Abstract
Epidemiological studies have examined breast cancer risk in relation to sex hormone concentrations measured by different methods: “extraction” immunoassays (with prior purification by organic solvent extraction, with or without column chromatography), “direct” immunoassays (no prior extraction or column chromatography), and more recently with mass spectrometry-based assays. We describe the associations of estradiol, estrone and testosterone with both body mass index and breast cancer risk in postmenopausal women according to assay method, using data from a collaborative pooled analysis of 18 prospective studies. In general, hormone concentrations were highest in studies that used direct assays and lowest in studies that used mass spectrometry-based assays. Estradiol and estrone were strongly positively associated with body mass index, regardless of the assay method; testosterone was positively associated with body mass index for direct assays, but less clearly for extraction assays, and there were few data for mass spectrometry assays. The correlations of estradiol with body mass index, estrone and testosterone were lower for direct assays than for extraction and mass spectrometry assays, suggesting that the estimates from the direct assays were less precise. For breast cancer risk, all three hormones were strongly positively associated with risk regardless of assay method (except for testosterone by mass spectrometry where there were few data), with no statistically significant differences in the trends, but differences may emerge as new data accumulate. Future epidemiological and clinical research studies should continue to use the most accurate assays that are feasible within the design characteristics of each study.
Keywords: Breast cancer, Estradiol, Body mass index, Extraction immunoassay, Direct immunoassay, Mass spectrometry
1. Introduction
Prospective epidemiological studies of the relationships of endogenous estrogens and other sex hormones with the risk for breast cancer and other diseases have used a variety of assays to measure hormone concentrations in stored samples of serum or plasma. Results have now been published from ∼20 such studies since the late 1980s. The first such studies measured hormones with in-house radioimmunoassays, which generally used a relatively large volume of sample and incorporated an organic extraction step and usually also purification by column chromatography. In the 1990s and 2000s the use of commercially produced immunoassays without extraction or chromatography (“direct assays”) became popular with many epidemiologists because these assays were easier to perform (and therefore faster and cheaper) and use less sample than the extraction assays. Some of the direct assays provided results considered adequate for epidemiological studies, which seek mainly to rank individuals rather than to provide accurate estimates of hormone concentrations [1], but the direct methods tend to overestimate concentrations and suffer from cross-reactivity with other steroids [2–5]. More recently mass spectrometry methods have been developed to measure sex hormones. However, the effect of these assay differences on the associations of sex hormones with other factors is unclear.
The aim of this paper is to describe the relationships of circulating estradiol, estrone and testosterone in postmenopausal women with body mass index (BMI) and breast cancer risk according to the type of assay used, using data from the international Endogenous Hormones and Breast Cancer Collaborative Group [6]. These analyses were prepared for presentation at the workshop “Measuring Estrogen Exposure and Metabolism” in Bethesda, Maryland, March 2014.
2. Methods
2.1. Data collection
Studies were eligible for the collaborative re-analysis if they included data on endogenous hormones and breast cancer risk using prospectively collected blood samples from postmenopausal women, as described previously [6–8]. Studies were identified by computer-aided literature searches, within relevant review articles, and through discussions with colleagues. The studies included were: Breast and Bone Follow-up to the Fracture Intervention Trial (B∼FIT), USA [9]; CLUE I study “Give us a clue to cancer and heart disease”; Washington County, MD, USA [10]; Cancer Prevention Study-II Nutrition Cohort (CPS-II Nutrition Cohort), USA [11]; Columbia Missouri Serum Bank, MO, USA [12,13]; European Prospective Investigation into Cancer and Nutrition (EPIC), Europe [14]; Guernsey, UK [15]; Malmö/Umeå, Sweden [16]; the Melbourne Collaborative Cohort Study (MCCS), Australia [17]; the Multi-Ethnic Cohort (MEC), USA [18]; Nurses’ Health Study (NHS I), USA [19,20]; New York University Women’s Health Study (NYU WHS), USA [21–23]; Study of Hormones and Diet in the Etiology of Breast Tumors (ORDET), Italy [24]; Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial cohort (PLCO), USA [25]; Rancho Bernardo, USA [26]; Radiation Effects Research Foundation (RERF), Japan [27,28]; Study of Osteoporotic Fractures (SOF), USA [29]; United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS), UK [30]; and the Women’s Health Initiative, Observational Study (WHI-OS), USA [31]. Details of the recruitment of participants, informed consent, and definitions of reproductive variables are in the original publications. Women who were using menopausal hormone therapy or other exogenous sex hormones at the time of blood collection were excluded. Collaborators provided data on concentrations of the hormones estradiol, estrone and testosterone, where available, as well as data on reproductive and anthropometric factors.
