Abstract
Purpose
MicroRNA (miR) expression is altered in urologic malignancies, including bladder cancer (BC). Individual miRs have been shown to modulate multiple signaling pathways that contribute to BC. We reviewed the primary literature on the role of miRs in BC and provide a general introduction to the processing, regulation, and function of miRs as tumor suppressors and oncogenes; and critically evaluate the literature on the implications of altered miR expression in BC.
Materials and Methods
We searched the English language literature for original and review articles in PubMed® from 1993 to March 2013, using the terms “microRNA” and “bladder cancer,” “transitional cell carcinoma,” or “urothelial carcinoma.” This search yielded 133 unique articles with greater than 85% published within the last three years.
Results
To date, the majority of miR studies in BC use profiling to describe dynamic changes in miR expression across stage and grade. Generalized down regulation of miRs, including those that target the fibroblast growth factor 3 (FGFR3) pathway such as miR-145, miR-101, miR-100 and miR-99a, has been observed in low grade, non-muscle invasive bladder cancer (NMIBC). In contrast, generalized increased expression of miRs is observed in high grade, muscle invasive bladder cancer (MIBC) compared with adjacent normal bladder urothelium, including miRs predicted to target p53, such as miR-21 and miR-373. Furthermore, p53 suppresses transcriptional factors which promote mesenchymal differentiation, ZEB-1 and ZEB-2, through regulation of the miR200 family.
Conclusions
Aberrations in miR expression identified between NMIBC and MIBC support and provide insight into molecular alterations known to distinguish the two parallel pathways of bladder carcinogenesis. The heterogeneity of tumor specimens and research methods limits the reproducibility of changes in miR expression profiles between studies and underscores the importance of in vivo validation in a field that utilizes in silico miR target prediction models.
Keywords: microRNAs, urinary bladder neoplasm, biomarkers
I. Introduction
MicroRNAs (miRs) are a class of small, conserved, non-protein-coding RNAs that regulate gene expression by controlling translation of target mRNAs. Greater than 60% of mammalian mRNAs contain conserved regions that serve as targets for miRs. In addition, one mRNA can be the target of multiple miRs, and each individual miR has the capacity to target hundreds of genes, with an average of 500 targets per miR [1]. As of its most recent release in August of 2012, over 2100 mature miRs were identified in humans and cataloged in the miRBase database [2]. With an emphasis on bladder cancer (BC), this review will provide a general introduction to miRs as tumor suppressors and oncogenes and critique the primary literature published to date on miR expression profiles and the roles of select individual miRs in BC.
II. Materials and Methods
We searched the English language literature for original and review articles in PubMed® from 1993 through March 2013, using the terms “microRNA” and “bladder cancer,” “transitional cell carcinoma,” or “urothelial carcinoma”. This search yielded 133 unique articles with greater than 85% published within the last three years. We selected articles for inclusion based on the presentation of miR expression profiling data from human bladder cancers or cell lines (rather than urine or serum), mechanistic validation of miRs identified within observational studies, or discussion of epigenetic regulation of miR expression. We cross-referenced articles for relevant missing reports.
III. Results and Discussion
A. Introduction to miRs
i. Biogenesis and mechanism of action of miRs
Approximately 1% of the mammalian genome encodes miRs in exons or introns of protein-coding genes or intergenic regions, and miRs may be clustered or found in isolation [3]. miRs are processed through multiple steps, starting in the nucleus and continuing in the cytoplasm (Figure 1). miRs are first transcribed in the nucleus from introns or exons of protein coding or non-protein coding RNA genes by RNA polymerase II into long primary transcripts (pri-miRNAs) of 1-3 kilobases. These transcripts are further processed into shorter pre-miRNAs by the RNA polymerase III Drosha and its cofactor Pasha, which comprise the DiGeorge syndrome critical region gene 8 (DGCR8) complex [3, 4]. (Of note, alternative mechanisms to miR biogenesis exist that do not require cleavage by Drosha [5].) Pre-miRNAs are imperfect hairpins of 70-100 nucleotides that are transported by exportin 5 into the cytoplasm where Dicer, another ribonuclease, cleaves the pre-miR hairpin to produce a mature, double-stranded miR of 18-25 nucleotides. The mature miR, or guide strand, is preferentially incorporated into an enzyme complex named RISC (RNA-induced silencing complex), which is comprised of the argonaute proteins (argonaute 1-4) and the target miR. The passenger strand, in contrast, is usually degraded. The mature miR provides mRNA target recognition for RISC and enables the complex to bind the 3′ untranslated region (UTR) of a complementary target mRNA to its “seed region,” which refers to a short sequence of less than 10 basepairs beginning at position 2 to 8 from the 5′ miRNA that binds to the 3′ UTR of mRNA transcripts. The fate of the target mRNA is determined by the degree of complementarity relative to the miR: a perfect match inhibits translation of the mRNA, which is then eliminated via RNA-induced silencing complex (RISC)-mediated destruction, whereas less perfect pairing slows protein translation but preserves the transcript [1]. Both mechanisms ultimately down-regulate target protein expression.
Figure 1.
