Abstract
We studied two non-neurotoxic amphetamine derivatives (methyl-thioamphetamine, MTA and N,N-dimethylMTA, DMMTA) interacting with serotonin (5-HT) transporters (SERTs) with affinities comparable to that of p-Cl-amphetamine (pCA). The rank order for their maximal effects in inducing both [3H]5-HT release from rat brain synaptosomes or hSERT-expressing HEK-293 cells, and currents in hSERT-expressing oocytes, was pCA » MTA ≥ DMMTA. A correlation between drug-induced release and currents is also strengthened by the similar bell shape of the dose–response curves. Release experiments indicated that MTA and DMMTA are SERT substrates although MTA is taken up by HEK-293 cells with a Vmax 40% lower than pCA. The weak effects of MTA and DMMTA in vitro might therefore be due to their properties as ‘partial substrates’ on the mechanisms, other than translocation, responsible for currents and/or release. After either local or systemic in vivo administration, MTA and DMMTA release 5-HT in a manner comparable to pCA. These findings confirm that the neurotoxic properties of some amphetamine derivatives are independent of their 5-HT-releasing activity in vivo. It is worth noting that only those amphetamine derivatives with high efficiency in inducing 5-HT release and currents in vitro have neurotoxic properties.
Keywords: methyl-thioamphetamine; N,N-dimethyl-thioamphetamine; neurotoxicity; p-Cl-amphetamine; serotonin release; serotonin transporters
Serotonin transporters (SERTs) are pre-synaptic plasma membrane proteins responsible for the reuptake of serotonin (5-HT) from the synaptic cleft. Clinically, SERTs are important pharmacological targets for the treatment of a variety of human conditions like depression and, until recently, eating disorders.
Compounds interacting with SERTs are classified, on the basis of their main mode of action, as transporter ‘blockers’ or ‘substrates.’ The former are of particular clinical interest as they are used as antidepressant drugs (e.g., tricyclic antidepressants like imipramine or the selective 5-HT reuptake inhibitors, like citalopram). Prototypical SERT substrates, on the other hand, include some amphetamine derivatives such as the anorectic drug d-fenfluramine and the psychostimulant 3,4-methylene-dioxymethamphetamine, MDMA (Mennini et al. 1996; Green et al. 2003). Unlike SERT blockers, these compounds can be translocated by SERTs, thus competing with 5-HT. Depending on the degree of competition this results in reduced 5-HT reuptake and, subsequently, higher extracellular concentrations of 5-HT. However, the overall rise in extracellular 5-HT occurs as a result of a SERT-dependent outward transport (release) of cytoplasmic 5-HT (Crespi et al. 1997).
The mechanism underlying this releasing effect of amphetamine derivatives has long been explained according to the model of ‘facilitated exchange diffusion’ or ‘transporter-mediated hetero-exchange’ (Fischer and Cho 1979; Levi and Raiteri 1993), often simplified by visualizing transporters as ‘revolving doors.’ However, recent data suggest that the operating modes of transporters, as well as the mechanisms of action of the ‘substrates,’ are very likely much more complex (Gnegy 2003; Seidel et al. 2005), and therefore, cast doubt on the sole dependence on these models. The oligomer-based counter-transport model has recently been introduced, explaining carrier-mediated efflux as dependent on current induction as well as on the quaternary structure (Sitte et al. 2004; Seidel et al. 2005).
3,4-Methylenedioxy-methamphetamine, d-fenfluramine and p-Cl-amphetamine (pCA, another selective 5-HT-releasing amphetamine derivative) induce long-term deficits of 5-HT nerve endings (Mamounas and Molliver 1988; Molliver and Molliver 1990; Green et al. 2003). However, acute 5-HT release, while possibly necessary, is presumably not sufficient to induce 5-HTergic neurotoxicity. Some SERT substrates induce SERT-dependent 5-HT release in vivo and/or in brain slices comparable to that elicited by neurotoxic amphetamine derivatives but, importantly, they lack their 5-HT neurotoxic potential. These compounds include an amphetamine derivative, 4-methyl-thioamphetamine (MTA), a conformationally restricted amphetamine analogue, 5-methoxy-6-methyl-2-aminoindan (MMAI) (Johnson et al. 1991; Huang et al. 1992; Rudnick and Wall 1992, 1993; Scorza et al. 1999; Gobbi et al. 2002), and a non-amphetamine compound 1-(m-chlorophenyl)piperazine (mCPP) (Pettibone and Williams 1984; Eriksson et al. 1999; Baumann et al. 2001).
It had been suggested that the lack of neurotoxic properties of MTA and MMAI might be related to the lack of dopamine (DA)-releasing properties (Johnson and Nichols 1991; Huang et al. 1992; Rudnick and Wall 1993), in line with the possibility of a role of DA in the events leading to neurotoxicity (Sprague et al. 1998). However, this was not supported by our previous in vitro findings in synaptosomes that MTA is comparable with pCA and MDMA as regards its potency as a DA-releasing agent (Gobbi et al. 2002). In the same paper, we reported that neurotoxic and non-neurotoxic compounds markedly differ in their ability to induce [3H]5-HT release in vitro, with the former (pCA, MDMA and d-fenfluramine) being efficient 5-HT releasers and the latter (MTA and mCPP, but also tramadol) much less active (Gobbi et al. 2002). These findings, highlighting a discrepancy between in vivo and in vitro synaptosomal data, suggested that in vitro studies in synaptosomes might bring to light subtle differences between 5-HT-releasing agents in their mode of action at 5-HT nerve endings. A better understanding of these differences might be useful to clarify the mechanism underlying neurotoxic potential of some amphetamine derivatives for serotonergic neurons.
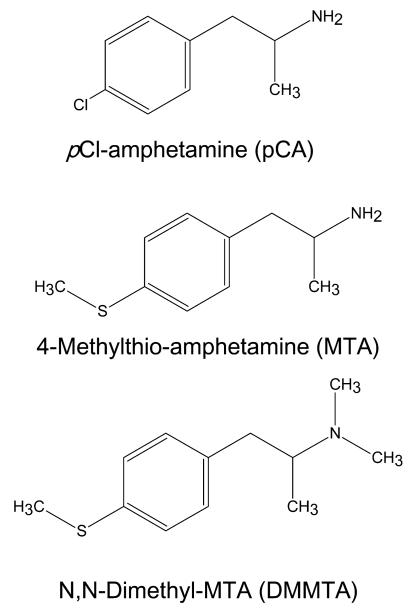
The present report extends our studies to another amphetamine derivative, N,N-dimethyl-MTA (DMMTA) (Hurtado-Guzman et al. 2003) (chemical structure in Fig. 1), selected because initial experiments identified it as a non-neurotoxic compound with very weak in vitro 5-HT-releasing properties in synaptosomes. The results strengthen the notion that the neurotoxicity of some amphetamine derivatives do not correlate with their ability to release 5-HT in vivo and suggest that the in vitro efficiency as 5-HT releasers can be predictive of their neurotoxic properties.
Fig. 1.
Chemical structures of the amphetamine derivatives used in the present study.
Materials and methods
Drugs
MTA and DMMTA, as hydrochloride salts (Hurtado-Guzman et al. 2003), were synthesized and kindly provided by Dr Bruce K. Cassels of the Universidad de Chile. pCA hydrochloride, citalopram hydrochloride and paroxetine were purchased, respectively, from Sigma Chemical Co (St. Louis, MO, USA), Lundbeck (Copenhagen, Denmark) and GlaxoSmithkline Pharma, Vienna, Austria.
Animals
Adult Male CRL:CD (SD) BR rats (Charles River, Italy) were used. They were kept in a controlled environment with a 12-h light-dark cycle and 21°C room temperature. Food and water were provided ad libitum. Procedures involving animals and their care were conducted in conformity with the institutional guidelines that are in compliance with national (D.L. n. 116, G.U., Suppl. 40, Feb. 18, 1992) and international laws and policies (EEC Council Directive 86/609, OJ L 358, 1, Dec.12, 1987; Guide for the Care and Use of Laboratory Animals, U.S. National Research Council, 1996).
