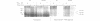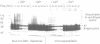Abstract
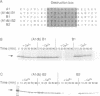
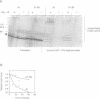
The destruction of mitotic cyclins by programmed proteolysis at the end of mitosis is an important element in cell cycle control. This proteolysis depends on a conserved motif of nine residues known as the 'destruction box', which is located 40-50 residues from the N-terminus. The sequences of the A- and B-type destruction boxes are slightly different, which might account for the differences in timing of their destruction. When the cyclin A-type destruction box was substituted for the normal one in cyclin B1 or B2, however, the resulting constructs were unexpectedly stable, although the converse substitution of B-type destruction boxes in cyclin A permitted normal degradation. We compared the ubiquitination of various cyclin constructs, and found that whereas mutation of the highly conserved residues in the destruction box strongly reduced the level of ubiquitinated intermediates, the stable destruction box 'swap' constructs did form such adducts. Thus, while ubiquitination is probably necessary for cyclin destruction, it is not sufficient. We also found that poly-ubiquitinated cyclin derivatives are still bound to p34cdc2, which is not detectably ubiquitinated itself, raising the questions of how cyclin and cdc2 dissociate from one another, and at what stage, in the process of degradation.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amon A., Irniger S., Nasmyth K. Closing the cell cycle circle in yeast: G2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell. 1994 Jul 1;77(7):1037–1050. doi: 10.1016/0092-8674(94)90443-x. [DOI] [PubMed] [Google Scholar]
- Beers E. P., Callis J. Utility of polyhistidine-tagged ubiquitin in the purification of ubiquitin-protein conjugates and as an affinity ligand for the purification of ubiquitin-specific hydrolases. J Biol Chem. 1993 Oct 15;268(29):21645–21649. [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994 Oct 7;79(1):13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Coleman T. R., Dunphy W. G. Cdc2 regulatory factors. Curr Opin Cell Biol. 1994 Dec;6(6):877–882. doi: 10.1016/0955-0674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Harper J. W. Cdk inhibitors: on the threshold of checkpoints and development. Curr Opin Cell Biol. 1994 Dec;6(6):847–852. doi: 10.1016/0955-0674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Finley D., Sadis S., Monia B. P., Boucher P., Ecker D. J., Crooke S. T., Chau V. Inhibition of proteolysis and cell cycle progression in a multiubiquitination-deficient yeast mutant. Mol Cell Biol. 1994 Aug;14(8):5501–5509. doi: 10.1128/mcb.14.8.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félix M. A., Labbé J. C., Dorée M., Hunt T., Karsenti E. Triggering of cyclin degradation in interphase extracts of amphibian eggs by cdc2 kinase. Nature. 1990 Jul 26;346(6282):379–382. doi: 10.1038/346379a0. [DOI] [PubMed] [Google Scholar]
