Abstract
Background
Transcatheter pulmonary valve replacement is currently performed in clinical trials, but is limited by the use of glutaraldehyde-treated bioprostheses. This feasibility study was performed to evaluate delivery-related tissue distortion during implantation of tissue-engineered (TE) heart valves.
Material/Methods
The injectable TE heart valve was mounted on a self-expanding nitinol stent (n=7) and delivered into the pulmonary position in 7 pigs, (weight 26 to 31 kg), performing a sternotomy or limited lateral thoracotomy. Prior to implantation, the injectable TE heart valves were crimped and inserted into an applicator. Positioning of the implants was guided by fluoroscopy, and after careful deployment, angiographic examination was performed to evaluate the correct delivered position. Hemodynamic measurements were performed by epicardial echocardiography. Finally, the animals were sacrificed and the injectable TE heart valves were inspected by gross examination and histological examination.
Results
Orthotopic deliveries of the injectable TE heart valves were all successful performed, expect in 1 where the valve migrated due to a discrepancy between pulmonary valve annulus size and injectable TE valve size. Angiographic evaluation (n=6) showed normal valve function, supported by epicardial echocardiography in which no increased flow velocity was measured, neither trans- nor paravalvular regurgitation. Histological evaluation demonstrated absence of tissue damage from the delivery process.
Conclusions
Transcatheter implantation of an injectable TE heart valve seems to be possible without tissue distortion due to the delivery system.
Keywords: Cardiac Catheters, Pulmonary Valve, Tissue Engineering
Background
The incidence of complex congenital heart disease is approximately 8.21/1000 live births in Canada, with involvement of the right ventricular outflow tract (RVOT) of approximately 10% [1]. Generally, initial RVOT reconstruction is performed by patch, but due to the absence of a sufficient functioning pulmonary valve, the right ventricle will be volume-overloaded and dilate over time. The volume overload of the right ventricle is usually well tolerated, but over time it might lead to decreased exercise tolerance, dyspnea, arrhythmia, symptoms of heart failure, and, eventually, sudden death [2,3]. At the time of ventricle dilatation, a valve conduit is chosen to reconstruct the RVOT, since today’s available valve conduits degenerate over time in absence of regeneration or growth potential [4–6].
The ideal would be a living autologous valve with regeneration, remodelling, and growth potential, implanted with minimal invasiveness, without tissue distortion or compromising valve function. Tissue engineering techniques allows us to construct valves with remodelling, regeneration, and growth potential, but these valves have only limited availability for clinical use and are conventionally implanted [7,8].
In 2000 Bonhoeffer et al. [9] began using glutaraldehyde-fixed bovine jugular veins mounted on balloon-expandable stainless stents to be percutaneously implanted. These valves were initially implanted in patients with pulmonary valve stenosis, but nowadays pulmonary valve regurgitations can also be treated with these valves if the current annulus size is still suitable and not over-dilated [10]. General disadvantages of the current clinically available transcatheter valves are availability for selected cases, high rate of stent fractures [11], and increased risk of endocarditis during follow-up [12]. Glutaraldehyde treatment is not an optimal option in congenital heart disease treatment because there is lack of growth potential as well as increased risk for early deterioration [13,14]. The aim of the present study was to evaluate the implantation of a newly designed injectable TE pulmonary heart valve with a self-expandable nitinol stent to reconstruct the RVOT.
Material and Methods
All experiments were performed in accordance to the “Principles of Laboratory Animal Care” prepared by the National Society of Medical Research, and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institute of Health (NIH, revised 1996). The study was approved by the institutional review and ethics committee at the University of Leipzig.
Valve design
Details on decellularization of porcine pulmonary heart valves were previously reported [15]. In brief, fresh porcine pulmonary heart valves were obtained from the slaughterhouse. After preparation and carefully trimming the muscle tissue to a minimum, the valves were placed in antibiotic solutions until decellularization with deoxycholic acid (Sigma Chemical Co, St. Louis, MO) was started. Extensive rinsing and ethanol treatment was performed before final sterilization. The decellularized pulmonary valves were sutured into a self-expendable nitinol stent (PFM Medical AG, Koeln, Germany) with a diameter of 16 mm (Figure 1A, 1B). This size was chosen because the diameter of the pulmonary valve annulus was preoperatively measured, adding 10% to ensure optimal fitting of the injectable TE heart valve into the pulmonary valve annulus.