Ex vivo [3H]citalopram binding
Rats were injected intraperitoneally with saline, 5 mg/kg pCA, 5 or 20 mg/kg DMMTA. One week later, they were killed by decapitation, their hippocampi dissected out and stored at −80 °C until the [3H]citalopram binding assay was conducted as described previously (Gobbi et al. 2002). Briefly, the hippocampi were resuspended in about 200 volumes of ice-cold Tris–HCl 50 mM, pH 7.4, containing 120 mM NaCl and 5 mM KCl, and incubated with 1 nM [3H]citalopram (85 Ci/mmol). Non-specific binding was determined using 1 μM fluoxetine. After 60 min at 25°C, incubation was stopped by rapid filtration through GF/B fiber filters pre-soaked in 0.5% polyethylenimine, which were then washed, dried and counted in a liquid scintillation counter. Inhibition curves were fitted as detailed below for uptake studies.
Preparation of the synaptosomal P2 fraction
Rats were killed by decapitation and their hippocampi were rapidly dissected out and homogenized in 40 vols of ice-chilled 0.32 M sucrose, pH 7.4, in a glass homogenizer with a Teflon pestle. The homogenates were centrifuged at 1000 g for 5 min and the supernatants centrifuged again at 12 000 g for 20 min to yield the crude synaptosomal pellet (P2).
Synaptosomal [3H]5-HT uptake
Synaptosomal uptake studies were carried out as described previously (Gobbi et al. 2002). Briefly, the P2 pellets were diluted with Krebs-Henseleit buffer to a concentration of about 5 mg wet weight tissue/mL, pre-incubated for 5 min at 30°C with or without the compounds to be tested, then incubated for another 5 min with 30 nM [3H]5-HT (NEN, 21.4 Ci/mmol). Non-specific uptake was determined in the presence of 0.3 μM citalopram. The reaction was stopped by adding 1 mL of ice-chilled Krebs-Henseleit buffer. Samples were immediately filtered through cellulose mixed ester filters (0.65 μm pore size, Millipore, Cork, Ireland) and washed twice. The radioactivity trapped on the filters was counted in 4 mL of Ultima Gold MV (Perkin Elmer, Groningen, The Netherlands) in a LKB 1214 Rackbeta liquid scintillation counter (Perkin Elmer, Turku, Finland) with a counting efficiency of about 60%. The inhibitory effect of five different DMMTA concentrations (from 30 to 3 μM), in triplicate, was determined in three different experiments. The log concentration/percentage inhibition curve was fitted using the one-site competition equation built into GraphPad Prism 4 for Windows (GraphPad Software, Inc, San Diego, CA, USA) to obtain the IC50 with 95% CI.
Release from [3H]5-HT-preloaded hippocampal synaptosomes
Synaptosomal release studies were carried out as described previously (Gobbi et al. 2002). Briefly, the P2 pellets were resuspended in Krebs-Henseleit buffer and incubated for 15 min at 37°C in the presence of 60 nM [3H]5-HT (NEN, 24.4 Ci/mmol). Synaptosomes were then layered onto cellulose mixed ester filters (0.65 μm pore size, Millipore) in a 20-chamber superfusion apparatus held at 37°C (Raiteri et al. 1974).
Superfusion was started (t = 0 min) at a rate of 0.5 mL/min with standard medium and, after a 44-min equilibration period, 4-min fractions were collected from each chamber. The first, collected from t = 44 to 48 min, was used to determine basal release. ‘Releasing’ drugs were normally added at t = 47 min and their effects were measured in the second fraction (from t = 48 to 52 min; the fluid takes about 1.5 min to flow from the filters to the collecting vials). When used, 1 μM citalopram was added from t = 40 min. The filters and separate fractions were put into scintillation vials and counted for radioactivity.
The fractional release rate (FRR) was calculated as 100 times the amount of radioactivity released into each 4-min fraction over the total radioactivity present on the filter at the start of that fraction. The FRRs before the releasing stimulus (i.e., t = 44–48 min), expressed as the percentage in 4 min, are reported as basal outflow. The overflow (%) was calculated as the difference between the FRR in the presence (t = 48–52 min) and absence of the drug (mean t = 44–48 min).
In previous studies (Gobbi et al. 1992), we showed that the transporter-dependent tritium overflow evoked by all the amphetamine derivatives tested (i.e., MDMA, pCA, d-fenfluramine, amphetamine) is mainly due to unmetabolized [3H]neurotransmitter. We therefore assumed here that the same held true for the transporter-dependent DMMTA-evoked tritium overflow, which is referred to as [3H]5-HT.
Release from [3H]5-HT-preloaded HEK-293 cells
Superfusion experiments were conducted as previously described (Scholze et al. 2000). In brief, HEK-293 cells stably expressing human SERT (hSERT) were grown overnight on round glass cover slips (diameter 5 mm) coated with poly-d-lysine (25 mg/mL) at 2 × 104 cells/well. hSERT cells were incubated with [3H]5-HT (0.4 μM, specific activity 28 Ci/mmol, New England Nuclear) for 20 min at 37°C in a final volume of 0.1 mL of a modified Krebs-Ringer-HEPES buffer [in mM]: 10 HEPES, 120 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 20 d-glucose, final pH 7.3. The coverslips were then transferred to small superfusion chambers (0.2 mL) and superfused with the same buffer (0.7 mL/min) at 25°C. The experiment was started after 45 min of washout with the collection of 2-min fractions. Amphetamine derivatives were added after collection of three baseline fractions; in some of the experiments, monensin was added a concentration of 10 μM concomitantly with the amphetamine derivatives (Scholze et al. 2000). At the end of the experiment, cells were lysed in 1% sodium dodecyl sulfate. Tritium in the superfusate fractions and in the sodium dodecyl sulfate lysates was determined by liquid scintillation counting. The tritium release was expressed as FRR (i.e., the radioactivity released during a 2-min fraction was expressed as a percentage of the total radioactivity present in the cells at the beginning of that fraction).
Drug-induced release was calculated by subtracting the mean FRR of the three baseline fractions from the mean FRR of the last three fractions after drug exposure, and is therefore expressed as the percentage in 2 min.
Uptake of PCA and MTA in HEK-293 cells
Uptake of p-chloroamphetamine (pCA) and MTA was determined as given in Seidel et al. (Seidel et al. 2005), with minor modifications. 5 × 105 cells stably expressing SERT were incubated for 15 min at 22°C in Krebs-HEPES buffer (10 mM Hepes, 120 mM NaCl, 3 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 20 mM glucose, final pH 7.3) with or without 10 μM paroxetine (in the wells for determination of non-specific uptake). After addition of different concentrations of amphetamine derivatives ranging from 1 to 30 μM and incubation for 1 min, the medium was aspirated and the cells were rapidly rinsed three times each with 1 mL ice-cold Krebs-Hepes buffer; 70 μL of double-distilled water was added and the cells were scraped off the wells using a rubber policeman and transferred to a reaction tube. pCA and MTA were extracted from the cells adding 150 μL acetonitrile. The cells were sonicated, frozen in liquid nitrogen and thawed directly afterwards. After centrifugation, 150 μL of the supernatant was spiked with internal standard (50 μL d-amphetamine, 1 mg/mL) and 6.25 μg dansyl chloride (Serva Feinbiochemika, Heidelberg, Germany) and mixed with 150 μL aqueous solution (pH 9.2; 15 mM Na2HPO4 final concentration). After 20 min at 65°C the samples were centrifuged to remove precipitate; 150 μL were injected into an HPLC system and resolved at a flow rate of 1 mL/min at 50°C [mobile phase 1 : 1, 30 mM KH2PO4 (pH 6): acetonitrile; column: Hewlett-Packard Hypersil MOS 200 × 2.1 mm; fluorescence detection: Shimadzu RF-551; excitation and emission wavelength, 318 and 510 nm].