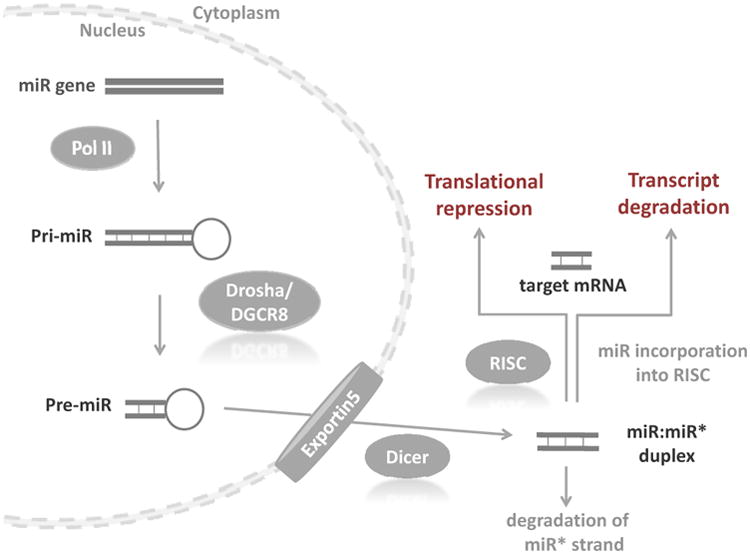
ii. Dysregulation of miRs in cancer
Genomic profiling studies demonstrate that the expression of groups of miRs is reproducibly increased or decreased in specific malignancies. miRs altered in malignancy (“oncomirs”) [4], target either oncogenes or tumor suppressors depending upon the cellular context. In turn, miRs can function as oncogenes or tumor suppressors depending on their relative expression and target. Loss of expression of a tumor suppressor miR through disordered miR processing, epigenetic alteration, or gene mutation or deletion may promote expression of a target oncogene. In contrast, increased expression of an oncogenic miR through amplification or translocation may result in reduced expression of a tumor suppressor.
While mutations in genomic regions encoding miRs have rarely been detected, miRs are often located at chromosomal breakpoint regions susceptible to translocations, amplifications, or deletions [6]. In addition, mutations in mRNA target regions of a miR may alter miR affinity for target transcripts or block binding altogether. miRs are often located near CpG islands or shores that may be silenced via methylation, an epigenetic phenomena that has been documented in BC [7]. Alterations in the miR processing machinery, such as defects in Dicer or Drosha activity, may also result in dysregulated miR expression, and this has been documented in BC [8]. Finally, genetic variants of miR-related genes in the form of single nucleotide polymorphisms (SNPs) have been shown to increase the risk of developing multiple malignancies, including BC [9].
iii. miR Isolation, Profiling and Target Identification
mRNA is often chemically modified and fragmented in FFPE samples making it difficult to quantify. In contrast, miRs are better able to withstand tissue processing because of their smaller size [10]. The study of miRs and their impact on gene regulation involves miR expression profiling and miR target identification. miR expression is commonly measured using either reverse transcription coupled with real time quantitative polymerase chain reaction (RT-qPCR) or commercially available miR microarray platforms. Recent work has addressed the lack of reference miRs for RT-qPCR expression studies in BC and provides the first recommendation for suitable BC miR gene reference signatures for normalization, specifically (miR-148b, miR-181b, and miR-874) or (miR-101, miR-125a-5p, miR-148b, and miR-151-5p) [11].
Target identification for miRs of interest is a significant challenge in this rapidly growing field due to the volume of new information, evolving nomenclature, and the fact that miRs bind targets with partial rather than complete complementarity. miR databases, such as TargetScan [12], search for target identification based on a smaller “seed” region in contrast to NCBI BLAST which solely relies on complete complementarity to identify a target. Because small sequences occur more frequently in the genome and because miR binding tolerates some degree of mismatch, miR target identification is an inexact science. While beyond the scope of this review, a comprehensive evaluation of Web-based databases of miR sequences and targets was recently published [13]. Experimental in vivo validation of miR targets is essential given that in silico prediction algorithms are imperfect. Confirmation of miR-mRNA interactions is most commonly performed by increasing or decreasing miR levels under a controlled set of experimental conditions and then assaying mRNA levels with mRNA expression arrays. Other methods of confirmation include correlation of miR levels and that of their predicted target genes by in situ hybridization and RT-qPCR.
B. Role of miRs in Bladder Cancer
MiR expression is altered in urologic malignancies and modulates multiple potentially oncogenic pathways. The majority of miR studies to date in BC consist of profiling experiments that yield a descriptive picture of dynamic expression changes across stage and grade. Working within the established paradigm of low-grade, non-muscle invasive bladder cancer (NMIBC) and high-grade, muscle invasive bladder cancer (MIBC) [14], most of these experiments have compared miR expression in normal bladder tissue versus NMIBC or MIBC. An increasing number of studies report the changed expression and predicted function of individual miR, though few have been independently validated. Thus far, a complex network of interactions is emerging that implicates novel mechanisms of gene modulation in BC pathogenesis. However, a comprehensive understanding of the role of miR is still required to reconcile apparently conflicting changes in miR expression in BC.
i. Comparative expression profiling in BC
MiRs known to be dysregulated in BC are predicted to target signal transduction pathways most highly implicated in the pathogenesis of BC, specifically fibroblast growth factor receptor 3 (FGFR3) and p53 [15] (Tables 1 and 2). Nearly 80% of papillary or NMIBC are characterized by mutation or overexpression of the fibroblast growth factor receptor (FGFR3) gene. In contrast, MIBC rarely harbor FGFR3 alterations. Instead, nearly 60% of these invasive tumors exhibit p53 mutations [16].
Table 1.