Figure 1.
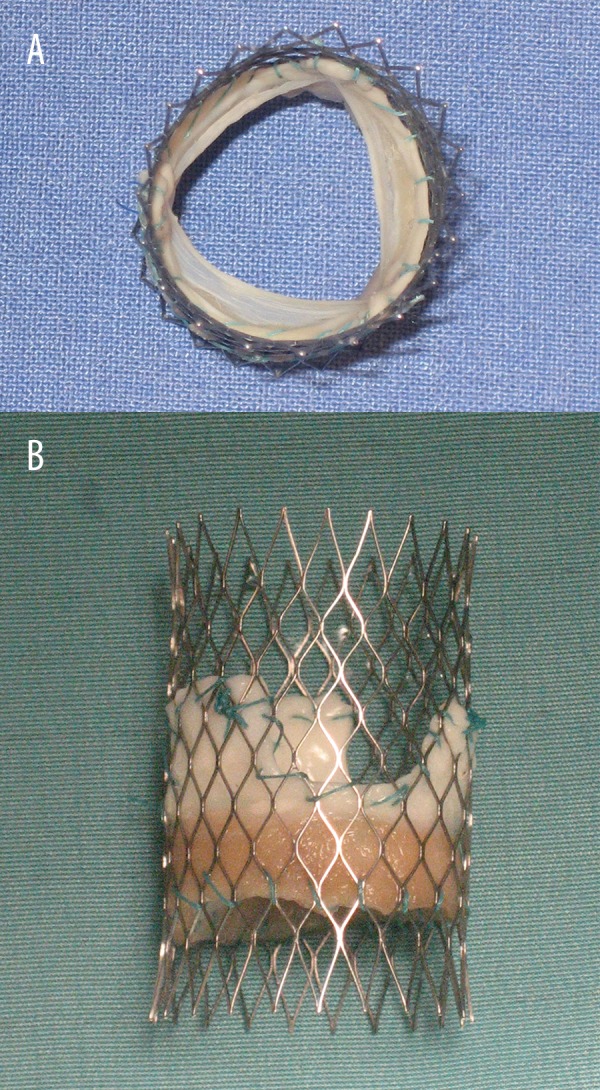
(A) View inside the outflow tract of the injectable TE heart valve. (B) Side view of the injectable TE heart valve mounted on a self-expanding nitinol stent.
Implantation procedure
Seven healthy pigs between 6 and 9 months of age with a median weight of 28 kg (range 26–31 kg) were included in this study. All animals were pre-medicated and, after general anesthesia was induced, mechanical ventilation was started. A median sternotomy or limited lateral thoracotomy was performed to implant the injectable TE pulmonary heart valve. A bolus of 5.000 IU heparin intravenously was administered and the pericardium was opened. One purse-string suture was placed at the level of the right ventricular outflow tract. After crimping the valve for at least 30 min, an introducer was used to implant the injectable TE pulmonary heart valve (Figure 2). The release procedure was guided by fluoroscopy and performed under rapid pacing. The implanted valve was studied by epicardial echocardiographic examination (Vivid; GE Healthcare, Munich Germany), invasive pressure, and angiographic measurements. Finally, 4–6 h after implantation, the animals were sacrificed and the injectable TE heart valves were explanted, rinsed, and subjected to gross examination and histopathological analysis.
Figure 2.
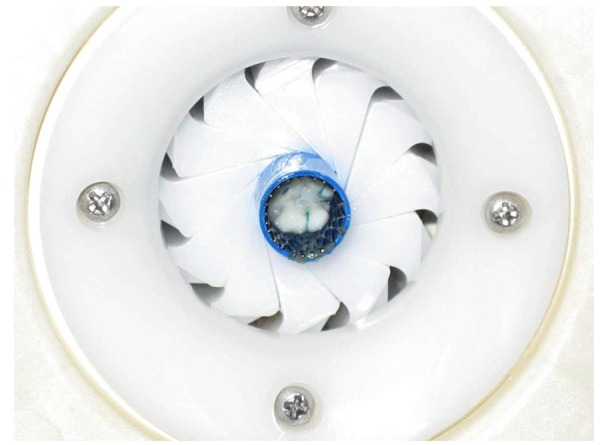
Crimping procedure of the injectable TE heart valve prior to delivery.