Preparation of Xenopus laevis oocytes and electrophysiological measurements
Oocytes were surgically removed from mature Xenopus laevis obtained from Xenopus Express (France), anesthetized with 0.2% tricaine. The follicular tissue containing the oocytes was washed with Ca2+-free modified Ringer’s buffer (R96 (mM): 96 NaCl, 2 KCl, 20 MgCl2, 5 Hepes, pH 7.4) und cut into small pieces. Portions of this tissue (approximately 10 mL) were added to 20 mL modified R96 with 0.1% collagenase 1A (Sigma–Aldrich, Vienna, Austria), and nutated for 45–60 min. Collagenase treatment was terminated by repeated washing in modified R96. The remaining follicular layer of the oocytes was manually removed and stage V–VI oocytes were collected for the subsequent cRNA injection.
The cDNA of the hSERT in pOTV (a kind gift from Dr M. Sonders, Columbia University, New York, USA) was linearized downstream of the poly(A) segment using NOT I or SPE I (Roche Austria, Vienna, Austria).
The cDNA was transcribed to cRNA using the Ambion mMessage Machine T7 kit (Ambion, Austin, TX, USA). The amount of cRNA was measured by determining the extinction coefficient at 260 nm. Each oocyte was injected with 10 ng cRNA with a Drummond Nanoject injector (Drummond Scientific Company, Broomall, PA, USA) and incubated at 19°C in standard oocyte buffer (SOS (mM): 96 NaCl, 2 KCl, 1 MgCl, 1.8 CaCl2, 5 Hepes, 2.5 NaPyruvat) supplemented with 50 μg/mL gentamicin, 50 μg/mL ampicillin and 5% dialyzed horse serum.
Electrophysiological experiments were performed using the two-electrode voltage-clamp technique with a Dagan Ca-1b amplifier. Oocytes were mounted on the RCP-10 Perfusion Recording Chamber and impaled with glass electrodes filled with potassium chloride (1 M). The resistance of the electrodes was approximately 1–2 MΩ. Oocytes were superfused with the Valve-Mate-8 Perfusion System at 1 mL/min using superfusion buffer [(mM): 100 NaCl, 2 KCl, 1 CaCl2, 1 MgCl2, 10 Hepes, pH 7.4]. The solution bath and superfused solutions were set to 19°C with a TC-10 temperature controller. The amplifier, perfusion chamber, perfusion control device and temperature control were purchased from Dagan Corporation (Minneapolis, MN, USA).
The voltage protocols were applied with an Axon Digidata 1322A D/A converter using Clampex 9.2 software (Axon Instruments, Union City, CA, USA) on a PC. Data were filtered at 20 Hz by the built-in low-pass filter of the amplifier, and digitalized at 500 Hz by the acquisition software.
After positioning and impaling the oocyte, the superfusion was turned on to rinse it with buffer, and the oocyte was clamped to a holding potential of −80mV. Typically, it took 1–3 min for the system to stabilize. The currents were elicited by superfusion buffer containing pCA, MTA or DMMTA at different concentrations, as indicated.
Baseline subtraction was performed with Clampfit 9.2 (Axon Instruments) by subtracting the leak current from the amphetamine-induced current. For further analysis and curve fitting, we used Prism 4.0 (Graphpad Software, San Diego, CA, USA).
Release from [3H]5-HT-preloaded hippocampal slices
Coronal hippocampal slices (0.4 mm thick) were prepared using a McIlwain tissue chopper and incubated in Krebs-Henseleit buffer with 80 nM [3H]5-HT for 15 min at 37°C. After washing with standard buffer, one slice was transferred to each of parallel superfusion chambers and superfused for 51 min at a rate of 0.5 mL/min. After washing for 36 min to achieve a constant base lane of tritium efflux, six 3-min fractions were collected. Drugs were added at t = 39 min, after the second fraction had been collected, and their effects were measured in the 45–48 or 48–51 min fraction, where the maximum effect was generally achieved. When used, 3 μM 6-nitroquipazine was present from t = 30 min. At the end of the experiments slices were dissolved in 0.5 mL Soluene 35 and tritium in superfusate samples and counted by liquid scintillation counting. The FRR was calculated as for synaptosomes and the drug-induced overflow as the difference between the FRR in the presence (t = 45–48 min) and absence of the drug (mean t = 36–39 min).
In vivo microdialysis
In all experiments, male Sprague–Dawley rats (250–300 g) anesthetized with chloral hydrate (400 mg/kg i.p.) were implanted with a dialysis probe (CMA/12, membrane length 2 mm, CMA Microdialysis, Sweden) into either the lateral septum (LS) or the dorsal hippocampus (coordinates with respect to bregma AP +0.2; ML −0.8, DV −5.5 and AP −3.8; ML 2.6, DV −3.6, respectively). We decided to study the effects of compounds in the LS since this region is part of the septohippocampal formation; it receives a significant serotonergic input from raphe nuclei (Dinopoulos et al. 1993) and has been considered an essential nodal point for integrating cognitive and emotional information and a target region for the action of SERT acting compounds such as antidepressants (Sheehan et al. 2004).
Artificial cerebrospinal fluid (NaCl 120 mM; KCl 2.4 mM; Na2HPO4 0.9 mM; NaH2PO4 1.4 mM; pH = 7.4) was perfused at 1 μL/min using a perfusion pump (Harvard Apparatus 22). Samples were collected every 20 min starting 120 min after beginning the perfusion. After the fourth basal fraction, drugs were administered locally (for 20 min) or systemically. Samples were received in HClO4 (4 μL) and the extracellular concentration of 5-HT was measured by HPLC-ED. The chromatographic conditions were as previously described (Sotomayor et al. 2005). At the end of the experiments, the rats were killed and the correct placement of the probe was verified. Results were expressed as femtomoles of 5-HT per fraction (uncorrected for recovery) and are presented (mean ± SEM) as a percentage of basal values.
Statistical analysis was done with one-way anova followed by the Student–Newman–Keuls multiple comparison test.
Under these conditions, 5-HT absolute basal values in LS and dorsal hippocampus were respectively 24.1 ± 1.6 fmol/20 μL (n = 41) and 5.0 ± 0.5 fmoles/20 μL (n = 24),. In experiments where citalopram (10 μM) was included in the perfusion fluid, the 5-HT absolute basal value in LS was 98.3 ± 14.1 fmol/20 μL (n = 9).
Results
Neurotoxic effects of DMMTA ex vivo
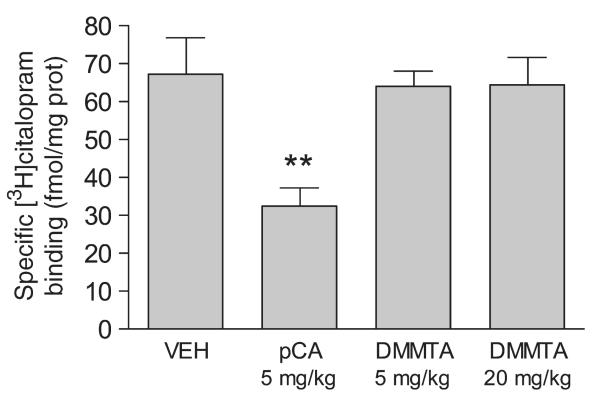
Figure 2 shows that treatment of rats with DMMTA up to 20 mg/kg i.p. was not neurotoxic, as indicated by [3H]citalopram binding to rat hippocampal SERTs 1 week after drug administration. In contrast, 1 week after treatment, 5 mg/kg pCA caused about a 51% decrease of SERTs.
Fig. 2.
In vivo treatment with DMMTA was not neurotoxic for 5-HTergic neurons in rat hippocampus. Male rats received one intraperitoneal injection of 5 or 20 mg/kg DMMTA or 5 mg/kg pCA. Control rats received vehicle alone (saline solution). One week later, rats were killed and [3H]citalopram binding was measured in their hippocampi. Each value represents the mean ± SE of five rats. **p < 0.01 versus vehicle-treated rats.