MicroRNAs with increased expression in bladder cancer compared with normal bladder urothelium.
| miR | mRNA Targets | Reference |
|---|---|---|
| miR-7 † | UBXN2B, SPATA2, C5orf22, ZNF828, POLE4 | [22, 25] |
| miR-10a | ARSJ,CADM2, SOBP, BDNF, FIGN | [22, 55] |
| miR-15a | FGF2, SCL11A2, PLAG1, ZBTB34, MGATA4 | [17] |
| miR-17/17-5p | FGD4, PKD2, MAP3K2, ZNFX1, PDCD1LG2 | [20, 25] |
| miR-18a | NEDD9, PHC3, INADL, TMEM170B, HCFC2 | [25, 56] |
| miR-19b | ZMYND11, LRP2, ARDB1, LONRF1, DSEL | [25, 57] |
| miR-20a | FGD4, PKD2, MAP3K2, ZNFX1, PDCD1LG2 | [25, 36, 56] |
| miR-21 | ZNF367, GPR64, YOD1, PHF14, PLEKHA1 | [17, 36] |
| miR-23a/b | KIAA1467, WBP2, PDE4B, ZIC4, MRC1 | [20] |
| miR-25 | CD69, FNIP1, SCL12A5, MAN2A1, ACTC1 | [25] |
| miR-26a/b/c † | KLHDC5, TET2, STRADB, CHORDC1, FAM98A | [20] |
| miR-92 | n/a | [25] |
| miR-93 | CROT, FGD4, LATS2, TGFBR2, ZKSCAN1 | [17, 56] |
| miR-96 | IRS1, MAP4K1 | [25, 26] |
| miR-100 † | (target validated with decreased miR expression) | [22] |
| miR-103-1 | DICER1, ANO3, NF1, EIF5, ARIH2 | [20] |
| miR-106a/b | n/a | [56] |
| miR-125b † | (target validated with decreased miR expression) | [22, 28] |
| miR-129 ‡ | SOX4, GALNT1 | [36] |
| miR-135b | ANGPT2, GK5, NR3C2, GULP1, LOC221710 | [17] |
| miR-141† | ZFR, RANBP6, ZEB2, ABL2, ARPC5 | [25, 53, 56] |
| miR-147 | ZNF677, C13orf36, NF1, MAP4K3, PDPK1 | [17] |
| miR-182 | RGS17, MITF, MFAP3, CTTN, TMEM20 | [25, 55] |
| miR-183 | ABAT, AKAP12, PIGX, PFN2, PTPN4 | [25, 55] |
| miR-184 | EPB41L5, ELN, SF1, ALDH4A1, NUS1 | [36] |
| miR-185 | EPB41L5, ELN, SF1, ALDH4A1, NUS1, LIMCH1 | [20] |
| miR-193a-3p ‡ | AB12, IL17RD, SCL10A6, DCAF7, FLI1 | [36] |
| miR-196a | HOXC8, HOXA7, SCL9A6, HOXA9, KLHL23 | [55] |
| miR-198 | UCN2, FAM134B, OTX1, NRIP1, APH1A | [36] |
| miR-199b-3p † | CELSR2, BCAR3, SH3GLB1, SLC24A2, KLHL3 | [22] |
| miR-200a/b/c † | (target validated with decreased miR expression) | [25, 56] |
| miR-203 † | (target validated with decreased miR expression) | [20, 55, 58] |
| miR-205 † | (target validated with decreased miR expression) | [20, 53] |
| miR-211 | SLC37A3, PHOX2B, AP1S2, RAB22A, HGSNAT | [17] |
| miR-221 | TRAIL pathway | [20, 59] |
| miR-222 | SNZ4, RGS5, CDKN1B, CXCL12, TCF12 | [22] |
| miR-223 † | FBXW7, SP3, PAX6, C13orf31, PURB | [20] |
| miR-224 | ZDHHC20, AFF3, U2SURP, C8orf44, TTC3 | [55] |
| miR-236 | n/a | [20] |
| miR-296-5p ‡ | HMGA1, RNF44, KCTD15, SLC25A22, WNT7B | [36] |
| miR-320a ‡ | LPPR1, KITLG, DNER, PBX3, IRF6 | [36] |
| miR-328 ‡ | KLHDC5, EIF4EBP1, ZAK, RANBP10, PGM2 | [36] |
| miR-373 ‡ | TMEM22, AKR1D1, ZPF14, ARFIP1, EDEM1 | [36] |
| miR-429 † | n/a | [25, 53, 56] |
| miR-452 | FAM8A1, DACT1, CASD1, PURA, IMMP2L | [22] |
| miR-483-3p ‡ | GAN, PGAM1, PGAM4, ZBTB26, C5orf42 | [36] |
| miR-489 †,‡ | n/a | [36] |
| miR-490-3p ‡ | ZC3H6, THEM4, TNKS2, USP37, TMOD3 | [36] |
| miR-492 | RPSGKA6, SPOPL, PPFIA4, CCBE1, CLDN19 | [36] |
| miR-498 †,‡ | NPA1L3, TIMM17A, CXorf1, C90rf5, DUSP4 | [36] |
| miR-503 †,‡ | FGF2, ZNFR2, MAPK8IP2, MGAT4A, KIF1C | [36] |
| miR-507 | CAMK4, PRKCE, ADAM17, SCN1A, BACH2 | [17] |
| miR-510 | PPP2R5E, SPOCK1, ADCY5, DPCD, THOC2 | [36] |
| miR-516b ‡ | GYS1, APOLD1, TOX3, RIC3, PKP2 | [36] |
| miR-517a | ZNF521, TMCC1, TNIP1, VSTM2B, ISL1 | [17] |
| miR-519e (‡) | n/a | [36] |
| miR-520b/d | n/a | [17] |
| miR-526b ‡ | TBX21, CREB1, KLHDC1, ABCB10, CDADC1 | [36] |
| miR-542-5p | TBR1, HSPG2, NETO1, RCE1, MYLK2 | [22] |
| miR-549 | FASTKD2, UBE2D3, KIAA0430, CASP14, PTRHD1 | [17] |
| miR-556 | CSRNP2, RNMT, WFIKKN2, CREB5, CCDC18 | [17] |
| miR-601 | SGK494, LHFPL2, PRKACB, B3GNT9, EEA1 | [17] |
| miR-639 | TBR1, KIAA1267, PKNOX1, QKI, POU2F2 | [17] |
| miR-644 | MYLK2, B3GNT9, CHMP7, RNF130, TFAP2B | [17] |
| miR-646 | SLC11A2, MGAT4A, PURA, FGF2, MTAP | [17] |
| miR-649 | HNRNPA1L2, HNRNPA1, CAND1, COPS7B, SLC2A4RG | [17] |
Bolded targets have been validated in the listed references. Otherwise, top 5 putative targets predicted based on sequence homology using TargetScan[12].