Results
The injectable TE heart valve was guided by fluoroscopy (Figure 3) and successfully delivered in all animals, except in 1 were the valve migrated due to the discrepancy between the implanted valve and the native pulmonary valve annulus. Angiography and epicardial echocardiography proved that all injectable TE heart valves were competent, and no trans- or paravalvular leakage was seen in any of the 6 successful implantations (Figure 4). Invasive pressure measurement showed a median maximum pressure gradient of 4 mm Hg (range 3–5 mm Hg) and median maximum pressure gradient of 8 mm Hg (range 6–12 mm Hg), confirmed by epicardial echocardiographic examination. During follow-up, none of the valves migrated and hemodynamic behavior of the implanted valves was stable.
Figure 3.
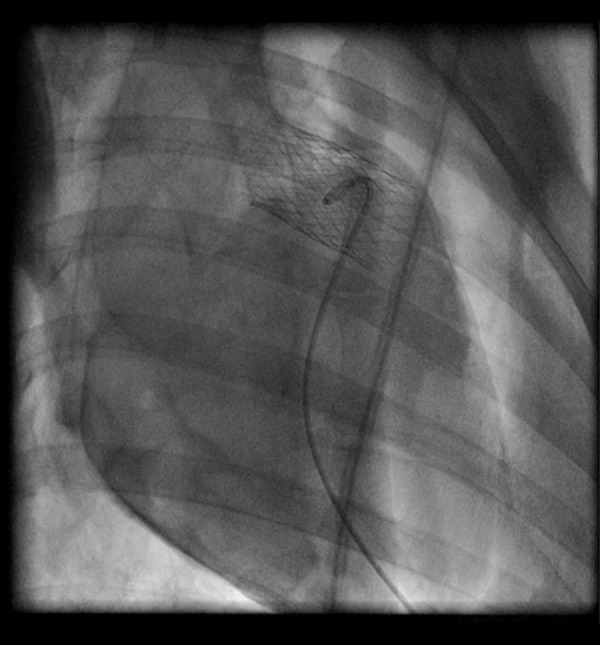
Angiographic examination shows the optimal position of the injected TE heart valve.
Figure 4.
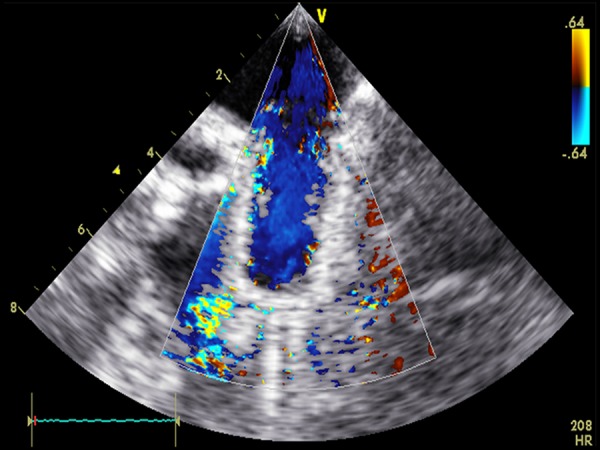
Echocardiographic examination shows the absence of para- and transvalvular regurgitation.
Gross examination of the injectable TE heart valve showed pliable leaflets without tears or bleeding. The leaflets were all intact and showed no thrombus formation (Figure 5A, 5B).
Figure 5.
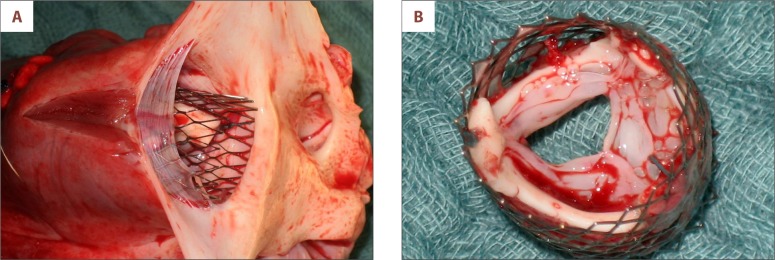
(A) Gross examination of the injectable TE valve implanted into the native pulmonary valve. (B) Pliable leaflets of the injectable TE heart valve without tissue distortion or biofilm.