2.2. Statistical analysis
For the analyses of hormones and BMI, hormone concentrations were logarithmically transformed to normalize the distributions. Geometric mean hormone concentrations by categories of BMI (calculated as weight in kilograms divided by the square of height in metres and categorized as <22.5, 22.5–24.9, 25.0–27.4, 27.5–29.9, and 30.0+ kg/m2), together with their 95% confidence intervals (CIs), were calculated using the predicted values from analysis of variance models, adjusted for study, age at blood collection (<55, 55–59, 60–64, 65–69, and 70+ years), and type of menopause (natural, hysterectomy without ovariectomy, bilateral ovariectomy, other or unknown). Partial correlations of estradiol with estrone, testosterone and BMI were computed using study-specific standardized values: (xjk − mj)/sj where mj and sj denote the mean and standard deviation of the log-transformed hormone concentrations in study j and xjk is an observation from that study. These standardized values were adjusted for age at blood collection and type of menopause (same categories as above).
Logistic regression conditioned on study-specific matching variables and stratified by study was used to calculate the odds ratio (OR) for breast cancer in relation to serum/plasma hormone concentrations, categorizing women in each study according to the quintiles of hormone concentration for the controls in that study. Adjustments were not made for reproductive, anthropometric or lifestyle risk factors for breast cancer because hormones may mediate the effects of some of these risk factors and previous analyses have shown that adjustments for these risk factors do not materially change the associations of hormones with breast cancer risk in postmenopausal women [6,7]. Most of the original studies used a nested case-control design with controls matched to cases on age and date at blood collection and other relevant factors, and the original matching was retained in the current analyses. Study-specific cut-points were used because the absolute concentrations of hormones vary substantially between studies, partly due to laboratory variation and different assay methods [6]. Tests for linear trend were calculated scoring the fifths as 0, 0.25, 0.5, 0.75, and 1. Heterogeneity in linear trends between studies using different assay methods was assessed using chi-square tests.
For the studies using mass spectrometry, we used the values for unconjugated steroids, where available; for B∼FIT, the mass spectrometry data for estradiol and estrone were available for total steroids, which sums the sulphated, glucuronidated, and unconjugated forms, but not for unconjugated steroids, and are not included in the analyses of steroids by BMI.
All statistical tests were two-sided, and statistical significance was taken as P < 0.05. All analyses were performed using Stata Statistical Software release 10 (Stata Corp., College Station, TX).
3. Results
3.1. Collaborating studies
Eighteen studies contributed data, eleven in the USA, two in the UK, one each in Australia, Italy, Japan and Sweden, and the multi-centre European study EPIC. Geometric mean hormone concentrations in controls are shown in Table 1. For estradiol, six studies had used extraction assays (of which all except Guernsey also used purification by column chromatography), ten studies had used direct assays and four studies had used mass spectrometry assays. Some studies had assay results from more than one phase of follow-up, and in the Columbia study results were available from an early phase of follow-up using direct assays, and from a later time of follow-up using mass spectrometry assays (which included some of the same women as the first follow-up).
Table 1.