“n/a” miR not annotated in TargetScan, release 6.2.
miR has been shown to be both increased and decreased in BC.
miR change identified in T2-4 tissue compared with Ta.
miR change identified in T2-4 tissue compared with Ta and normal urothelium.
Table 2.
Selected ongoing clinical trials of targeted agents in advanced bladder cancer.
| Target | Agent | Trial design | NCT |
|---|---|---|---|
| pan-FGFR | BGJ398 | phase 1b; BGJ398/BYL719 | NCT01928459 |
| phase II, 2nd line; BGJ398 | NCT02160041 | ||
| BGJ398 | phase I; BGJ398 | NCT01004224 | |
| EGFR | cetuixmab | phase II; 1st line; gemcitabine/cisplatin +/- cetuximab | NCT00645593 |
| HER2 | lafatinib | phase II/III, 2nd line; single-agent lapatinib | NCT01928459 |
| Ad/Her2/Neu dendritic cell vaccine | phase I; Ad/Her2/Neu vaccine | NCT01928459 | |
| VEGF | bevacizumab | phase III, 1st line; gemcitabine/cisplatin +/- bevacizumab | NCT01928459 |
| sorafanib | phase II, 1st line; gemcitabine/carboplatin/sorafenib, followed by sorafenib maintenance | NCT00461851 | |
| sorafenib | phase I, 1st line (platinum-ineligible) or 2nd line; vinflunine/sorafenib | NCT01844947 | |
| pazopanib | phase II, 1st line (cisplatin-ineligible); gemcitabine/pazopanib | NCT01622660 | |
| pazopanib | phase II, 2nd line; weekly paclitaxel/pazopanib | NCT01108055 | |
| MET | cabozantinib | phase II, 2nd line; single-agent cabozantinib | NCT 01688999 |
| mTOR | everolimus | phase I, 1st line; gemcitabine/split-dose cisplatin/everolimus | NCT01182168 |
| everolimus | phase II, 1st line (cisplatin-ineligible); everolimus +/-paclitaxel | NCT01215136 | |
| temsirolimus | phase II, 2nd line; single-agent temsirolimus | NCT01827943 | |
| pan-PI3K | buparlisib (BKM120) | phase II, 2nd line; single-agent buparlisib | NCT01090466 |
| HSP27 | OGX-427 | phase II, 1st line; gemcitabine/cisplatin +/-OGX-427 | NCT02160041 |
| OGX-427 | phase II, 2nd line; docetaxel +/-OGX-427 | NCT02160041 |
Abbreviations: EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; HER2, human epidermal growth factor receptor 2; HSP, heat shock protein; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol-3-kinase; VEGF, vascular endothelial growth factor.
Catto et al. were among the first to report distinct profiles of miR expression between NMIBC and MIBC [17]. They measured the expression levels of 322 miRs in six BC cell lines, 20 normal bladder samples, and 52 bladder tumors (23 low-grade NMIBC; 12 high-grade NMIBC and 18 MIBC) using a microarray platform. NMIBC tumors demonstrated down-regulation of miR-99a, miR-100, miR-101 and miR-145 compared with MIBC. miR-99a and miR-100 were experimentally validated to target FGFR3, and expression of either of these miRs would be predicted to result in increased expression of FGFR3 in NMIBC. Thus, FGFR3 expression may be increased in the absence of FGFR3 mutation or copy number gain. Catto et al. also discovered that miR-21, which down-regulates the p53 pathway in glioblastoma cells [18], and miR-373, which promotes pro-metastatic pathways in multiple tumor types [19], were among the most up-regulated in MIBC [17]. These findings are consistent with the current model of bladder cancer oncogenesis in which oncogene activation is associated with NMIBC and tumor suppressor gene inactivation with MIBC.
Catto et al. found generalized down-regulation of miRs in NMIBC compared with normal bladder urothelium, whereas MIBC demonstrated increased expression [17]. An earlier report by Gottardo et al. also noted up-regulation of miRs in bladder cancers compared with normal urothelial tissue (specifically miR-17-5p, miR-23a/b, miR-26b, miR-103-1, miR-185, miR-203, miR-205, miR-221, and miR-223; 1.2 fold change cut-off; P < 0.05), though they combined both NMIBC and MIBC in their analysis [20].
In contrast with Gottardo et al. [20], Catto revealed more shared alterations shared between high-grade NMIBC and MIBC than between low-grade and high-grade NMIBC tumors [17], suggesting that despite the lack of muscle-invasion, high-grade NMIBC may warrant aggressive therapy similar to the approach used for MIBC. In addition some miR aberrations identified in MIBC may occur earlier in the spectrum of disease. Baffa et al. demonstrated additional differences in miR expression between paired primary MIBC and metastatic lymph nodes [21]. They profiled 10 primary bladder cancers and paired lymph nodes using a miR microarray of 326 human miR genes and qRT-PCR confirmation and observed reduced expression of miR-143, miR-145 and miR-320 and increased expression of miR-10b, miR-29b, 142-5p in primary tumors compared with metastases. Thus, miR expression becomes progressively deranged as urothlelial cells develop an invasive and then metastatic phenotype.
