGFP as a reporter we show that all promoters can be repressed under specific conditions, but allow expression in media supplemented with respective inducers. Having a choice of 5, one may ask which promoter is best suited for use in Z. tritici? Pnar1, PlaraB, and Pex1A are relatively “tight” when their repressors are present (Table 2), suggesting that they are suitable to investigate the cellular roles of essential genes. Moreover, all three promoters drive relatively weak expression under induced conditions, which reduces the risk of over-expression artifacts when essential genes are under their control. However, depending on the native expression level of a gene of interest, Pgal7 may be a better choice, as its expression level under ON conditions is significantly higher than that of PlaraB, Pnar1 and Pex1A, while the promoter is still clearly repressed in the presence of glucose. Furthermore, experimental conditions could dictate the use of a specific promoter. For example, analysis of sugar metabolic pathways precludes the usage of PlaraB, but suggests the usage of Pnar1. On the other hand, Pex1A is the tightest promoter, as it shows no eGFP fluorescence, suggesting that eGFP expression is strongly repressed under OFF-conditions. Therefore, this promoter may be useful in investing signaling cascade components, which could act in very low concentrations in the cell and, therefore, require a near-complete shutdown of their transcription. PlaraB, on the other hand, shows relatively weak expression under induced conditions. This could be useful to avoid over-expression of genes that have naturally low cellular expression. Finally, Picl1 showed exceptionally strong expression under induced conditions, but was not tightly “shut-down” in the presence of its repressor. Thus, Picl1 may be a useful tool for over-expression studies, which, again, will depend on the native expression level of the genes of interest. Taken together, the set of conditional promoters are a powerful tool to perform in-depth analysis of essential genes in Z. tritici.
Acknowledgments
The authors are grateful for funding for this work from the Biotechnology & Biological Sciences Research Council (BB/I020667/1). We acknowledge Dr. M. Guo for help with Z. tritici transformations and M. Latz for analysis of fluorescence intensities. We thank Prof. S.J. Gurr for improving the manuscript.
Footnotes
All material and protocols described here are available upon request.
Contributor Information
S. Kilaru, Email: S.Kilaru@exeter.ac.uk.
G. Steinberg, Email: G.Steinberg@exeter.ac.uk.
References
- Banks G.R., Shelton P.A., Kanuga N., Holden D.W., Spanos A. The Ustilago maydis nar1 gene encoding nitrate reductase activity: sequence and transcriptional regulation. Gene. 1993;131:69–78. doi: 10.1016/0378-1119(93)90670-x. [DOI] [PubMed] [Google Scholar]
- Bottin A., Kamper J., Kahmann R. Isolation of a carbon source-regulated gene from Ustilago maydis. Mol. Gen. Genet. 1996;253:342–352. doi: 10.1007/pl00008601. [DOI] [PubMed] [Google Scholar]
- Giaever G., Chu A.M., Ni L., Connelly C., Riles L., Veronneau S. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- Gouka R.J., Hessing J.G., Punt P.J., Stam H., Musters W., Van den Hondel C.A. An expression system based on the promoter region of the Aspergillus awamori 1,4-beta-endoxylanase A gene. Appl. Microbiol. Biotechnol. 1996;46:28–35. doi: 10.1007/s002530050779. [DOI] [PubMed] [Google Scholar]
- Holliday R. Ustilago maydis. In: King R.C., editor. Handbook of Genetics. Plenum Press; New York: 1974. pp. 575–595. [Google Scholar]
- Holsters M., de Waele D., Depicker A., Messens E., van Montagu M., Schell J. Transfection and transformation of Agrobacterium tumefaciens. Mol. Gen. Genet. 1978;163:181–187. doi: 10.1007/BF00267408. [DOI] [PubMed] [Google Scholar]
- Hood E., Gelvin S.B., Melchers L., Hoekema A. New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res. 1993;2:208–218. [Google Scholar]
- Kafer E. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv. Genet. 1977;19:33–131. doi: 10.1016/s0065-2660(08)60245-x. [DOI] [PubMed] [Google Scholar]
- Kamath R.S., Fraser A.G., Dong Y., Poulin G., Durbin R., Gotta M., Kanapin A., Le Bot N., Moreno S., Sohrmann M., Welchman D.P., Zipperlen P., Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Kema G.H.J., van Silfhout C.H. Genetic variation for virulence and resistance in the wheat-Mycosphaerella graminicola pathosystem. III. Comparative seedling and adult plant experiments. Phytopathology. 1997;87:266–272. doi: 10.1094/PHYTO.1997.87.3.266. [DOI] [PubMed] [Google Scholar]