Effect of DMMTA on synaptosomal [3H]5-HT uptake
N,N-dimethylMTA inhibited [3H]5-HT uptake in rat hippocampal synaptosomes with an IC50 of 171 nM (95% CI 128–230 nM). For comparison, the IC50 values of pCA and MTA, determined under identical conditions, were 216 and 102 nM (Gobbi et al. 2002).
Releasing effect of DMMTA on synaptosomes preloaded with [3H]5-HT
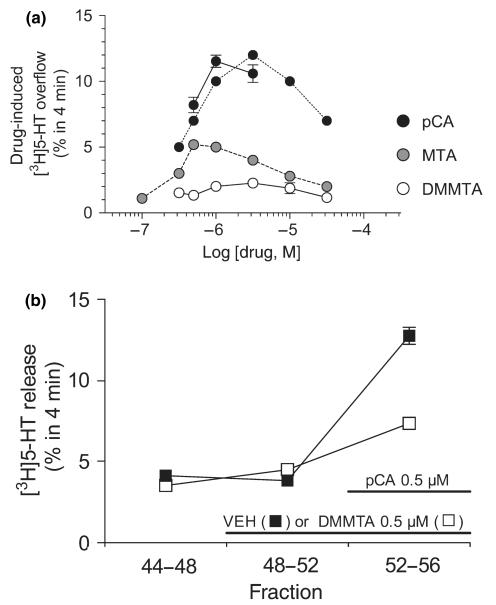
Figure 3(a) shows the concentration–response curve for the releasing effect of DMMTA applied to superfused rat hippocampal synaptosomes preloaded with [3H]5-HT. The releasing effect of selected concentrations of pCA, measured in parallel with DMMTA, is also shown. For comparison, the concentration–response curves of MTA and pCA determined previously are also indicated (Gobbi et al. 2002). [3H]5-HT release induced by DMMTA was low, less than the MTA-induced release and much lower than the pCA-induced release. As previously demonstrated for pCA and MTA (Crespi et al. 1997; Gobbi et al. 2002), the releasing effect of DMMTA was SERT-dependent, since it was almost completely abolished by 1 μM citalopram (data not shown).
Fig. 3.
Panel (a). Releasing effect of DMMTA from superfused rat hippocampal synaptosomes preloaded with [3H]5-HT. Release was calculated by subtracting the fractional release rate (FRR) immediately before the stimulus (basal release) from that immediately after. Each value is the mean ± SEM of 3–12 replications from 1 to 4 experiments; in most cases SEM is within the size of the symbols. In each experiment testing DMMTA derivatives, pCA was always added as a positive internal control and the results are shown. Dashed lines show the concentration-effect curves previously reported with pCA and MTA (Gobbi et al. 2002), for comparison. Panel (b) DMMTA (0.5 μM) antagonized the [3H]5-HT release induced by 0.5 μM pCA. Synaptosomes were exposed to DMMTA, or its vehicle (VEH) from t = 48 min onward and pCA was added from t = 52 min. Three 4-min fractions were collected: t = 44–48 (basal release), t = 48–52 (DMMTA-induced release) and t = 52–56 (pCA-induced release in the absence or presence of DMMTA). Ordinates indicate the [3H]5-HT released in each 4-min fraction as a percentage of the total radioactivity in the synaptosomes at the start of that fraction. Each value is the mean ± SEM of four replications (four chambers in parallel). Two-way anova was done using the data of fractions 2 and 3, and the results indicated significant interactions (p < 0.01) between DMMTA and pCA.
Figure 3(b) shows that 0.5 μM DMMTA (causing very little releasing effect per se) interacted with SERTs, since it markedly (68%) antagonized the releasing effect of an equimolar concentration of pCA. This datum agrees with the 5-HT uptake data, indicating similar affinities of the two compounds for SERTs.
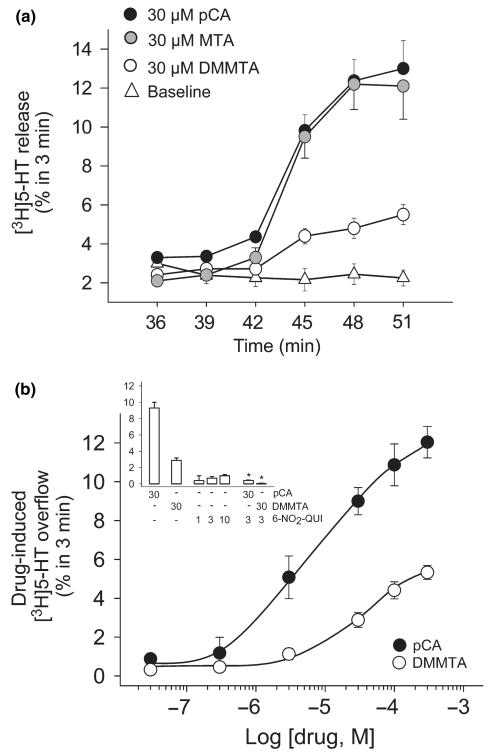
Releasing effect of DMMTA, MTA and pCA on hSERT-expressing HEK-293 cells preloaded with [3H]5-HT
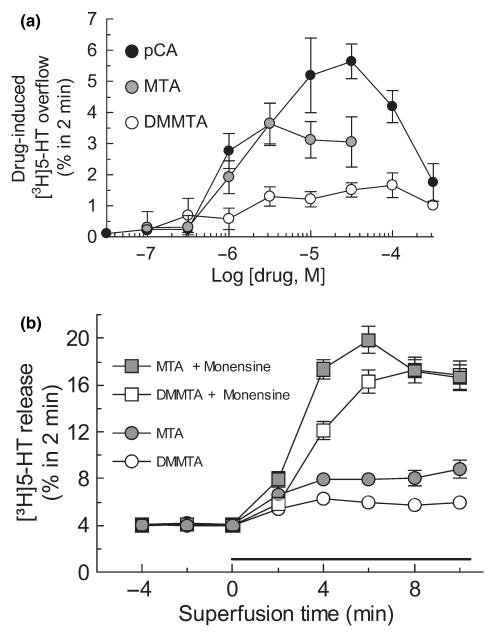
Figure 4(a) shows the releasing effect of the three amphetamine derivatives applied to superfused hSERT-expressing HEK-293 cells preloaded with [3H]5-HT. Like in synaptosomes, pCA was a much more potent [3H]5-HT releaser than MTA, and gave bell-shaped dose–response curve. [3H]5-HT release induced by DMMTA was even lower than the release induced by MTA.
Fig. 4.
Panel (a) Releasing effect of DMMTA, MTA, and pCA from superfused SERT-expressing HEK-293 cells preloaded with [3H]5-HT. Drug-induced release is the difference between the mean fractional release rate (FRR) after and before cells are exposed to drugs. Symbols represent mean ± SEM of 6–9 observations from two or three independent experiments. Panel (b) Effect of monensin on the [3H]5-HT-releasing effects of MTA and DMMTA. After three fractions of basal efflux, the cells were exposed, as indicated, to 10 μM MTA or DMMTA, in the absence or presence of 10 μM monensin. Data are presented as FRR. Symbols represent mean ± SEM of nine observations from three separate experiments.
The [3H]5-HT release induced by both MTA and DMMTA was markedly enhanced by co-application of the Na+/H+-ionophore monensin (Fig. 4b). Previously, we had found that monensin, which raises the intracellular sodium concentration, only enhanced efflux triggered by transporter substrates such as pCA, and that this measure sufficed to clearly distinguish transport uptake inhibitors from transporter substrates (Scholze et al. 2000). Thus, the results presented in Fig. 4(b) strongly argue for DMMTA and MTA meeting the criteria to be regarded as transporter substrates.