Age-adjusted geometric mean (95% CI) hormone concentrations for controls, by assay type and study.
| Assay type and study | Sample | Estradiol (pmol/L) |
Estrone (pmol/L) |
Testosterone (nmol/L) |
|||
|---|---|---|---|---|---|---|---|
| N | Mean (95% CI) | N | Mean (95% CI) | N | Mean (95% CI) | ||
| Extractiona | |||||||
| CLUE-I | Serum | 58 | 51.9 (44.3–60.8) | 58 | 134 (118–153) | ||
| Guernsey | Serum | 177 | 37.5 (34.2–41.1) | ||||
| MEC | Plasma | 302 | 30.1 (28.0–32.3) | 303 | 118 (111–125) | 303 | 0.79 (0.73–0.85) |
| NHS I | Plasma | 637 | 24.9 (23.8–26.2) | 623 | 90 (86–93) | 626 | 0.74 (0.71–0.78) |
| Rancho Bernardo | Plasma | 127 | 42.3 (38.0–47.1) | 131 | 109 (100–119) | 128 | 0.78 (0.70–0.88) |
| SOF | Serum | 365 | 21.2 (19.8–22.7) | 245 | 72 (67–77) | 372 | 0.62 (0.58–0.67) |
| Sweden Malmö/Umeå | Plasma | 230 | 1.17 (1.07–1.27) | ||||
| Direct | |||||||
| Columbia (direct assay) | Serum | 133 | 48.4 (43.6–53.8) | 133 | 120 (110–131) | 133 | 0.55 (0.49–0.61) |
| EPIC phase 1 | Serum | 1152 | 91.5 (88.2–94.8) | 1106 | 140 (136–145) | 1319 | 1.14 (1.10–1.18) |
| EPIC phase 2 | Serum | 818 | 71.6 (68.6–74.7) | 575 | 140 (135–147) | 808 | 1.10 (1.05–1.15) |
| Guernsey | Serum | 178 | 0.93 (0.84–1.02) | ||||
| MCCS | Plasma | 707 | 60.3 (57.6–63.1) | 714 | 0.66 (0.62–0.69) | ||
| NYU WHS phase 1 | Serum | 558 | 84.7 (80.4–89.3) | 562 | 95 (91–99) | 562 | 0.63 (0.60–0.67) |
| NYU WHS phase 2 | Serum | 347 | 98 (93–103) | ||||
| ORDET | Serum | 681 | 18.2 (17.4–19.1) | 681 | 0.78 (0.74–0.82) | ||
| RERF phase 1 | Serum | 45 | 69.7 (58.3–83.5) | ||||
| RERF phase 2 | Serum | 124 | 68.0 (61.0–75.8) | 126 | 0.37 (0.33–0.42) | ||
| Sweden Malmö/Umeå | Plasma | 239 | 73 (69–78) | ||||
| UKCTOCS | Serum | 375 | 59.4 (55.8–63.2) | 381 | 302 (287–318) | 377 | 0.83 (0.78–0.89) |
| WHI-OS | Serum | 436 | 41.1 (38.7–43.5) | ||||
| Mass spectrometry | |||||||
| B∼FIT | Serum | 490 | [40.0 (37.8–42.3)]b | 490 | [291 (277–305)]b | ||
| CPS-II Nutrition Cohort | 249 | 24.4 (22.5–26.4) | 268 | 65 (61–69) | 254 | 0.68 (0.63–0.74) | |
| Columbia (mass spec.) | Serum | 217 | 11.8 (10.9–12.8) | 217 | 45 (42–48) | ||
| PLCO | Serum | 445 | 15.9 (15.0–16.8) | 445 | 55 (53–58) | ||
Abbreviations for study names: B∼FIT = Breast and Bone Follow-up to the Fracture Intervention Trial; CLUE = Washington County, MD study “Give us a clue to cancer and heart disease”; CPS-II = Cancer Prevention Study-II; EPIC = European Prospective Investigation into Cancer and Nutrition; MCCS = Melbourne Collaborative Cohort Study; MEC = Multi-ethnic Cohort; NHS I = Nurses’ Health Study I; NYU WHS = New York University Women’s Health Study; ORDET = Study of Hormones and Diet in the Etiology of Breast Tumors; PLCO = Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; RERF = Radiation Effects Research Foundation; SOF = Study of Osteoporotic Fractures; UKCTOCS = United Kingdom Collaborative Trial of Ovarian Cancer Screening; WHI-OS = Women’s Health Initiative, Observational Study.