- Kilaru, S., Steinberg, G., 2015. Yeast recombination-based cloning as an efficient way of constructing vectors for Zymoseptoria tritici. Fungal Genet. Biol. 79, 76–83. [DOI] [PMC free article] [PubMed]
- Kilaru, S., Schuster, M., Latz, M., Das Gupta, S., Steinberg, N., Fones, H., Gurr S., Talbot, N.J., Steinberg, G., 2015a. A gene locus for targeted ectopic gene integration in Zymoseptoria tritici. Fungal Genet. Biol. 79, 118–124. [DOI] [PMC free article] [PubMed]
- Kilaru, S., Schuster, M., Studholme, D., Soanes, D., Lin, C., Talbot, N.J., Steinberg, G., 2015b. A codon-optimised green fluorescent protein for live cell imaging in Zymoseptoria tritici. Fungal Genet. Biol. 79, 125–131. [DOI] [PMC free article] [PubMed]
- Kristiansson E., Thorsen M., Tamas M.J., Nerman O. Evolutionary forces act on promoter length: identification of enriched cis-regulatory elements. Mol. Biol. Evol. 2009;26:1299–1307. doi: 10.1093/molbev/msp040. [DOI] [PubMed] [Google Scholar]
- Meyer V., Wanka F., van Gent J., Arentshorst M., van den Hondel C.A., Ram A.F. Fungal gene expression on demand: an inducible, tunable, and metabolism-independent expression system for Aspergillus niger. Appl. Environ. Microbiol. 2011;77:2975–2983. doi: 10.1128/AEM.02740-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond C.K., Pownder T.A., Sexson S.L. General method for plasmid construction using homologous recombination. Biotechniques. 1999;26 doi: 10.2144/99261rr02. 134–141. [DOI] [PubMed] [Google Scholar]
- Rohel E.A., Payne A.C., Fraaije B.A., Hollomon D.W. Exploring infection of wheat and carbohydrate metabolism in Mycosphaerella graminicola transformants with differentially regulated green fluorescent protein expression. Mol. Plant Microbe Interact. 2001;14:156–163. doi: 10.1094/MPMI.2001.14.2.156. [DOI] [PubMed] [Google Scholar]
- Ruff J.A., Lodge J.K., Baker L.G. Three galactose inducible promoters for use in C. neoformans var. grubii. Fungal Genet. Biol. 2009;46:9–16. doi: 10.1016/j.fgb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Russell D.W. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2001. Molecular Cloning. [Google Scholar]
- Schatz P.J., Solomon F., Botstein D. Isolation and characterization of conditional-lethal mutations in the TUB1 α-tubulin gene of the yeast Saccharomyces cerevisiae. Genetics. 1988;120:681–695. doi: 10.1093/genetics/120.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster, M., Kilaru, S., Latz, M., Steinberg, G., 2015. Fluorescent markers of the microtubule cytoskeleton in Zymoseptoria tritici. Fungal Genet. Biol. 79, 141–149. [DOI] [PMC free article] [PubMed]
- Schuster M., Kilaru S., Ashwin P., Lin C., Severs N.J., Steinberg G. Controlled and stochastic retention concentrates dynein at microtubule ends to keep endosomes on track. EMBO J. 2011;30:652–664. doi: 10.1038/emboj.2010.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Halleck M.S., Schlegel R.A., Williamson P. A subfamily of P-type ATPases with aminophospholipid transporting activity. Science. 1996;272:1495–1497. doi: 10.1126/science.272.5267.1495. [DOI] [PubMed] [Google Scholar]
- Van der Veen P., Flipphi M.J., Voragen A.G., Visser J. Induction of extracellular arabinases on monomeric substrates in Aspergillus niger. Arch. Microbiol. 1993;159:66–71. doi: 10.1007/BF00244266. [DOI] [PubMed] [Google Scholar]
- Vu B.V., Pham K.T., Nakayashiki H. Substrate-induced transcriptional activation of the MoCel7C cellulase gene is associated with methylation of histone H3 at lysine 4 in the rice blast fungus Magnaporthe oryzae. Appl. Environ. Microbiol. 2013;79:6823–6832. doi: 10.1128/AEM.02082-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.Y., Thornton C.R., Kershaw M.J., Debao L., Talbot N.J. The glyoxylate cycle is required for temporal regulation of virulence by the plant pathogenic fungus Magnaporthe grisea. Mol. Microbiol. 2003;47:1601–1612. doi: 10.1046/j.1365-2958.2003.03412.x. [DOI] [PubMed] [Google Scholar]
- Zwiers L.H., De Waard M.A. Efficient Agrobacterium tumefaciens-mediated gene disruption in the phytopathogen Mycosphaerella graminicola. Curr. Genet. 2001;39:388–393. doi: 10.1007/s002940100216. [DOI] [PubMed] [Google Scholar]