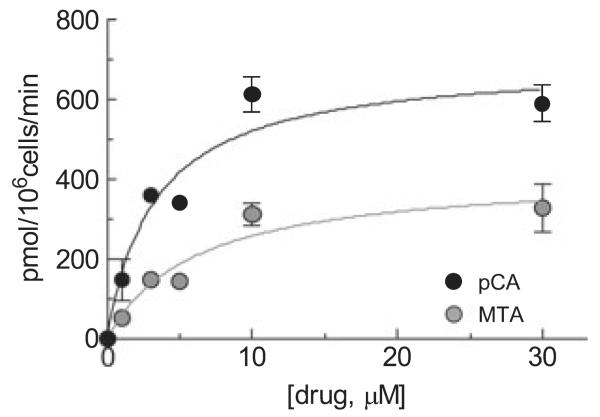
Uptake of pCA and MTA in SERT-expressing HEK-293 cells
To further confirm the substrate properties of MTA we measured its paroxetine-specific uptake in SERT-expressing HEK-293 cells. Figure 5 shows that MTA is taken up by SERTs with a Km of 6.1 ± 2.3 μM and Vmax 416 ± 58 pmol/min/106 cells. This Vmax was, however, significantly lower (40%, p < 0.02) than that found, in parallel, for pCA (695 ± 57 μM). The Km of pCA uptake was 3.3 ± 0.9 μM, consistent with t measurements (Seidel et al. 2005). The uptake of DMMTA could not be measured because a free amino group is needed for derivatization, rendering HPLC analysis impossible.
Fig. 5.
Concentration dependence of uptake of MTA and pCA in HEK-293 cells stably expressing hSERT. Cells were incubated for 1 min with MTA and pCA at the concentrations indicated. Amphetamines were then extracted from the cells and determined by HPLC as described under Materials and methods. Symbols show the specific (i.e., paroxetine-sensitive) uptake and are means ± SEM of three experiments run in triplicate. The saturation curves were fitted using the ‘one-site model’ hyperbola equation (GraphPad Prism 4.0a). The calculated Vmax of MTA (416 ± 58) was significantly lower (p < 0.01) than the Vmax of pCA (695 ± 58) whereas Km (6.0 ± 2.3 vs. 3.3 ± 1.0 μM, respectively) were not significantly different (compared with F-tests included in the GraphPad Prism 4.0a software).
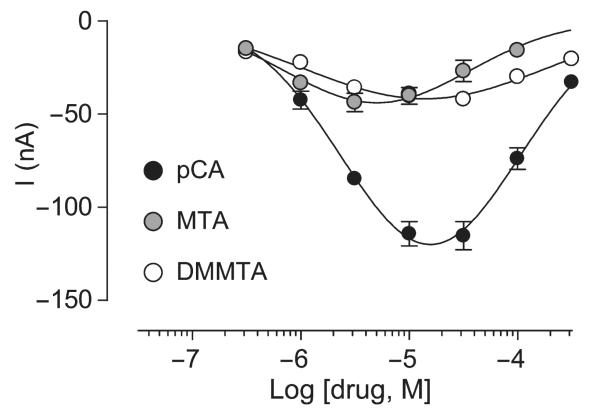
Induction of inward currents in hSERT-expressing oocytes
Application of amphetamine derivatives to hSERT-expressing Xenopus laevis oocytes led to distinct, robust generation of current. Clearly, pCA induces much higher inward currents than DMMTA and MTA (Fig. 6). All three amphetamine derivatives induced bell-shaped concentration–response curves.
Fig. 6.
Inward currents induced by application of different concentrations of pCA, DMMTA and MTA to hSERT expressing Xenopus laevis oocytes. Electrophysiological experiments were done using the two-electrode voltage-clamp technique with oocytes clamped to a holding potential of −80 mV. Data are means ± SEM of three experiments performed in triplicate.
Releasing effect of DMMTA from brain slices preloaded with [3H]5-HT
Figure 7, panel (a) illustrates the time-course of the releasing effects brought about by 30 μM DMMTA, MTA, and pCA, when applied to superfused rat hippocampal brain slices preloaded with [3H]5-HT. As previously reported, the releasing effect of MTA was similar to that of pCA (Huang et al. 1992) whereas DMMTA had much less effect. The complete concentration–response curves of DMMTA and pCA are compared in panel (b): at the highest concentration (300 μM) the releasing effect of DMMTA was about half that of pCA. The releasing effect of 30 μM of both compounds was almost completely abolished by 3 μM 6-nitroquipazine, indicating the SERT-dependence of this effect (panel b, inset); 6-nitro-quipazine by itself significantly increased [3H]5-HT release only at 10 μM.
Fig. 7.
Releasing effect of DMMTA from rat brain hippocampal slices preloaded with [3H]5-HT. Slices were prepared, labeled with the radioactive tracer and exposed to drugs in superfusion. DMMTA, MTA or pCA were added at t = 39 min of superfusion and maintained until the end of the experiments. 6-Nitro-quipazine (6-NO2-QUI) was introduced at t = 30 min. Panel (a) Time course of the release of [3H]5-HT from slices exposed to 30 μM DMMTA, MTA or pCA. Data are expressed as FRR (see Materials and methods). Panel (b) and inset. Concentration–response curve of the effects of DMMTA or pCA and block by 6-NO2-QUI. Data are expressed as drug-induced [3H]5-HT overflow, calculated by subtracting the basal release (second fraction collected; t = 36–39 min) from the release measured in the fifth fraction collected (t = 45–48 min), where the drugs generally reached maximum effect. The data presented are mean ± SEM of 4–6 experiments in triplicate. *p < 0.001 compared to the effect of pCA or DMMTA alone (two-tailed Student’s t-test).
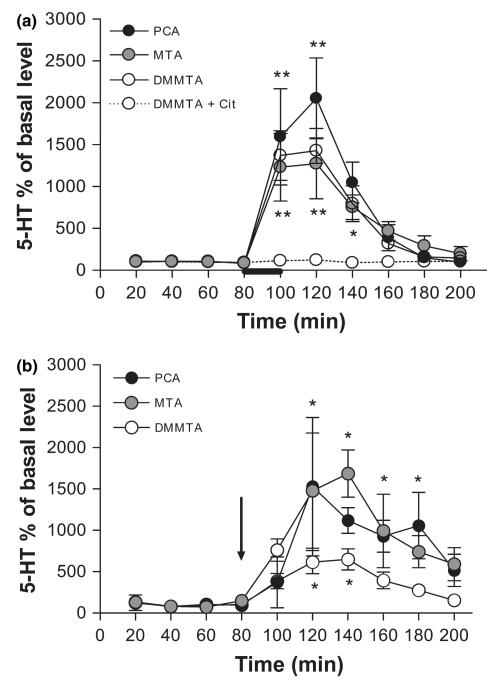
In vivo effect of DMMTA on brain 5-HT extracellular levels
Figure 8(a) shows the effects of DMMTA, MTA or pCA applied through the dialysis probe, on extracellular 5-HT in the LS of anesthetized rats. In view of the structural similarities between 5-HT and the three drugs (an aromatic ring separated from an amino group by a two-carbon atom chain and molecular weights in the 170–209 range), concentrations were chosen assuming that the probe recoveries for both drugs would be similar to that for 5-HT (8–11%). Thus, a concentration of 1 mM in the perfusion fluid would be expected to give approximately 100 μM in the proximity of the cannula.
Fig. 8.
Microdialysis data showing the effects of DMMTA, MTA or pCA either applied locally (panel a, drugs infused at a concentration of 1 mM as indicated by the bar) or given systemically (panel b, i.p. injection of 5 mg/kg is indicated by the arrow) on extracellular 5-HT in the rat lateral septum (LS) and in the dorsal hippocampus. Data are mean ± SEM (n = 4–6 for each condition). Asterisks indicate a significant difference between samples after treatment and the corresponding basal values, calculated by one-way anova-Student–Newman–Keuls: *p < 0.05, and **p < 0.01.
When administered through the probe, DMMTA raised extracellular 5-HT in the LS. This effect was similar in magnitude to that observed with MTA and slightly lower than with pCA (Fig. 8a). In addition, the effect of MTA was similar to that previously reported in the dorsal hippocampus (Gobbi et al. 2002). The releasing effect of DMMTA involves 5-HT transporters, since SERT blockade by 10 μM citalopram, completely abolished it (Fig. 8a, dashed line).