Given the differences in miR expression between NMIBC and MIBC, attempts have been made to define a miR expression profile that can accurately classify pathologic stage [22-24]. However, a consistent signature has yet to be identified, likely due to tissue heterogeneity and differences in experimental methods. Recent work from Veerla et al. and the Summerhayes group illustrate how differences in methodology may result in disparate findings from independent groups. Veerla et al. measured the expression of 300 miRs in a panel of 34 BC tumors [7 Ta, 10 T1, and 17 T2/3; 10 low-grade (grade G1 and G2) and 24 high-grade (G3)] using the Illumina microRNA Expression Profiling Assay based on the miRBase release 9.0 and then updated the miR targets of the probe set for miRBase release 11.0 [22]. Using a hierarchical cluster analysis, they identified an expression profile of 51 miRs that was able to distinguish Ta, T2 and T2-T3 tumors with 82% concordance between pathologic stage and cluster assignment. miR-10a had high expression in Ta tumors, and miR-125b and miR-222 had increased expression in T2-T3 tumors.
In contrast, the group led by Summerhayes found that a ratio of miRs not identified by Veerla was able to discriminate between muscle-invasive and non-invasive BC [24]. Using a panel of two invasive and two non-invasive human BC cell lines, they measured differences in expression of 343 miRs and subsequently defined a ratio of miR-21 to miR-205 that identified invasive cell lines in a larger panel of 14 different BC cell lines. When applied to a set of 53 bladder tumor samples (28 non-muscle invasive tumors and 25 muscle-invasive tumors), the ratio demonstrated higher miR-21:miR-205 ratio for invasive versus non-invasive tumors with 100% sensitivity and 79% specificity. In a follow-up study, they validated the utility of the miR-21:miR-205 ratio in an independent cohort of 57 bladder tumor samples [23].
Importantly, the miR-21:miR-205 ratio identified by the Summerhayes group was based on expression in cell lines with in vitro invasive properties rather than being derived from muscle-invasive tumors [23, 24], which may explain why Veerla et al. did not observe the same discriminatory ability of these miRs in their work [22]. Additional discrepancies in methodology included the definition of noninvasive tumors; Veerla et al. combined Ta with T1 tumors in their noninvasive cohort and 94% of the tumors were G2-G3, while Summerhayes' group restricted noninvasive tumors to Ta and 100% were G1. Furthermore, findings were confirmed by qRT-PCR by Summerhayes et al. while this was not performed by Veerla et al.
The differences between these studies highlight some of the ways in which seemingly similar experiments may arrive at disparate conclusions that limit the applicability of the work. Furthermore, they illustrate the importance of confirming the results of microarray studies with a second technology, such as qRT-PCR, and validating results in an external cohort. While these points are relevant for all biomarker studies, they are of particular concern for miRs; since the field is still young, these early reports will provide the framework for future experimental designs.
ii. Deep sequencing in BC
Comprehensive deep RNA sequencing is an alternative to microarray-based technology that has been utilized to identify miR species that distinguish benign and malignant bladder tissue. This approach allows for the measurement of absolute abundance of a miR rather than relative levels and has the additional benefit of revealing novel sequences not detected by microarrays. Han et al. were the first to use high-throughput deep sequencing to generate genome-wide miR expression profiles of nine pairs of MIBC (5 low-grade, 4 high-grade) and adjacent matched histologically normal bladder urothelium [25]. Similar to the microarray-based findings of Catto et al. [17] and Gottardo et al. [20], they noted that up-regulated miRs were more common than down-regulated sequences in MIBC. miR-96 was identified as the most significantly up-regulated miR, and miR-490-5p was the most significantly down-regulated miR. Wang et al. confirmed increased expression of miR-96 in BC compared with normal tissue and demonstrated that this miR species increased cellular proliferation and invasion in the human BC cell line T24, possibly through induction of IRS1, a protein involved in insulin signaling, and MAP4K1, which belongs to the STE/Ser/Thr protein kinase family and mediates growth regulation and environmental stress response [26].
Han et al. also identified clusters of miRs that were significantly up-regulated in BC, specifically clusters miR-96, miR-182 and miR-183 (chromosome 7); miR-200b and miR-429 (chromosome 1); miR-200c and miR-141 (chromosome 12); and miR-17 and miR-92 (chromosome 13) [25]. In addition, a cluster on chromosome 5 encoding miR-143 and miR-145 was significantly down-regulated. The similar co-expression patterns of these miRs within a cluster suggest common regulatory factors or cooperative roles that may contribute to the pathogenesis of BC. In contrast, Wszolek et al. reported down-regulation of miR-200a/b/c and miR-141 [23]. However, these results were from invasive immortalized BC cell lines growing in vitro and may not be representative of in vivo tumors. Strengths of the work by Han et al. include the use of human tumor tissue that was immediately snap-frozen in liquid nitrogen at the time of resection and validation of results using RT-PCR [25]. Han et al. went on to combine their miR expression data from BC with similar whole genome expression profiling of clear cell renal cell carcinoma and testicular germ cell tumors to demonstrate cancer-specific miR alterations, as well as a more limited number of common miR changes, including reduced expression of the miR-199a/b across all three tumors [27].