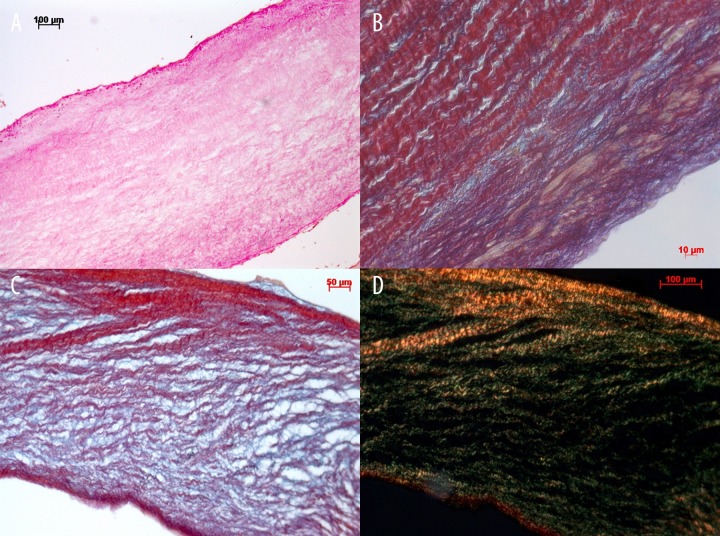
Histopathological examination showed a preserved extracellular matrix with absence of any tissue damage due to the delivery system or implantation process (Figure 6A–6D).
Figure 6.
(A) Global histological tissue examination shows an intact extracellular matrix after crimping and catheter delivery (hematoxylin & eosin staining). (B, C) Detailed examination shows absence of tissue distortion of the extracellular matrix (picrosirius red staining). (D) Detailed examination of staining of the intact collagen I (red in polarized light) and collagen III (green in polarized light) of the injectable TE heart valve.
Discussion
Today’s treatment options for complex congenital RVOT disease are not yet optimal and need to be improved. Allografts are still an excellent option, but availability is limited; therefore, alternative materials are used such as glutaraldehyde-fixed conduits to correct congenital malformation of the RVOT in neonates and infants. Homann et al. [6] showed similar survival rates for patients treated with allografts and xenografts, but freedom of re-operation was different for both groups, at 28% for xenografts versus 62% for allografts (p<0.01). These xenografts showed serious deterioration during long-term follow-up, such as sclerosis and calcification. In a more recent study by Vitanova et al. [16], different types of conduits for RVOT reconstruction were compared in patients below the age of 1 year. They showed similar rates of freedom for reoperation for allografts, Contegra, and Hancock conduits at 38.1±8.3%, 38.0±11.3%, and 20.3±7.5%, respectively (p=0.4). The Hancock conduit, however, was associated with a significantly higher rate of thrombosis (P=0.01) and the patients receiving a Contegra conduit developed faster conduit regurgitation or stenosis than patients with allografts (p=0.01). Nevertheless, all these heart valve substitutes have no growth potential.
New technologies are therefore needed that are free of glutaraldehyde, to allow tissue remodelling. Tissue engineering could overcome this problem, which clinically started with autologous endothelial cell seeded grafting [17]. Several TE heart valves have been developed and are now clinically used. The reported clinical outcomes, however, strongly varied [7,8,18–21]. One reason for this could be the different origins of tissue used for the extracellular matrix [22,23]. Excellent clinical results are achieved with human-origin tissue [24,25]. Another issue is the performed decellularization process itself, which can be very different and has enormous influence on the extracellular matrix [8,19,20]. Conventional surgical implantation techniques have been used for these TE heart valves [7,8,18], but it would be desirable to implant these valves minimally invasively by using a catheter.
Percutaneous pulmonary valve implantation (PPVI) was started in 2000 by Bonhoeffer et al. [9] in patients with degenerated conduits within the RVOT, showing initially limited benefit [26]. Nordmeyer et al. [27] reported a high number of stent fractures, which can be dramatically reduced by performing pre-stenting using a strong stent [28] or performing a valve-in-valve procedure [29]. After modifications were done, this treatment option become promising, but only for selected patients.