Systemically administered 5 mg/kg doses of the three amphetamine derivatives (i.p.) markedly raised extracellular 5-HT, measured by microdialysis in the dorsal hippocampus (Fig. 8b). The releasing effect of DMMTA was about half that after either MTA and pCA.
Discussion
In the present study, we further characterized MTA and DMMTA, two amphetamine derivatives which, despite their high affinity for SERTs, lack the neurotoxic properties typical of this class of compounds (e.g., pCA, MDMA, and d-fenfluramine). The effects of pCA were determined for comparison. Most of the MTA’s effects, both in vitro and in vivo, have already been published, but we can now add some interesting data.
DMMTA is an MTA derivative recently described for its anti-MAO-A activity (10-times less potent, however, than MTA) (Hurtado-Guzman et al. 2003). The 5-HT-releasing effects of DMMTA and its parent compounds were evaluated in vivo, in microdialysis studies, and in vitro, in superfused synaptosomes and brain tissue slices and in heterologously transfected cells stably expressing hSERTs. In particular, the latter system gave data that can be more easily interpreted as pure effects of the compounds at SERTs, avoiding the confounding factor represented by the presence of synaptic vesicles and vesicular release. Since SERT substrates not only induce release but also SERT-mediated uncoupled charge transfer (Mager et al. 1995), we also measured the currents induced by DMMTA, MTA, and pCA in voltage-clamped Xenopus oocytes expressing hSERT (Gerstbrein and Sitte 2006).
In vitro, MTA, DMMTA and pCA have different ‘efficacies’ for induction of 5-HT release and currents: evidence for a correlation between these SERT-mediated effects
When tested on superfused synaptosomes preloaded with [3H]5-HT, DMMTA-induced tritium release was significantly lower than that induced by pCA or MTA: if the maximal release induced by pCA is set to 100%, then the maximal release induced by MTA and DMMTA is about 50% and 25%, respectively. Although low, these effects exclude that these compounds behave as pure SERT blockers. In fact, in our experimental model of superfused synaptosomes, the sole inhibition of reuptake by prototypical SERT blockers did not result in any ‘apparent’ releasing effect (Raiteri et al. 1974; Gobbi et al. 1992).
When the three amphetamine derivatives were tested for induction of [3H]5-HT release from superfused hSERT-expressing HEK-293 cells, or for induction of inwardly-directed currents in hSERT-transfected oocytes, the results were very similar to those in synaptosomes. Since HEK-293 cells, like oocytes, lack synaptic vesicles, these results rule out the possibility that the differences between the maximal releasing effect of pCA, MTA and DMMTA are related to differences in the compounds’ ability to interact with – or induce release from – the vesicular pool (Gobbi et al. 2002). We therefore suggest that these differences might arise as a consequence of a different interaction with SERTs or with other intracellular mechanisms involved in the SERT-dependent release/currents.
The finding that the [3H]5-HT release induced by both MTA and DMMTA from superfused cells was greatly enhanced in the presence of monensin, as was pCA-induced release (Scholze et al. 2000), confirm that these amphetamine derivatives are all SERT substrates. However, some substrates may be more efficiently taken up (high Vmax) than others (lower Vmax, ‘partial’ substrates), and that differences in the Vmax might result in differences in the transporter-dependent effects, such as release and currents (Jensen and Brauner-Osborne 2004). Our finding that the Vmax of SERT-mediated MTA uptake, measured in HEK-293 cells, is significantly lower (about 40%) than that of pCA might thus help explain to the lower effects of MTA than pCA.
The availability of compounds with ‘partial’ effects highlights the correlation between amphetamine-induced currents and amphetamine-induced 5-HT release (at least in synaptosomes and HEK-293 cells), which is also strengthened by the similar bell-shaped dose–response curves found, especially with pCA, in all these in vitro assays (for a possible explanation of bell-shaped curves see (Gobbi et al. 2002; Seidel et al. 2005). This correlation agrees with the theory that substituted amphetamine-induced release might involve ion fluxes through the transporter. Accordingly, in HEK-293 cells expressing the DA transporter (DAT) Zn2+ stimulates both amphetamine-induced inward currents (Meinild et al. 2004; Pifl et al. 2004) and amphetamine-induced DA release (Scholze et al. 2002; Pifl et al. 2004). On the other hand, Zn2+ inhibits DAT-mediated DA uptake (and thus, probably, DAT-mediated amphetamine uptake), dissociating the amphetamine-induced release/currents from the amphetamine uptake (Scholze et al. 2002; Pifl et al. 2004). Analogously, the down-regulation or inhibition of protein kinase C (PKC) activity resulted in a significant decrease in amphetamine-induced monoamine release, with no apparent effect on [3H]monoamine uptake (Kantor et al. 2001; Seidel et al. 2005).
These findings challenged the classical ‘facilitated exchange diffusion’ model (Fischer and Cho 1979; Levi and Raiteri 1993) and suggest that currents and/or release can be induced independently by the translocation of the drug (i.e., the property of being a substrate might be either not necessary or not sufficient). Activation of PKC plays a fundamental role in amphetamine-induced monoamine release (Kantor et al. 2001; Gnegy 2003; Seidel et al. 2005) and N-terminal phosphorylation of DAT is essential for amphetamine-induced DA efflux (Khoshbouei et al. 2004).
Thus, the low maximal effects of MTA and DMMTA for induction of 5-HT release and currents may not (only) be associated with a low Vmax of translocation, but also with low efficacy (lower than pCA) for activating those mechanisms (i.e., phosphorylation) responsible for the inward currents and/or release of the intracellular substrate.
The relative efficacies of pCA, MTA, and DMMTA for induction of 5-HT release are different in brain slices or in vivo after local application
In superfused rat hippocampal slices, the maximal [3H]5-HT release induced by DMMTA is about half the maximal release induced by pCA, thus partly confirming the results in synaptosomes. Previous data in brain slices showed that MTA is as potent as pCA as a [3H]5-HT releaser (Huang et al. 1992) and this was confirmed in the present study. Thus, the normalized maximal releasing effects of the three amphetamine derivatives (pCA 100%, MTA 100%, and DMMTA 50%) indicate less pronounced differences than those found in synaptosomes and cells. Another discrepancy with the data obtained in synaptosomes and cells is that the dose–response curve of pCA in brain slices was not bell-shape, at least in the range of concentrations tested (present data and Huang et al. 1992).
Local application of DMMTA into the LS in vivo resulted in a marked, citalopram-sensitive increase of extracellular 5-HT, quantitatively similar to that induced by MTA, and slightly lower than that elicited by pCA. The MTA-induced 5-HT release in the LS was similar in magnitude to the MTA-induced release previously described in the hippocampus which in turn was similar to or only slightly lower than the pCA-induced release (Gobbi et al. 2002). Altogether, these findings suggest that, when locally applied in vivo, both MTA and DMMTA release 5-HT in a manner quantitatively and qualitatively comparable to the neurotoxic amphetamine derivative pCA.
The evidence that MTA and DMMTA significantly differ from pCA as regards their maximal 5-HT-releasing effect in synaptosomes and cells as well as their maximal attainable effect for induction of SERT-mediated currents in oocytes, confirms and extends the observation, described in our previous paper (Gobbi et al. 2002), of a discrepancy between in vivo and in vitro synaptosomal data. If, in vitro, MTA and DMMTA (but also mCPP, Gobbi et al. 2002) behave as ‘partial substrates’ on targets relevant for the induction of reverse transport, they appear to act as ‘full substrates’ when applied locally in vivo.
This should not be so surprising, however, because similar observations have often been reported for ligands of G protein-coupled receptors, which can act as full agonists in some experimental systems and as partial agonists in others, and theoretical models have been proposed to account for this behavior (Hoyer and Boddeke 1993). Further work is, however, necessary to substantiate this possibility.