- Glotzer M., Murray A. W., Kirschner M. W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991 Jan 10;349(6305):132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Gordon C., McGurk G., Dillon P., Rosen C., Hastie N. D. Defective mitosis due to a mutation in the gene for a fission yeast 26S protease subunit. Nature. 1993 Nov 25;366(6453):355–357. doi: 10.1038/366355a0. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ganoth D., Pehrson J., Palazzo R. E., Cohen L. H. Methylated ubiquitin inhibits cyclin degradation in clam embryo extracts. J Biol Chem. 1991 Sep 5;266(25):16376–16379. [PubMed] [Google Scholar]
- Hershko A., Ganoth D., Sudakin V., Dahan A., Cohen L. H., Luca F. C., Ruderman J. V., Eytan E. Components of a system that ligates cyclin to ubiquitin and their regulation by the protein kinase cdc2. J Biol Chem. 1994 Feb 18;269(7):4940–4946. [PubMed] [Google Scholar]
- Hershko A. The ubiquitin pathway for protein degradation. Trends Biochem Sci. 1991 Jul;16(7):265–268. doi: 10.1016/0968-0004(91)90101-z. [DOI] [PubMed] [Google Scholar]
- Holloway S. L., Glotzer M., King R. W., Murray A. W. Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell. 1993 Jul 2;73(7):1393–1402. doi: 10.1016/0092-8674(93)90364-v. [DOI] [PubMed] [Google Scholar]
- Hunt T., Luca F. C., Ruderman J. V. The requirements for protein synthesis and degradation, and the control of destruction of cyclins A and B in the meiotic and mitotic cell cycles of the clam embryo. J Cell Biol. 1992 Feb;116(3):707–724. doi: 10.1083/jcb.116.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irniger S., Piatti S., Michaelis C., Nasmyth K. Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell. 1995 Apr 21;81(2):269–278. doi: 10.1016/0092-8674(95)90337-2. [DOI] [PubMed] [Google Scholar]
- Jeffrey P. D., Russo A. A., Polyak K., Gibbs E., Hurwitz J., Massagué J., Pavletich N. P. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature. 1995 Jul 27;376(6538):313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- Jennissen H. P. Ubiquitin and the enigma of intracellular protein degradation. Eur J Biochem. 1995 Jul 1;231(1):1–30. [PubMed] [Google Scholar]
- King R. W., Peters J. M., Tugendreich S., Rolfe M., Hieter P., Kirschner M. W. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell. 1995 Apr 21;81(2):279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Stewart E., Poon R. Y., Hunt T. Cyclin A and cyclin B dissociate from p34cdc2 with half-times of 4 and 15 h, respectively, regardless of the phase of the cell cycle. J Biol Chem. 1994 Nov 18;269(46):29153–29160. [PubMed] [Google Scholar]
- Kobayashi H., Stewart E., Poon R., Adamczewski J. P., Gannon J., Hunt T. Identification of the domains in cyclin A required for binding to, and activation of, p34cdc2 and p32cdk2 protein kinase subunits. Mol Biol Cell. 1992 Nov;3(11):1279–1294. doi: 10.1091/mbc.3.11.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer M. A., Richards J. P., De Silva-Udawatta M. N., Temenak J. J., Knoblich J. A., Lehner C. F., Bennett K. L. Caenorhabditis elegans cyclin A- and B-type genes: a cyclin A multigene family, an ancestral cyclin B3 and differential germline expression. J Cell Sci. 1995 Jun;108(Pt 6):2415–2424. doi: 10.1242/jcs.108.6.2415. [DOI] [PubMed] [Google Scholar]