Assays which included an extraction step; all except two (Guernsey and Sweden Malmö/Umeå) also included purification by chromatography.
Geometric mean concentrations of estradiol and estrone for B∼FIT are for total steroids, versus unconjugated steroids in the other studies.
3.2. Associations of hormones with BMI
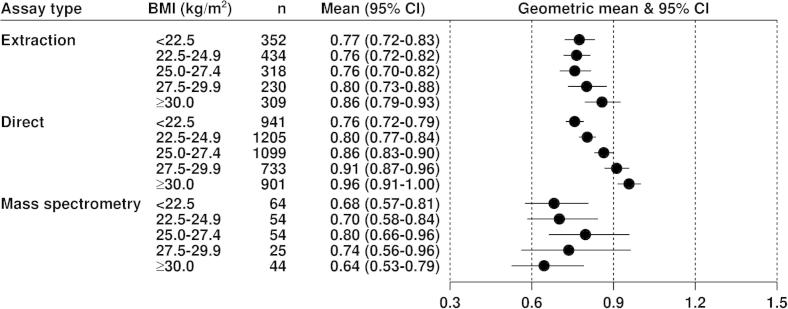
Figs. 1–3 show the geometric mean hormone concentrations in relation to BMI for estradiol, estrone, and testosterone. Among all controls for estradiol (Fig. 1) and estrone (Fig. 2), the mean values were highest for the direct assays and lowest for the mass spectrometry assays, with the extraction assays intermediate. For all assay types the mean concentrations of both estrogens were positively associated with BMI in an approximately linear fashion. Geometric mean concentrations of estradiol were 82%, 31% and 43% higher in obese (BMI ⩾ 30) than in lean (BMI < 22.5) women for data from extraction, direct and mass spectrometry assays, respectively (Fig. 1); the corresponding differences for estrone were 57%, 47% and 33% (Fig. 2). Geometric mean concentrations of testosterone were 12% and 26% higher in obese (BMI ⩾ 30) than in lean (BMI < 22.5) women for data from extraction and direct assays, respectively, with few data from mass spectrometry (Fig. 3). The correlations of estradiol with BMI, estrone and testosterone were substantially larger for extraction and mass spectrometry assays than for direct assays (Table 2).
Fig. 1.
Geometric mean estradiol (pmol/L, with 95% confidence intervals) in postmenopausal control women by assay type in relation to BMI, adjusted for study, age at blood collection and type of menopause.
Fig. 2.
Geometric mean estrone (pmol/L, with 95% confidence intervals) in postmenopausal control women by assay type in relation to BMI, adjusted for study, age at blood collection and type of menopause.
Fig. 3.
Geometric mean testosterone (nmol/L, with 95% confidence intervals) in postmenopausal control women by assay type in relation to BMI, adjusted for study, age at blood collection and type of menopause.
Table 2.
Partial correlations of log estradiol with BMI, log estrone and log testosterone: values standardized to study-specific distribution and adjusted for age and type of menopause.
| Assay | BMI | Estrone | Testosterone |
|---|---|---|---|
| Extraction | 0.42 | 0.66 | 0.40 |
| Direct | 0.19 | 0.38 | 0.32 |
| Mass spectrometry | 0.41 | 0.84 | 0.41 |
3.3. Associations of hormones with breast cancer risk
Figs. 4–6 show the associations of the hormones with breast cancer risk. With the exception of testosterone measured by mass spectrometry, for which there was limited data, all measures of the three hormones showed highly statistically significant associations with breast cancer risk, with odds ratios in the highest versus the lowest fifth between 1.46 and 2.66. There was no statistically significant heterogeneity between the different assay methods for the linear associations of each hormone with breast cancer risk.