Independently from the underlying molecular mechanisms, our data suggest that the maximal 5-HT-releasing effect in vitro (superfused rat brain synaptosomes and SERT-expressing cells), but not in vivo, might be a valuable indicator of the compound’s efficacy for activating the uncoupled mechanisms involved in reverse transport.
The different neurotoxic properties of amphetamine derivatives appear to correlate with their in vitro efficacies for induction of 5-HT release or currents
The present findings also confirm and extend the suggestion that the different neurotoxic properties of the amphetamine derivatives are unlikely to be due to a difference in their 5-HT-releasing effects in vivo (Huang et al. 1992; Rudnick and Wall 1993). Thus, microdialysis studies showed that 5 mg/kg i.p. of both MTA and DMMTA induce marked 5-HT release in the dorsal hippocampus, comparable (specially in the case of MTA) to the 5-HT release elicited by a neurotoxic dose of pCA. No neurotoxic effects were observed, even at the higher MTA and DMMTA dose of 20 mg/kg [(Gobbi et al. 2002) and present data] and even lower effects on extracellular 5-HT have been reported for neurotoxic PCA doses (Iyer et al. 1994; Muchimapura et al. 2002).
The possibility that the differences in the neurotoxicity between these amphetamine derivatives could be due to a different effect on MAO-A is also unlikely, because the potency of DMMTA as MAO-A inhibitor is only twofold higher than that of pCA (Hurtado-Guzman et al. 2003).
The different neurotoxic properties of the amphetamine derivatives appear to correlate with their effects (5-HT release and currents) in vitro in synaptosomes and cells. mCPP is another non-neurotoxic compound which in vivo and in brain slices raises extracellular 5-HT by acting as a SERT substrate (Pettibone and Williams 1984; Eriksson et al. 1999; Baumann et al. 2001) whereas it has negligible 5-HT-releasing effect in synaptosomes (Gobbi et al. 2002) and SERT-expressing cells (M.H. and H.H.S., unpublished data). Thus, all the compounds tested so far that induce maximal 5-HT release in vitro (pCA but also MDMA and dfenfluramine, ‘full substrates’) are neurotoxic for 5-HTergic nerve endings, whereas the compounds that, in synaptosomes, are poor 5-HT releasers (MTA, DMMTA, mCPP, and tramadol, ‘partial substrates’) are not neurotoxic.
The substrate-induced inward (Na+) currents may play a large part, resulting in sustained depolarization which, in turn, could favor the free radical formation and oxidative stress previously found to be involved in the serotonergic neurotoxicity of some amphetamine derivatives (Shankaran et al. 1999, 2001). Alternatively, it is tempting to suggest that neurotoxicity is a consequence of full activation of those mechanisms which, in synaptosomes and cells, lead to inward currents and/or 5-HT release. Activation of PKC might be a candidate mechanism since, as mentioned above, it may play a fundamental role in amphetamine-induced monoamine release (Kantor et al. 2001; Gnegy 2003; Khoshbouei et al. 2004; Seidel et al. 2005). Drug-induced 5-HT release in vivo is likely to involve additional mechanisms, similar for the neurotoxic and non-neurotoxic drugs considered in the present study.
In conclusion, in vitro studies in synaptosomes and cells might predict neurotoxic properties of SERT substrates, by highlighting differences that cannot be detected by measuring their 5-HT-releasing properties in vivo.
Acknowledgments
We thank Veronica Noches for technical assistance with MD experiments. This work was partially supported by FONDECYT (grant 1011017 MIF and KG). The support of DICYT-USACH (MRP) is also gratefully acknowledged, as well as a grant from the Austrian Science Fund/FWF P18706 (to HHS).
Abbreviations used
- 5-HT
serotonin
- DA
dopamine
- DAT
dopamine transporter
- DMMTA
N,N-dimethyl,4-methyl-thioamphetamine
- FRR
fractional release rate
- LS
lateral septum
- mCPP
1-(m-chlorophenyl)-piperazine
- MDMA
3,4-methylenedioxy-methamphetamine
- MMAI
5-methoxy-6-methyl-2-aminoindan
- MTA
4-methyl-thioamphetamine
- pCA
p-chloroamphetamine
- PKC
protein kinase C
- SERT
serotonin transporter
References
- Baumann MH, Ayestas MA, Dersch CM, Rothman RB. 1-(m-chlorophenyl)piperazine (mCPP) dissociates in vivo serotonin release from long-term serotonin depletion in rat brain. Neuropsychopharmacology. 2001;24:492–501. doi: 10.1016/S0893-133X(00)00221-9. [DOI] [PubMed] [Google Scholar]
- Crespi D, Mennini T, Gobbi M. Carrier-dependent and Ca(2 + )-dependent 5-HT and dopamine release induced by (+)-amphetamine, 3,4-methylendioxymethamphetamine, p-chloroamphetamine and (+)-fenfluramine. Br. J. Pharmacol. 1997;121:1735–1743. doi: 10.1038/sj.bjp.0701325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinopoulos A, Dori I, Parnavelas JG. Serotonergic innervation of the mature and developing lateral septum of the rat: a light and electron microscopic immunocytochemical analysis. Neuroscience. 1993;55:209–222. doi: 10.1016/0306-4522(93)90467-t. [DOI] [PubMed] [Google Scholar]
- Eriksson E, Engberg G, Bing O, Nissbrandt H. Effects of mCPP on the extracellular concentrations of serotonin and dopa-mine in rat brain. Neuropsychopharmacology. 1999;20:287–296. doi: 10.1016/S0893-133X(98)00070-0. [DOI] [PubMed] [Google Scholar]
- Fischer JF, Cho AK. Chemical release of dopamine from striatal homogenates: evidence for an exchange diffusion model. J. Pharmacol. Exp. Ther. 1979;208:203–209. [PubMed] [Google Scholar]
- Gerstbrein K, Sitte HH. Currents in neurotransmitter transporters. Handb. Exp. Pharmacol. 2006;175:95–111. doi: 10.1007/3-540-29784-7_5. [DOI] [PubMed] [Google Scholar]
- Gnegy ME. The effect of phosphorylation on amphetamine-mediated outward transport. Eur. J. Pharmacol. 2003;479:83–91. doi: 10.1016/j.ejphar.2003.08.059. [DOI] [PubMed] [Google Scholar]
- Gobbi M, Frittoli E, Mennini T, Garattini S. Releasing activities of d-fenfluramine and fluoxetine on rat hippocampal synaptosomes preloaded with [3H]serotonin. Naunyn-Schmied. Arch. Pharmacol. 1992;345:1–6. doi: 10.1007/BF00175461. [DOI] [PubMed] [Google Scholar]
- Gobbi M, Moia M, Pirona L, Ceglia I, Reyes-Parada M, Scorza C, Mennini T. p-Methylthioamphetamine and 1-(mchlorophenyl)piperazine, two non-neurotoxic 5-HT releasers in vivo, differ from neurotoxic amphetamine derivatives in their mode of action at 5-HT nerve endings in vitro. J. Neurochem. 2002;82:1435–1443. doi: 10.1046/j.1471-4159.2002.01073.x. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) Pharmacol. Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Boddeke HW. Partial agonists, full agonists, antagonists: dilemmas of definition. Trends Pharmacol. Sci. 1993;14:270–275. doi: 10.1016/0165-6147(93)90129-8. [DOI] [PubMed] [Google Scholar]
- Huang X, Marona-Lewicka D, Nichols DE. p-Methylthioamphetamine is a potent new non-neurotoxic serotonin-releasing agent. Eur. J. Pharmacol. 1992;229:31–38. doi: 10.1016/0014-2999(92)90282-9. [DOI] [PubMed] [Google Scholar]