- Lorca T., Abrieu A., Means A., Dorée M. Ca2+ is involved through type II calmodulin-dependent protein kinase in cyclin degradation and exit from metaphase. Biochim Biophys Acta. 1994 Sep 29;1223(3):325–332. doi: 10.1016/0167-4889(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Lorca T., Cruzalegui F. H., Fesquet D., Cavadore J. C., Méry J., Means A., Dorée M. Calmodulin-dependent protein kinase II mediates inactivation of MPF and CSF upon fertilization of Xenopus eggs. Nature. 1993 Nov 18;366(6452):270–273. doi: 10.1038/366270a0. [DOI] [PubMed] [Google Scholar]
- Lorca T., Devault A., Colas P., Van Loon A., Fesquet D., Lazaro J. B., Dorée M. Cyclin A-Cys41 does not undergo cell cycle-dependent degradation in Xenopus extracts. FEBS Lett. 1992 Jul 13;306(1):90–93. doi: 10.1016/0014-5793(92)80844-7. [DOI] [PubMed] [Google Scholar]
- Lorca T., Galas S., Fesquet D., Devault A., Cavadore J. C., Dorée M. Degradation of the proto-oncogene product p39mos is not necessary for cyclin proteolysis and exit from meiotic metaphase: requirement for a Ca(2+)-calmodulin dependent event. EMBO J. 1991 Aug;10(8):2087–2093. doi: 10.1002/j.1460-2075.1991.tb07741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca F. C., Shibuya E. K., Dohrmann C. E., Ruderman J. V. Both cyclin A delta 60 and B delta 97 are stable and arrest cells in M-phase, but only cyclin B delta 97 turns on cyclin destruction. EMBO J. 1991 Dec;10(13):4311–4320. doi: 10.1002/j.1460-2075.1991.tb05009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey D. T., Yoo Y., Rechsteiner M. Ubiquitination of full-length cyclin. FEBS Lett. 1995 Aug 14;370(1-2):109–112. doi: 10.1016/0014-5793(95)00799-f. [DOI] [PubMed] [Google Scholar]
- Masui Y., Markert C. L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971 Jun;177(2):129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- Minshull J., Golsteyn R., Hill C. S., Hunt T. The A- and B-type cyclin associated cdc2 kinases in Xenopus turn on and off at different times in the cell cycle. EMBO J. 1990 Sep;9(9):2865–2875. doi: 10.1002/j.1460-2075.1990.tb07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin N., Abrieu A., Lorca T., Martin F., Dorée M. The proteolysis-dependent metaphase to anaphase transition: calcium/calmodulin-dependent protein kinase II mediates onset of anaphase in extracts prepared from unfertilized Xenopus eggs. EMBO J. 1994 Sep 15;13(18):4343–4352. doi: 10.1002/j.1460-2075.1994.tb06754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Solomon M. J., Kirschner M. W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989 May 25;339(6222):280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. Control of the yeast cell cycle by the Cdc28 protein kinase. Curr Opin Cell Biol. 1993 Apr;5(2):166–179. doi: 10.1016/0955-0674(93)90099-c. [DOI] [PubMed] [Google Scholar]