Fig. 4.
Odds ratios (95% confidence intervals) for breast cancer by fifth of estradiol in postmenopausal cases and matched controls by assay type, conditioned on study-specific matching variables and stratified by study.
Fig. 5.
Odds ratios (95% confidence intervals) for breast cancer by fifth of estrone in postmenopausal cases and matched controls by assay type, conditioned on study-specific matching variables and stratified by study.
Fig. 6.
Odds ratios (95% confidence intervals) for breast cancer by fifth of testosterone in postmenopausal cases and matched controls by assay type, conditioned on study-specific matching variables and stratified by study.
4. Discussion
The findings from the different assay types (direct, extraction, mass spectrometry) were broadly similar. For all three hormones examined, the largest amount of data was from direct assays. Where enough data were available, all three assay methods showed that BMI was strongly positively associated with the estrogens and moderately positively associated with testosterone, and all three hormones were strongly positively associated with breast cancer risk, regardless of assay method.
The cross-sectional analyses of geometric mean hormone concentrations in relation to BMI showed that on average the direct assays give concentrations that are substantially higher than those from the other assay methods. This difference has been discussed previously, and may be in part due to cross-reactivity between steroids in the direct assays leading to estimated absolute concentrations which may be greater than the true value [2–5]. The direct assays still showed the expected strong positive associations between BMI and estrogens, but the relative increase in estrogens from lean to obese women was larger for the extraction assays than for the other methods. Conversely the direct assays showed a stronger positive association between testosterone and BMI than the extraction assays. This could perhaps be due to cross-reactivity with estrogenic compounds in the direct assay methods. The correlations of estradiol with BMI, estrone and testosterone were lower for the direct assays than for the extraction and mass spectrometry assays; the reason for this is not known but might again be due to cross-reactivity in the direct assay methods.
The relative risk analyses showed strong positive associations of all three hormones with breast cancer risk, as previously reported by this collaborative group [6] and by subsequent individual studies [9,13,14,16,18,20,23,25,30,31]. There were no striking or statistically significant differences between the results from the different assay methods, but this should be re-evaluated as prospective data accumulate because further data from the more accurate assay methods might reveal differences.
In conclusion, the existing data from prospective studies show that estrogens are strongly associated with breast cancer risk, regardless of the assay method. Direct assays are in general less accurate than extraction assays or mass spectrometry assays [2–5], and it is possible that the estrogen measures from direct assays may partly reflect cross-reactivity with a number of estrogens and the detection of overall estrogenicity. Recent technological advances have made it possible to apply mass spectrometry methods to small sample volumes in large-scale studies, and future epidemiological and clinical research studies should continue to use the most accurate assays that are feasible within the design characteristics of each study.
Acknowledgements
The central pooling and analysis of these data was supported by Cancer Research UK (C570/A5028). Details of funding for the original studies are in the relevant publications. The NYU WHS was supported by National Cancer Institute Grants R01 CA098661 and P30 CA016087 and National Institute of Environmental Health Sciences center grant ES000260.
UKCTOCS researchers were supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
The funders of this pooled analysis had no role in the study design, data collection, data analysis, data interpretation or writing of the report. The authors had full access to all the data and had final responsibility for the decision to submit the manuscript. We thank the women who participated, the research staff and the collaborating laboratories in each of the studies.
Appendix A. Authors
A.1. Co-authors at secretariat
T.J. Key, P.N. Appleby, G.K. Reeves, R.C. Travis, Cancer Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, Oxford, UK.
A.2. Co-authors from collaborating studies
B∼FIT, USA: L.A. Brinton, Hormonal and Reproductive Epidemiology Branch, National Cancer Institute, Bethesda, MD, USA; C.M. Dallal, Department of Epidemiology and Biostatistics, University of Maryland School of Public Health, College Park, MD, USA.