ii. Functional validation of miRs identified in expression analyses
Functional validation studies are essential to confirm the findings of any method of expression analysis. Ichimi et al. identified 27 miRs that were differentially expressed in 14 BCs, including NMIBC (pTa) and invasive (>pT1) cases, compared with normal bladder tissue and confirmed these results in a larger cohort of 104 BCs and 31 normal specimens [28]. Subsequent work by this group has validated these findings and demonstrated functional implications in BC. Using TargetScan, Chiyomaru et al. determined that miR-133a and miR-145 contain target sequences for fascin homologue 1 (FSCN1), a protein that organizes actin and is involved in cell motility in BC [29], and functional studies revealed that each miR decreased the mRNA and protein expression of FSCN1. They also demonstrated that transient transfection of BC cell lines with miR-133a, miR-1, and miR-218, reduced the expression of mRNA and protein levels of LIM and SH3 protein 1 (LASP1), an actin binding protein, and reduced cell viability [30]. This group showed that miR-1 and miR-133a targets the genes encoding transgelin 2 (TAGLN2), a protein with unknown function but an observed clinical correlation with BC tumor grade [31], as well as prothymosin-α (PTMA), which is involved in cellular proliferation and differentiation, and purine nucleoside phosphorylase (PNP), an enzyme that catalyzes phosphorolysis of purine nucleosides and when deficient results in impaired cell-mediated immunity [32]. In addition, they reported that miR-1 may mediate apoptosis through inhibition of splicing factor arginine/serine-rich 9 (SRSF9/SRp30s) [33] and that miR-133a induces apoptosis via regulation of glutathione S-transferase π1 (GSTP1) where transfection of cell lines with miR-133a reduced mRNA and protein expression levels of GSTP1 [27].
Orntoft's group investigated the down-regulation of miR-145 in invasive and in situ carcinomas compared with normal urothelium. Expression of this miR resulted in caspase-dependent and -independent apoptosis in BC cell lines, and the same miR was down-regulated in Ta bladder tumors, thus supporting its role as a likely tumor suppressor [34]. Further, they showed that the miR-143/-145 cluster directly suppressed the proto-oncogene plasminogen activator inhibitor-1 (PAI-1), an enzyme that inhibits apoptosis in vitro and promotes angiogenesis and cell proliferation. They also demonstrated that expression of the miR-143/-145 cluster was inversely related to PAI-1 expression across the spectrum of superficial to muscle-invasive bladder tumors [35]. These results may help build a case for the prognostic use of these miRs to assess the risk of progression in patients with non-invasive and minimally invasive tumors.
In addition to miR-145, Dyrskjot also identified miR-129 as a possible prognostic marker [36]. Putative miR-129 targets included GALNT1, a protein involved in signaling of transforming growth factor-β and known to be increased in BC, and SRY-related HMG-box 4 (SOX4), which is located next to the E2F3 oncogene that is critical for the G1/S transition and is implicated in the pathogenesis of BC as a target of miR-125 [37]. While the exact function of SOX4 remains unknown, it has been shown to activate the p53 pathway in the setting of DNA damage [38], and increased SOX4 expression correlates with improved overall survival [39]. In support of these findings, transfection of T24 and SW780 BC cell lines with miR-129 suppressed growth and induced apoptosis [36].
More recent publications have assessed the prognostic role of miR expression in BC. Pignot et al. used RT-PCR to identify a signature of three miRs (miR-9, miR-182 and miR-200b) that were associated with MIBC recurrence-free and overall survival from within a larger set of 15 miRs found to be deregulated in a cohort of 166 bladder tumors (86 NMIBC and 80 MIBC) and 11 normal bladders [40]. Using Rosetta Genomics' microRNA expression profiling platform and microdissection of tumor tissue, Rosenberg et al. measured expression in 108 NMIBC and 29 MIBC for whom clinical data were available [41]. They identified miR-29c* as the most differentially expressed miR between NMIBC tumors that eventually progressed to MIBC versus those that did not, with increased expression associated with improved prognosis. This miR is known to reduce expression of DNA methyltransferase in methothelioma [42], and thus reduced levels of miR-29c* may lead to aberrant methylation patterns in BC.
iii. Alterations in miR expression
Catto et al. revealed changes in components of the miR processing machinery with up-regulation of Dicer and Drosha in normal urothelium from tumor-bearing bladder but reduced expression in tumors [17]. Furthermore, rather than correlating with the expression of individual miRs, suppressed miR processing caused global rather than specific alterations in miR expression. Recently a second group demonstrated significantly reduced Dicer mRNA expression in BC compared with paired normal adjacent urothelial tissue and normal bladder samples [8]. In contrast, Han et al. reported increased expression of Dicer, Drosha, and Exportin 5 in BC, with higher expression correlating with increased tumor grade [43]. This finding provides a potential mechanism for their previous work which demonstrated increased miR levels in BC compared with normal tissue [25]. Therefore, conflicting data exist regarding whether miR processing machinery is increased or decreased in BC. Furthermore, altered RNA polymerase II affinity for miR transcripts, potentially due to reduction in transcription factors, could theoretically alter miR expression despite up-regulation of the miR processing machinery.
In addition to changes in miR processing, copy number alteration of miR genes could have profound implications on miR expression. Lamy et al. reported that miRs in BC are over-represented in regions of copy number loss [44]. Tatarano et al. demonstrated that deletion of 4p15.31, which is commonly observed in BC cell lines, results in the loss of tumor suppressor miR-218, and restoration of miR-218 expression inhibits BC proliferation, migration, invasion, and apoptosis [45]. Likewise, one could hypothesize that copy number gain of an oncomir could promote BC oncogenesis.