An additional patient group – those with pulmonary valve regurgitation of different etiology – could also benefit from this method. Prospective randomized studies on the optimal timing for intervention of pulmonary valve regurgitation are not currently available. Today, cardiac magnetic resonance imaging (cMRI) is the criterion standard for diagnosing ventricular function, mass, and volumes [30], which is also important in evaluating the postoperative development of right ventricular volume reduction and cardiac improvement, resulting in better exercise tolerance [31]. The improvement of right ventricular function after pulmonary valve implantation, however, did not decrease death in recent studies [32]. cMRI examination will also provide additional information of value in avoiding fatal complications of percutaneous pulmonary valve implantation by left coronary occlusion, which can occur immediately after or hours after the procedure has been completed (33). McKenzie et al. [34] showed comparative data of surgical pulmonary valve replacement to PPVI, showing freedom of reoperation was 94%, and in the expanded Melody valve trial [35] freedom of re-intervention was 53%. If PPVI is advocated in patients because it is less invasive and is suggested to be done at an earlier stage, modification of materials is needed to achieve this. Kiefer et al. [36] showed changes of the extracellular matrix, which were related to the crimping time. However, in a subcutaneous rat model, which is a passive screening model for calcification, there were no sign of increased tissue calcification. Therefore, examinations in a large circulating animal model are needed to investigate the impact of tissue crimping on leaflet durability and calcification during long-term follow-up. The present study was performed by using a decellularized porcine extracellular matrix in the porcine model, which simulates the situation of implanting a decellularized allograft in humans. To implant this injectable TE heart valve into the pulmonary position using a limited thoracotomy, the valve does not need to be extensively crimped. To implant an injectable TE heart valve percutaneously, the material of choice is pericardial tissue. In a previous study [37], we showed that decellularized pericardium withstands systemic circulation pressure without any effects. Additionally, because the decellularization of the pericardial tissue stays very pliable, and due to the absence of glutaraldehyde to cross-link, no tissue rupture can occur. The present feasibility study shows that injectable TE heart valves can be implanted safely and that crimping for the delivery process can be safely performed without tissue distortion. Long-term follow-up will be needed to evaluate functionality and absence of tissue distortion to eventually expand the use of this less invasive approach in humans.
Limitations of the study
This feasibility study was performed to evaluate the influence of delivery procedure on an injectable TE heart valve. However, due to the limitation of the acute setting of these experiments, long-term implantation studies will be needed to evaluate the injectable TE heart valve behavior under permanent burden.
Conclusions
Transcatheter implantation of an injectable TE heart valve seems to be feasible, without tissue distortion due to the delivery procedure.
Footnotes
Source of support: German Federal Minister of Economics and Technology (project number: KF2802705CR4)
References
- 1.Hoffmann IJ. Incidence of congenital heart disease. I. Postnatal incidence. Pediatr Cardiol. 1995;16:103–13. doi: 10.1007/BF00801907. [DOI] [PubMed] [Google Scholar]
- 2.Diller GP, Breithardt G, Baumgartner H. Congenital heart defects in adulthood. Dtsch Aztebl Int. 2011;108:452–59. doi: 10.3238/arztebl.2011.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marelli A, Mackie A, Ionescu-Ittu R, et al. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–72. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 4.Zubairi R, Malik S, Jaquiss RD, et al. Risk factors for prosthesis failure in pulmonary valve replacement. Ann Thorac Surg. 2011;91:561–65. doi: 10.1016/j.athoracsur.2010.07.111. [DOI] [PubMed] [Google Scholar]