CLUE I, Washington County, MD, USA: K.J. Helzlsouer, The Prevention and Research Center, Mercy Medical Center, Baltimore, MD, USA; J. Hoffman-Bolton, K. Visvanathan, The George W. Comstock Center for Public Health Research and Prevention, Johns Hopkins University, Hagerstown, MD, USA.
Columbia, MO, USA: J.F. Dorgan, University of Maryland School of Medicine, Baltimore, MD, USA; R.T. Falk, Hormonal and Reproductive Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
CPS-II Nutrition Cohort, USA: S.M. Gapstur, M.M. Gaudet, Epidemiology Research Program, American Cancer Society, Atlanta, GA, USA.
EPIC, Europe: R. Kaaks, DKFZ, Heidelberg, Germany; E. Riboli, School of Public Health, Imperial College, London, UK; S. Rinaldi, International Agency for Research on Cancer, Lyon, France.
Guernsey, UK: T. Key, Cancer Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Malmö/Umeå, Sweden: J. Manjer, Department of Surgery, Malmö University Hospital, Malmö, Sweden; G. Hallmans, Department of Clinical Medicine and Public Health, Umeå University Hospital, Umeå, Sweden.
MCCS, Australia: G.G. Giles, Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Australia.
MEC, USA: L. Le Marchand, L.N. Kolonel, Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI, USA; B.E. Henderson, University of Southern California, Health Sciences Campus, Los Angeles, CA, USA.
Nurses’ Health Study, USA: S.S. Tworoger, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School and Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA; S.E. Hankinson, Division of Biostatistics and Epidemiology, University of Massachusetts, Amherst, MA, and Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School and Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA.
NYU WHS, USA: A. Zeleniuch-Jacquotte, K. Koenig, Department of Environmental Medicine, New York University School of Medicine, New York, NY, USA.
ORDET, Italy: V. Krogh, S. Sieri, Fondazione IRCCS Istituto Nazionale dei Tumori, Milano, Italy; P. Muti, Department of Oncology, McMaster University, Hamilton, Canada.
PLCO, USA: R.G. Ziegler, C. Schairer, Epidemiology and Biostatistics Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA; B.J. Fuhrman, Department of Epidemiology, Fay W Boozman College of Public Health, University of Arkansas for Medical Sciences, Little Rock, AR, USA.
Rancho Bernardo, USA: E. Barrett-Connor, G.A. Laughlin, Division of Epidemiology, Department of Family and Preventive Medicine, University of California, San Diego, CA, USA.
RERF, Japan: E.J. Grant, Department of Epidemiology, J Cologne, Department of Statistics, W. Ohishi, Department of Clinical Studies, Radiation Effects Research Foundation, Hiroshima, Japan; A. Hida, Department of Clinical Studies, Radiation Effects Research Foundation, Hiroshima, Nagasaki, Japan.
SOF, USA: J.A. Cauley, Department of Epidemiology, University of Pittsburgh, PA, USA.
UKCTOCS, UK: E.-O. Fourkala, U. Menon, Women’s Cancer, Institute for Women’s Health, University College, London, UK.
Women’s Health Initiative Observational Study, USA: T.E. Rohan, H.D. Strickler, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA; M.J. Gunter, School of Public Health, Imperial College, London, UK.