Epigenetic silencing of miRs via hypermethylation of CpG shore regions has been documented in BC [7], though some have reported that the genome-wide methylation patterns between BC and normal adjacent tissue are similar [46]. Saito showed that tumor suppressor miR-126, which was down-regulated in cell lines and BC, could be up-regulated in cell lines by DNA methylation and histone deacetylation inhibitors [47]. Similarly, Yoshitomi et al. demonstrated that restoration of expression of the tumor suppressor miR-517 in BC cell lines with the demethylating agent 5-aza-2′-deoxycytidine induced apoptosis [48]. Furthermore, hypermethylation of miR-1224 correlated with BC tumor grade, stage, and prognosis, and raises the potential for a novel therapeutic approach with hypomethylating agents in this disease [7].
iv. Epithelial versus mesenchymal differentiation
Cancer cells, even those arising in epithelial compartments such as the urothelium, differ in their degree of epithelial versus mesenchymal differentiation. Mesenchymal differentiation has been linked with increased ability of cancer cells to invade, migrate, and metastasize [49]. Mesenchymal cancer cells lose cellular polarity and adhesion, a phenomenon that has been implicated in the pathogenesis of muscle-invasive BC [50]. On a molecular level, mesenchymal differentiation is associated with the expression of a variety of transcription factors including Twist, ZEB-1, and ZEB-2; loss of the adhesion protein E-cadherin; and increased expression of matrix metalloproteinases. The tumor suppressor p53 suppresses ZEB-1 and ZEB2 via p53-induced up-regulation of miR-200 and miR-192 [51]. The miR-200 family (miR-200a, -200b, -200c, -141 and -429) can promote epithelial differentiation across a spectrum of cancers [38], and multiple reports link this family of miRs to mesenchymal differentiation and BC. For example, stable expression of miR-200 in the high-grade BC cell line UMUC3 yielded decreased expression of ZEB-1/2, increased E-cadherin expression, and reduced cell migration [52]. Furthermore, sensitivity of this cell line, as well as T24 and UMUC5, to epidermal growth factor receptor (EGFR) inhibitor was enhanced by forced miR-200 expression. The regulation of miR-200 family and miR-205 expression in MIBC appears to occur through coordinated promoter CpG hypermethylation and repressive chromatin marks [53]. Expression of these miRs is inversely correlated with Twist expression, but interestingly no target sequences for miR-200 or miR-205 binding are found on Twist1 mRNA. Therefore, this transcription factor may globally repress the miR-200 family rather than individual members. In addition to its potential effects on Twist, increased miR-200c expression correlates with reduced migration and invasion of invasive BC cell lines and loss of ZEB-1 expression, consistent with the role of ZEB-1 in promoting mesenchymal differentiation [54].
IV. Conclusion
The field of miR research has advanced significantly within the last decade, and more recent investigations are focusing on the role of miRs in BC. Aberrations in miR expression identified in NMIBC and MIBC support and provide insight into molecular alterations known to distinguish the two parallel pathways of bladder carcinogenesis. miRs that target the FGFR3 pathway, specifically miR-99a, miR-100, miR-101, and miR-145, are among the most altered in non-muscle invasive disease with reduced expression. In contrast, miRs predicted to target p53, including miR-21 and miR-373 are the most up-regulated in MIBC, and p53 suppresses transcriptional factors which promote mesenchymal differentiation, ZEB-1 and ZEB-2, through regulation of the miR-200 family. The discovery of additional changes in miR expression in BC may ultimately expand what is known about molecular drivers of BC pathogenesis outside of the FGFR3 and p53 pathways. A number of questions remain regarding factors that regulate miR expression, including changes in miR processing machinery and potentially dynamic alterations in response to stressors such as chemotherapy. Thus far, a complex network of interactions is emerging that implicates novel mechanisms of gene modulation in BC. It is hoped that better understanding of these mechanisms will lead to unique opportunities for the development of diagnostic biomarkers and targeted therapy across the spectrum of BC.
Acknowledgments
The authors would like to gratefully acknowledge the Bladder Cancer Advocacy Network for supporting this work.
References
- 1.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775–89. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152–7. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 5.Li MA, He L. microRNAs as novel regulators of stem cell pluripotency and somatic cell reprogramming. Bioessays. 2012;34:670–80. doi: 10.1002/bies.201200019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dudziec E, Miah S, Choudhry HM, et al. Hypermethylation of CpG islands and shores around specific microRNAs and mirtrons is associated with the phenotype and presence of bladder cancer. Clin Cancer Res. 2011;17:1287–96. doi: 10.1158/1078-0432.CCR-10-2017. [DOI] [PubMed] [Google Scholar]
- 8.Wu D, Tao J, Xu B, Li P, Lu Q, Zhang W. Downregulation of Dicer, a component of the microRNA machinery, in bladder cancer. Mol Med Report. 2012;5:695–9. doi: 10.3892/mmr.2011.711. [DOI] [PubMed] [Google Scholar]
- 9.Yang H, Dinney CP, Ye Y, Zhu Y, Grossman HB, Wu X. Evaluation of genetic variants in microRNA-related genes and risk of bladder cancer. Cancer Res. 2008;68:2530–7. doi: 10.1158/0008-5472.CAN-07-5991. [DOI] [PubMed] [Google Scholar]