- 5.Mohannadi S, Belli E, Martinovic I, et al. Surgery for right ventricle to pulmonary artery conduit obstruction: risk factors for further reoperation. Eur J Cardiothorac Surg. 2005;28:217–22. doi: 10.1016/j.ejcts.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Homann M, Haehnel JC, Mendler N, et al. Reconstruction of the RVOT with valved biological conduits: 25 years experience with allografts and xenografts. Eur J Cardiothorac Surg. 2000;17:624–30. doi: 10.1016/s1010-7940(00)00414-0. [DOI] [PubMed] [Google Scholar]
- 7.Konertz W, Angeli E, Tarusinov G, et al. Right ventricular outflow tract reconstruction with decellularized porcine xenografts in patients with congenital heart disease. J Heart Valve Dis. 2011;20:341–47. [PubMed] [Google Scholar]
- 8.Cebotari S, Tudorache I, Ciubotaru A, et al. Use of fresh decellularized allografts for pulmonary valve replacement may reduce the reoperation rate in children and young adults: early report. Circulation. 2011;124:S115–23. doi: 10.1161/CIRCULATIONAHA.110.012161. [DOI] [PubMed] [Google Scholar]
- 9.Bonhoeffer Ph, Boudjemline Y, Saliba Z, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356:1403–5. doi: 10.1016/S0140-6736(00)02844-0. [DOI] [PubMed] [Google Scholar]
- 10.Boudjemline Y, Brugada G, Van-Aerschot I, et al. Outcome and safety of transcatheter pulmonary valve replacement in patients with large patched right ventricular outflow tracts. Arch Cardiovasc Dis. 2012;105:404–13. doi: 10.1016/j.acvd.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 11.McElhinney DB, Cheatham JP, Jones TK, et al. Stent fracture, valve dysfunction, and right ventricular oputflow tract reintervention after transcatheter pulmonary valve implantation. Patient-related and procedural risk factors in the US Melody valve trial. Circ Cardiovasc Interv. 2011;4:602–14. doi: 10.1161/CIRCINTERVENTIONS.111.965616. [DOI] [PubMed] [Google Scholar]
- 12.Fraisse A, Aldebert P, Malekzadeh-Milani S, et al. Medoly transcatheter pulmonary valve implantation: Results from a French registry. Arch Cardiovasc Dis. 2014;107:607–14. doi: 10.1016/j.acvd.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Meyns B, Van Garsse L, Boshoff D, et al. The Contegra conduit in the right ventricular outflow tract induces supravalvular stenosis. J Thorac Cardiovasc Surg. 2004;128:834–40. doi: 10.1016/j.jtcvs.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Dohmen PM, da Costa F, Holinski S, et al. Is there a possibility for a glutaraldehyde-free heart valve to grow? Eur Surg Res. 2006;38:54–61. doi: 10.1159/000091597. [DOI] [PubMed] [Google Scholar]
- 15.Erdbrügger W, Konertz W, Dohmen PM, et al. Decellularized xenogenic heart valves reveal remodelling and growth potential in vivo. Tissue Eng. 2006;12:2059–68. doi: 10.1089/ten.2006.12.2059. [DOI] [PubMed] [Google Scholar]
- 16.Vitanova K, Cleuziou J, Hörer J, et al. Which type of conduit to choose for right ventricular outflow tract reconstruction in patients below 1 year of age? Eur J Cardiothorac Surg. 2014;46:961–66. doi: 10.1093/ejcts/ezu080. [DOI] [PubMed] [Google Scholar]
- 17.Smit FE, Dohmen PM. Cardiovascular tissue engineering: where we come from and where are we now? Med Sci Monit Basic Res. 2015;21:1–3. doi: 10.12659/MSMBR.893546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dohmen PM, Lembcke A, Hotz H, et al. Ross operation with a tissue-engineered heart valve. Ann Thorac Surg. 2002;74:1438–42. doi: 10.1016/s0003-4975(02)03881-x. [DOI] [PubMed] [Google Scholar]
- 19.Dohmen PM, Lembcke A, Holinski S, et al. Ten years of clinical results with a tissue-engineered pulmonary valve. Ann Thorac Surg. 2011;92:1308–14. doi: 10.1016/j.athoracsur.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Rüffer A, Purbojo A, Cicha I, et al. Early failure of xenogenous de-cellularsed pulmonary valve conduits – a word of caution! Eur J Cardiothorac Surg. 2010;38:78–85. doi: 10.1016/j.ejcts.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 21.da Costa FD, Dohmen PM, Duarte D, et al. Immunological and echocardiographic evaluation of decellularized versus cryopreserved allografts during the Ross operation. Eur J Cardiothorac Surg. 2005;27:572–78. doi: 10.1016/j.ejcts.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 22.Simon P, Kasimir MT, Seebacher G, et al. Early failure of the tissue engineered porcine heart valve SYNERGRAFTTM in pediatric patients. Eur J Cardiothorac Surg. 2003;23:1002–6. doi: 10.1016/s1010-7940(03)00094-0. [DOI] [PubMed] [Google Scholar]