References
- 1.Rinaldi S., Déchaud H., Biessy C., Morin-Raverot V., Toniolo P., Zeleniuch-Jacquotte A. Reliability and validity of commercially available, direct radioimmunoassays for measurement of blood androgens and estrogens in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2001;10(7):757–765. [PubMed] [Google Scholar]
- 2.Stanczyk F.Z., Cho M.M., Endres D.B., Morrison J.L., Patel S., Paulson R.J. Limitations of direct estradiol and testosterone immunoassay kits. Steroids. 2003;68(14):1173–1178. doi: 10.1016/j.steroids.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Lee J.S., Ettinger B., Stanczyk F.Z., Vittinghoff E., Hanes V., Cauley J.A., Chandler W., Settlage J., Beattie M.S., Folkerd E., Dowsett M., Grady D., Cummings S.R. Comparison of methods to measure low serum estradiol levels in postmenopausal women. J Clin Endocrinol Metab. 2006;91(10):3791–3797. doi: 10.1210/jc.2005-2378. [DOI] [PubMed] [Google Scholar]
- 4.Stanczyk F.Z., Lee J.S., Santen R.J. Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol Biomarkers Prev. 2007;16(9):1713–1719. doi: 10.1158/1055-9965.EPI-06-0765. [DOI] [PubMed] [Google Scholar]
- 5.Rosner W., Hankinson S.E., Sluss P.M., Vesper H.W., Wierman M.E. Challenges to the measurement of estradiol: an Endocrine Society position statement. J Clin Endocrinol Metab. 2013;98(4):1376–1387. doi: 10.1210/jc.2012-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endogenous Hormones and Breast Cancer Collaborative Group Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 7.Endogenous Hormones and Breast Cancer Collaborative Group Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95(16):1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 8.Endogenous Hormones and Breast Cancer Collaborative Group Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer. 2011;105(5):709–722. doi: 10.1038/bjc.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dallal C.M., Tice J.A., Buist D.S., Bauer D.C., Lacey J.V., Jr., Cauley J.A. Estrogen metabolism and breast cancer risk among postmenopausal women: a case-cohort study within B∼FIT. Carcinogenesis. 2014;35(2):346–355. doi: 10.1093/carcin/bgt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helzlsouer K.J., Alberg A.J., Bush T.L., Longcope C., Gordon G.B., Comstock G.W. A prospective study of endogenous hormones and breast cancer. Cancer Detect Prev. 1994;18(2):79–85. [PubMed] [Google Scholar]
- 11.Gaudet M.M., Patel A.V., Teras L.R., Sun J., Campbell P.T., Stevens V.L. Obesity-related markers and breast cancer in CPS-II Nutrition Cohort. Int J Mol Epidemiol Genet. 2013;4(3):156–166. [PMC free article] [PubMed] [Google Scholar]
- 12.Dorgan J.F., Longcope C., Stephenson H.E., Jr., Falk R.T., Miller R., Franz C. Relation of prediagnostic serum estrogen and androgen levels to breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5(7):533–539. [PubMed] [Google Scholar]
- 13.Falk R.T., Brinton L.A., Dorgan J.F., Fuhrman B.J., Veenstra T.D., Xu X. Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: a nested case-control study. Breast Cancer Res. 2013;15(2):R34. doi: 10.1186/bcr3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaaks R., Rinaldi S., Key T.J., Berrino F., Peeters P.H., Biessy C. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12(4):1071–1082. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 15.Thomas H.V., Key T.J., Allen D.S., Moore J.W., Dowsett M., Fentiman I.S. A prospective study of endogenous serum hormone concentrations and breast cancer risk in post-menopausal women on the island of Guernsey. Br J Cancer. 1997;76(3):401–405. doi: 10.1038/bjc.1997.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manjer J., Johansson R., Berglund G., Janzon L., Kaaks R., Agren A. Postmenopausal breast cancer risk in relation to sex steroid hormones, prolactin and SHBG (Sweden) Cancer Causes Control. 2003;14(7):599–607. doi: 10.1023/a:1025671317220. [DOI] [PubMed] [Google Scholar]