- Hurtado-Guzman C, Fierro A, Iturriaga-Vasquez P, Sepulveda-Boza S, Cassels BK, Reyes-Parada M. Monoamine oxidase inhibitory properties of optical isomers and N-substituted derivatives of 4-methylthioamphetamine. J. Enzyme Inhib. Med. Chem. 2003;18:339–347. doi: 10.1080/1475636031000118437. [DOI] [PubMed] [Google Scholar]
- Iyer RN, Sprouse JS, Aghajanian GK, Roth RH, Brad-berry CW. Tryptophan pretreatment augmentation of p-chloroamphetamine-induced serotonin and dopamine release and reduction of long-term neurotoxicity. Biochem. Pharmacol. 1994;48:1501–1508. doi: 10.1016/0006-2952(94)90576-2. [DOI] [PubMed] [Google Scholar]
- Jensen AA, Brauner-Osborne H. Pharmacological characterization of human excitatory amino acid transporters EAAT1, EAAT2 and EAAT3 in a fluorescence-based membrane potential assay. Biochem. Pharmacol. 2004;67:2115–2127. doi: 10.1016/j.bcp.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Nichols DE. Combined administration of a non-neurotoxic 3,4-methylenedioxymethamphetamine analogue with amphetamine produces serotonin neurotoxicity in rats. Neuropharmacology. 1991;30:819–822. doi: 10.1016/0028-3908(91)90192-e. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Conarty PF, Nichols DE. [3H]monoamine releasing and uptake inhibition properties of 3,4-methylenedioxymethamphetamine and p-chloroamphetamine analogues. Eur. J. Pharmacol. 1991;200:9–16. doi: 10.1016/0014-2999(91)90659-e. [DOI] [PubMed] [Google Scholar]
- Kantor L, Hewlett GH, Park YH, Richardson-Burns SM, Mellon MJ, Gnegy ME. Protein kinase C and intracellular calcium are required for amphetamine-mediated dopamine release via the norepinephrine transporter in undifferentiated PC12 cells. J. Pharmacol. Exp. Ther. 2001;297:1016–1024. [PubMed] [Google Scholar]
- Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, Galli A, Javitch JA. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi G, Raiteri M. Carrier-mediated release of neurotransmitters. [Review] [52 refs] Trends Neurosci. 1993;16:415–419. doi: 10.1016/0166-2236(93)90010-j. [DOI] [PubMed] [Google Scholar]
- Mager S, Min C, Henry DJ, Chavkin C, Hoffman BJ, Davidson N, Lester HA. Conducting states of a mammalian serotonin transporter. Neuron. 1995;12:845–859. doi: 10.1016/0896-6273(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Mamounas LA, Molliver ME. Evidence for dual serotonergic projections to neocortex: axons from the dorsal and medial raphe nuclei are differentially vulnerable to the neurotoxin pchloroamphetamine (PCA) Exp.Neurol. 1988;102:23–36. doi: 10.1016/0014-4886(88)90075-1. [DOI] [PubMed] [Google Scholar]
- Meinild AK, Sitte HH, Gether U. Zinc potentiates an uncoupled anion conductance associated with the dopamine transporter. J. Biol. Chem. 2004;279:49671–49679. doi: 10.1074/jbc.M407660200. [DOI] [PubMed] [Google Scholar]
- Mennini T, Fracasso C, Cagnotto A, Bergami A, Frittoli E, Gobbi M, Caccia S, Garattini S. In vitro and in vivo effects of the anorectic agent dexfenfluramine on the central serotoninergic neuronal systems of non-human primates. A comparison with the rat. Naunyn. Schmiedebergs Arch. Pharmacol. 1996;353:641–647. doi: 10.1007/BF00167183. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Molliver ME. Anatomic evidence for a neurotoxic effect of (±)-fenfluramine upon serotonergic projections in the rat. Brain Res. 1990;511:165–168. doi: 10.1016/0006-8993(90)90237-6. [DOI] [PubMed] [Google Scholar]
- Muchimapura S, Fulford AJ, Mason R, Marsden CA. Isolation rearing in the rat disrupts the hippocampal response to stress. Neuroscience. 2002;112:697–705. doi: 10.1016/s0306-4522(02)00107-0. [DOI] [PubMed] [Google Scholar]
- Pettibone DJ, Williams M. Serotonin-releasing effects of substituted piperazines in vitro. Biochem. Pharmacol. 1984;33:1531–1535. doi: 10.1016/0006-2952(84)90424-6. [DOI] [PubMed] [Google Scholar]
- Pifl C, Rebernik P, Kattinger A, Reither H. Zn2 + modulates currents generated by the dopamine transporter: parallel effects on amphetamine-induced charge transfer and release. Neuropharmacology. 2004;46:223–231. doi: 10.1016/j.neuropharm.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Angelini F, Levi G. A simple apparatus for studying the release of neurotransmitters from synaptosomes. Eur. J. Pharmacol. 1974;25:411–414. doi: 10.1016/0014-2999(74)90272-6. [DOI] [PubMed] [Google Scholar]
- Rudnick G, Wall SC. p-Chloroamphetamine induces serotonin release through serotonin transporters. Biochemistry. 1992;31:6710–6718. doi: 10.1021/bi00144a010. [DOI] [PubMed] [Google Scholar]
- Rudnick G, Wall SC. Non-neurotoxic amphetamine derivatives release serotonin through serotonin transporters. Mol. Pharmacol. 1993;43:271–276. [PubMed] [Google Scholar]
- Scholze P, Zwach J, Kattinger A, Pifl C, Singer EA, Sitte HH. Transporter-mediated release: a superfusion study on human embryonic kidney cells stably expressing the human serotonin transporter. J. Pharmacol. Exp. Ther. 2000;293:870–878. [PubMed] [Google Scholar]
- Scholze P, Norregaard L, Singer EA, Freissmuth M, Gether U, Sitte HH. The role of zinc ions in reverse transport mediated by monoamine transporters. J. Biol. Chem. 2002;277:21505–21513. doi: 10.1074/jbc.M112265200. [DOI] [PubMed] [Google Scholar]
- Scorza C, Silveira R, Nichols DE, Reyes-Parada M. Effects of 5-HT-releasing agents on the extracellullar hippocampal 5-HT of rats. Implications for the development of novel antidepressants with a short onset of action. Neuropharmacology. 1999;38:1055–1061. doi: 10.1016/s0028-3908(99)00023-4. [DOI] [PubMed] [Google Scholar]
- Seidel S, Singer EA, Just H, et al. Amphetamines take two to tango: an oligomer-based counter-transport model of neurotransmitter transport explores the amphetamine action. Mol. Pharmacol. 2005;67:140–151. doi: 10.1124/mol.67.1.. [DOI] [PubMed] [Google Scholar]
- Shankaran M, Yamamoto BK, Gudelsky GA. Involvement of the serotonin transporter in the formation of hydroxyl radicals induced by 3,4-methylenedioxymethamphetamine. Eur. J. Pharmacol. 1999;385:103–110. doi: 10.1016/s0014-2999(99)00728-1. [DOI] [PubMed] [Google Scholar]
- Shankaran M, Yamamoto BK, Gudelsky GA. Ascorbic acid prevents 3,4-methylenedioxymethamphetamine (MDMA)-induced hydroxyl radical formation and the behavioral and neuro-chemical consequences of the depletion of brain 5-HT. Synapse. 2001;40:55–64. doi: 10.1002/1098-2396(200104)40:1<55::AID-SYN1026>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res. Brain Res. Rev. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Sitte HH, Farhan H, Javitch JA. Sodium-Dependent Neurotransmitter transporters: oligomerization as a determinant of transporter function and trafficking. Mol. Interv. 2004;4:38–47. doi: 10.1124/mi.4.1.38. [DOI] [PubMed] [Google Scholar]
- Sotomayor R, Forray MI, Gysling K. Acute morphine administration increases extracellular DA levels in the rat lateral septum by decreasing the GABAergic inhibitory tone in the ventral tegmental area. J. Neurosci. Res. 2005;81:132–139. doi: 10.1002/jnr.20537. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Everman SL, Nichols DE. An integrated hypothesis for the serotonergic axonal loss induced by 3,4-methylenedioxymethamphetamine. Neurotoxicology. 1998;19:427–441. [PubMed] [Google Scholar]