- Norbury C., Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M., Roberts J. M. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science. 1993 Mar 26;259(5103):1908–1912. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
- Pines J. The cell cycle kinases. Semin Cancer Biol. 1994 Aug;5(4):305–313. [PubMed] [Google Scholar]
- Rechsteiner M. Natural substrates of the ubiquitin proteolytic pathway. Cell. 1991 Aug 23;66(4):615–618. doi: 10.1016/0092-8674(91)90104-7. [DOI] [PubMed] [Google Scholar]
- Resnitzky D., Gossen M., Bujard H., Reed S. I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994 Mar;14(3):1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Ruoff B., Wolf D. H. Proteasome and cell cycle. Evidence for a regulatory role of the protease on mitotic cyclins in yeast. FEBS Lett. 1993 Dec 20;336(1):34–36. doi: 10.1016/0014-5793(93)81603-w. [DOI] [PubMed] [Google Scholar]
- Sagata N., Oskarsson M., Copeland T., Brumbaugh J., Vande Woude G. F. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988 Oct 6;335(6190):519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Seufert W., Futcher B., Jentsch S. Role of a ubiquitin-conjugating enzyme in degradation of S- and M-phase cyclins. Nature. 1995 Jan 5;373(6509):78–81. doi: 10.1038/373078a0. [DOI] [PubMed] [Google Scholar]
- Seufert W., Jentsch S. Ubiquitin-conjugating enzymes UBC4 and UBC5 mediate selective degradation of short-lived and abnormal proteins. EMBO J. 1990 Feb;9(2):543–550. doi: 10.1002/j.1460-2075.1990.tb08141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
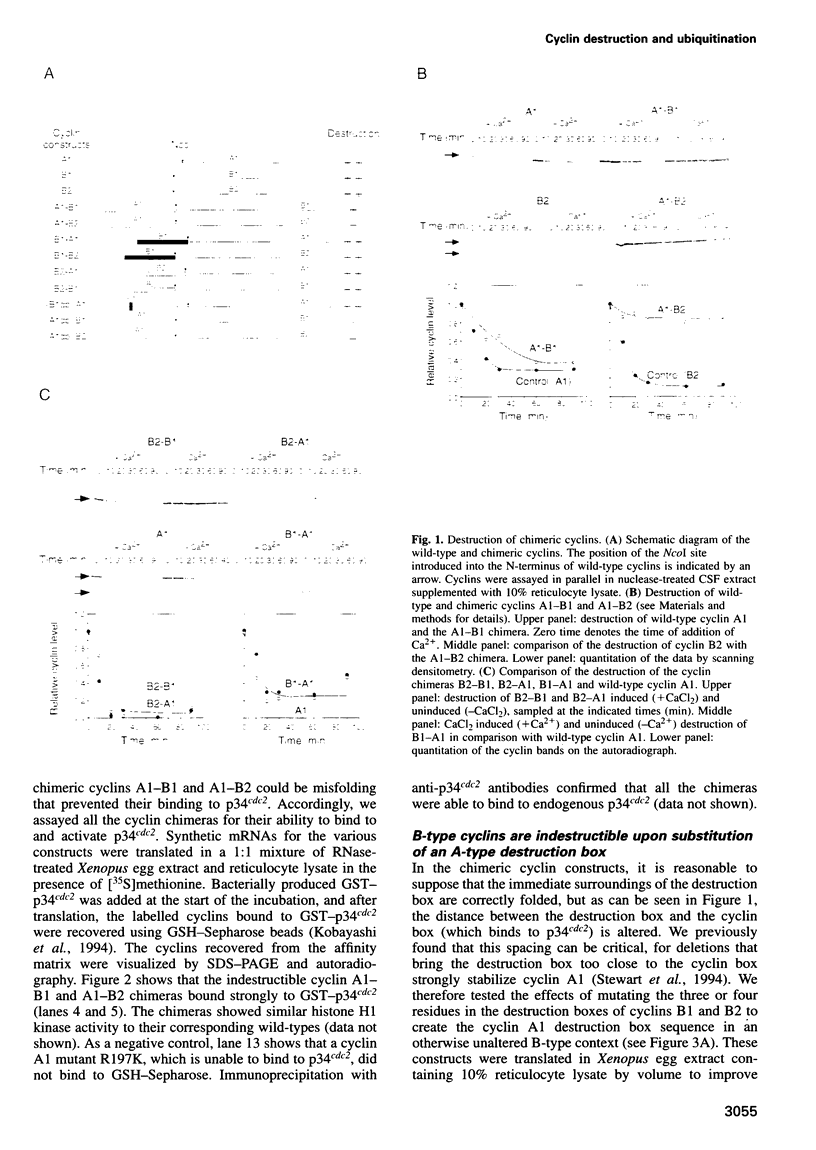
- Stewart E., Kobayashi H., Harrison D., Hunt T. Destruction of Xenopus cyclins A and B2, but not B1, requires binding to p34cdc2. EMBO J. 1994 Feb 1;13(3):584–594. doi: 10.1002/j.1460-2075.1994.tb06296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin V., Ganoth D., Dahan A., Heller H., Hershko J., Luca F. C., Ruderman J. V., Hershko A. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol Biol Cell. 1995 Feb;6(2):185–197. doi: 10.1091/mbc.6.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugendreich S., Tomkiel J., Earnshaw W., Hieter P. CDC27Hs colocalizes with CDC16Hs to the centrosome and mitotic spindle and is essential for the metaphase to anaphase transition. Cell. 1995 Apr 21;81(2):261–268. doi: 10.1016/0092-8674(95)90336-4. [DOI] [PubMed] [Google Scholar]
- Waldmann R., Hanson P. I., Schulman H. Multifunctional Ca2+/calmodulin-dependent protein kinase made Ca2+ independent for functional studies. Biochemistry. 1990 Feb 20;29(7):1679–1684. doi: 10.1021/bi00459a002. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Hunt T., Ikawa Y., Sagata N. Independent inactivation of MPF and cytostatic factor (Mos) upon fertilization of Xenopus eggs. Nature. 1991 Jul 18;352(6332):247–248. doi: 10.1038/352247a0. [DOI] [PubMed] [Google Scholar]
- van der Velden H. M., Lohka M. J. Cell cycle-regulated degradation of Xenopus cyclin B2 requires binding to p34cdc2. Mol Biol Cell. 1994 Jul;5(7):713–724. doi: 10.1091/mbc.5.7.713. [DOI] [PMC free article] [PubMed] [Google Scholar]