- 17.Baglietto L., English D.R., Hopper J.L., MacInnis R.J., Morris H.A., Tilley W.D. Circulating steroid hormone concentrations in postmenopausal women in relation to body size and composition. Breast Cancer Res Treat. 2009;115(1):171–179. doi: 10.1007/s10549-008-0069-3. [DOI] [PubMed] [Google Scholar]
- 18.Woolcott C.G., Shvetsov Y.B., Stanczyk F.Z., Wilkens L.R., White K.K., Caberto C. Plasma sex hormone concentrations and breast cancer risk in an ethnically diverse population of postmenopausal women: the Multiethnic Cohort Study. Endocr Relat Cancer. 2010;17(1):125–134. doi: 10.1677/ERC-09-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hankinson S.E., Willett W.C., Manson J.E., Colditz G.A., Hunter D.J., Spiegelman D. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1998;90(17):1292–1299. doi: 10.1093/jnci/90.17.1292. [DOI] [PubMed] [Google Scholar]
- 20.Missmer S.A., Eliassen A.H., Barbieri R.L., Hankinson S.E. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96(24):1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 21.Toniolo P.G., Levitz M., Zeleniuch-Jacquotte A., Banerjee S., Koenig K.L., Shore R.E. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Inst. 1995;87(3):190–197. doi: 10.1093/jnci/87.3.190. [DOI] [PubMed] [Google Scholar]
- 22.Zeleniuch-Jacquotte A., Bruning P.F., Bonfrer J.M., Koenig K.L., Shore R.E., Kim M.Y. Relation of serum levels of testosterone and dehydroepiandrosterone sulfate to risk of breast cancer in postmenopausal women. Am J Epidemiol. 1997;145(11):1030–1038. doi: 10.1093/oxfordjournals.aje.a009059. [DOI] [PubMed] [Google Scholar]
- 23.Zeleniuch-Jacquotte A., Shore R.E., Koenig K.L., Akhmedkhanov A., Afanasyeva Y., Kato I. Postmenopausal levels of oestrogen, androgen, and SHBG and breast cancer: long-term results of a prospective study. Br J Cancer. 2004;90(1):153–159. doi: 10.1038/sj.bjc.6601517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berrino F., Muti P., Micheli A., Bolelli G., Krogh V., Sciajno R., Pisani P., Panico S., Secreto G. Serum sex hormone levels after menopause and subsequent breast cancer. J Natl Cancer Inst. 1996;88(5):291–296. doi: 10.1093/jnci/88.5.291. [DOI] [PubMed] [Google Scholar]
- 25.Fuhrman B.J., Schairer C., Gail M.H., Boyd-Morin J., Xu X., Sue L.Y. Estrogen metabolism and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2012;104(4):326–339. doi: 10.1093/jnci/djr531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garland C.F., Friedlander N.J., Barrett-Connor E., Khaw K.T. Sex hormones and postmenopausal breast cancer: a prospective study in an adult community. Am J Epidemiol. 1992;135(11):1220–1230. doi: 10.1093/oxfordjournals.aje.a116228. [DOI] [PubMed] [Google Scholar]
- 27.Kabuto M., Akiba S., Stevens R.G., Neriishi K., Land C.E. A prospective study of estradiol and breast cancer in Japanese women. Cancer Epidemiol Biomarkers Prev. 2000;9(6):575–579. [PubMed] [Google Scholar]
- 28.Grant E.J., Neriishi K., Cologne J., Eguchi H., Hayashi T., Geyer S. Associations of ionizing radiation and breast cancer-related serum hormone and growth factor levels in cancer-free female A-bomb survivors. Radiat Res. 2011;176(5):678–687. doi: 10.1667/rr2631.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cauley J.A., Lucas F.L., Kuller L.H., Stone K., Browner W., Cummings S.R. Elevated serum estradiol and testosterone concentrations are associated with a high risk for breast cancer. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1999;130(4 Pt 1):270–277. doi: 10.7326/0003-4819-130-4_part_1-199902160-00004. [DOI] [PubMed] [Google Scholar]
- 30.Fourkala E.O., Zaikin A., Burnell M., Gentry-Maharaj A., Ford J., Gunu R. Association of serum sex steroid receptor bioactivity and sex steroid hormones with breast cancer risk in postmenopausal women. Endocr Relat Cancer. 2012;19(2):137–147. doi: 10.1530/ERC-11-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunter M.J., Hoover D.R., Yu H., Wassertheil-Smoller S., Rohan T.E., Manson J.E. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101(1):48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]