- 23.Rieder E, Seebacher G, Kasimir MT, et al. Tissue engineering of heart valves: Decellularized porcine and human valve scaffolds differe importantly in residual potential to attract monocytic cells. Circulation. 2005;111:2792–97. doi: 10.1161/CIRCULATIONAHA.104.473629. [DOI] [PubMed] [Google Scholar]
- 24.Kasimir MT, Rieder E, Seebacher G, et al. Comparison of different decellularization procedures of porcine heart valves. Int J Artif Organs. 2003;26:421–27. doi: 10.1177/039139880302600508. [DOI] [PubMed] [Google Scholar]
- 25.Rieder E, Kasimir MT, Silberhumer G, et al. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J Thorac Cardiovasc Surg. 2004;127:399–405. doi: 10.1016/j.jtcvs.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Khanbadkone S, Coats L, Taylor A, et al. Percutaneous pulmonary valve implantation in humans: results in 59 consecutive patients. Circulation. 2005;112:1189–97. doi: 10.1161/CIRCULATIONAHA.104.523266. [DOI] [PubMed] [Google Scholar]
- 27.Nordmeyer J, Khambadkone S, Coats L, et al. Risk stratification systemic classification and anticipatory management strategies for stent fracture after percutaneous pulmonary valve implantation. Circulation. 2007;115:1392–97. doi: 10.1161/CIRCULATIONAHA.106.674259. [DOI] [PubMed] [Google Scholar]
- 28.Eicken A, Ewert P, Hager A, et al. Percutaneous pulmonary valve implantation: two-centre experience with more than 100 patients. Eur Heart J. 2011;32:1260–65. doi: 10.1093/eurheartj/ehq520. [DOI] [PubMed] [Google Scholar]
- 29.Nordmeyer J, Coats L, Lurz P, et al. Percutaneous pulmonary valve-in-valve implantation: a successful treatment concept for early device failure. Eur Heart J. 2008;29:810–15. doi: 10.1093/eurheartj/ehn073. [DOI] [PubMed] [Google Scholar]
- 30.Helbing WA, Niezen RA, Le Cessie S, et al. Right ventricular diastolic function in children with pulmonary regurgitation after repair of tetralogy of Fallot: volumetric evaluation by magnetic resonance velocity mapping. J Am Coll Cardiol. 1996;28:1827–35. doi: 10.1016/S0735-1097(96)00387-7. [DOI] [PubMed] [Google Scholar]
- 31.Frigiola A, Tsang V, Bull C, et al. Biventricular response after pulmonary valve replacement for right ventricular outflow tract dysfunction: is age a predictor of outcome? Circulation. 2008;118:S182–90. doi: 10.1161/CIRCULATIONAHA.107.756825. [DOI] [PubMed] [Google Scholar]
- 32.Harrild DM, Berul CI, Cecchin F, et al. Pulmonary valve replacement in tetralogy of Fallot: impact on survival and ventricular tachycardia. Circulation. 2009;119:445–51. doi: 10.1161/CIRCULATIONAHA.108.775221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biermann D, Schönebeck J, Rebel M, et al. Left coronary artery occlusion after percutaneous pulmonary valve implantation. Ann Thorac Surg. 2012;94:e7–9. doi: 10.1016/j.athoracsur.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 34.McKenzie ED, Khan MS, Dietzman TW, et al. Surgical pulmonary valve replacement: A benchmark for outcomes comparisons. J Thorac Cardiovasc Surg. 2014;148:1450–53. doi: 10.1016/j.jtcvs.2014.02.060. [DOI] [PubMed] [Google Scholar]
- 35.McElhinney DB, Hennesen JT. The Melody valve and Ensemble delivery system for transcatheter pulmonary valve replacement. Ann NY Acad Sci. 2013;1291:77–85. doi: 10.1111/nyas.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiefer P, Gruenwald F, Kempfert J, et al. Crimping may affect the durability of transcatheter valves: an experimental analysis. Ann Thorac Surg. 2011;92:155–60. doi: 10.1016/j.athoracsur.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Dohmen PM, da Costa F, Lopes SV, et al. Successful implantation of a decellularized equine pericardial patch into the systemic circulation. Med Sci Monit Basic Res. 2014;20:1–8. doi: 10.12659/MSMBR.889915. [DOI] [PMC free article] [PubMed] [Google Scholar]