- 10.Liu A, Xu X. MicroRNA isolation from formalin-fixed, paraffin-embedded tissues. Methods Mol Biol. 2011;724:259–67. doi: 10.1007/978-1-61779-055-3_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratert N, Meyer HA, Jung M, et al. Reference miRNAs for miRNAome analysis of urothelial carcinomas. PLoS One. 2012;7:e39309. doi: 10.1371/journal.pone.0039309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan Gana NH, Victoriano AF, Okamoto T. Evaluation of online miRNA resources for biomedical applications. Genes Cells. 2012;17:11–27. doi: 10.1111/j.1365-2443.2011.01564.x. [DOI] [PubMed] [Google Scholar]
- 14.Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer. 2005;5:713–25. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 15.Fendler A, Stephan C, Yousef GM, Jung K. MicroRNAs as regulators of signal transduction in urological tumors. Clin Chem. 2011;57:954–68. doi: 10.1373/clinchem.2010.157727. [DOI] [PubMed] [Google Scholar]
- 16.Shariat SF, Tokunaga H, Zhou J, et al. p53, p21, pRB, and p16 expression predict clinical outcome in cystectomy with bladder cancer. J Clin Oncol. 2004;22:1014–24. doi: 10.1200/JCO.2004.03.118. [DOI] [PubMed] [Google Scholar]
- 17.Catto JW, Miah S, Owen HC, et al. Distinct microRNA alterations characterize high- and low-grade bladder cancer. Cancer Res. 2009;69:8472–81. doi: 10.1158/0008-5472.CAN-09-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papagiannakopoulos T, Shapiro A, Kosik KS. MicroRNA-21 targets a network of key tumor-suppressive pathways in glioblastoma cells. Cancer Res. 2008;68:8164–72. doi: 10.1158/0008-5472.CAN-08-1305. [DOI] [PubMed] [Google Scholar]
- 19.Huang Q, Gumireddy K, Schrier M, et al. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol. 2008;10:202–10. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- 20.Gottardo F, Liu CG, Ferracin M, et al. Micro-RNA profiling in kidney and bladder cancers. Urol Oncol. 2007;25:387–92. doi: 10.1016/j.urolonc.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Baffa R, Fassan M, Volinia S, et al. MicroRNA expression profiling of human metastatic cancers identifies cancer gene targets. J Pathol. 2009;219:214–21. doi: 10.1002/path.2586. [DOI] [PubMed] [Google Scholar]
- 22.Veerla S, Lindgren D, Kvist A, et al. MiRNA expression in urothelial carcinomas: important roles of miR-10a, miR-222, miR-125b, miR-7 and miR-452 for tumor stage and metastasis, and frequent homozygous losses of miR-31. Int J Cancer. 2009;124:2236–42. doi: 10.1002/ijc.24183. [DOI] [PubMed] [Google Scholar]
- 23.Wszolek MF, Rieger-Christ KM, Kenney PA, et al. A MicroRNA expression profile defining the invasive bladder tumor phenotype. Urol Oncol. 2011;29:794–801.e1. doi: 10.1016/j.urolonc.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Neely LA, Rieger-Christ KM, Neto BS, et al. A microRNA expression ratio defining the invasive phenotype in bladder tumors. Urol Oncol. 2010;28:39–48. doi: 10.1016/j.urolonc.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Han Y, Chen J, Zhao X, et al. MicroRNA expression signatures of bladder cancer revealed by deep sequencing. PLoS One. 2011;6:e18286. doi: 10.1371/journal.pone.0018286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Luo H, Li Y, Chen T, Wu S, Yang L. hsa-miR-96 up-regulates MAP4K1 and IRS1 and may function as a promising diagnostic marker in human bladder urothelial carcinomas. Mol Med Report. 2012;5:260–5. doi: 10.3892/mmr.2011.621. [DOI] [PubMed] [Google Scholar]
- 27.Uchida Y, Chiyomaru T, Enokida H, et al. MiR-133a induces apoptosis through direct regulation of GSTP1 in bladder cancer cell lines. Urol Oncol. 2013;31:115–23. doi: 10.1016/j.urolonc.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Ichimi T, Enokida H, Okuno Y, et al. Identification of novel microRNA targets based on microRNA signatures in bladder cancer. Int J Cancer. 2009;125:345–52. doi: 10.1002/ijc.24390. [DOI] [PubMed] [Google Scholar]
- 29.Chiyomaru T, Enokida H, Tatarano S, et al. miR-145 and miR-133a function as tumour suppressors and directly regulate FSCN1 expression in bladder cancer. Br J Cancer. 2010;102:883–91. doi: 10.1038/sj.bjc.6605570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiyomaru T, Enokida H, Kawakami K, et al. Functional role of LASP1 in cell viability and its regulation by microRNAs in bladder cancer. Urol Oncol. 2012;30:434–43. doi: 10.1016/j.urolonc.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Yoshino H, Chiyomaru T, Enokida H, et al. The tumour-suppressive function of miR-1 and miR-133a targeting TAGLN2 in bladder cancer. Br J Cancer. 2011;104:808–18. doi: 10.1038/bjc.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamasaki T, Yoshino H, Enokida H, et al. Novel molecular targets regulated by tumor suppressors microRNA-1 and microRNA-133a in bladder cancer. Int J Oncol. 2012;40:1821–30. doi: 10.3892/ijo.2012.1391. [DOI] [PubMed] [Google Scholar]
- 33.Yoshino H, Enokida H, Chiyomaru T, et al. Tumor suppressive microRNA-1 mediated novel apoptosis pathways through direct inhibition of splicing factor serine/arginine-rich 9 (SRSF9/SRp30c) in bladder cancer. Biochem Biophys Res Commun. 2012;417:588–93. doi: 10.1016/j.bbrc.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Ostenfeld MS, Bramsen JB, Lamy P, et al. miR-145 induces caspase-dependent and -independent cell death in urothelial cancer cell lines with targeting of an expression signature present in Ta bladder tumors. Oncogene. 2010;29:1073–84. doi: 10.1038/onc.2009.395. [DOI] [PubMed] [Google Scholar]
- 35.Villadsen SB, Bramsen JB, Ostenfeld MS, et al. The miR-143/-145 cluster regulates plasminogen activator inhibitor-1 in bladder cancer. Br J Cancer. 2012;106:366–74. doi: 10.1038/bjc.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dyrskjot L, Ostenfeld MS, Bramsen JB, et al. Genomic profiling of microRNAs in bladder cancer: miR-129 is associated with poor outcome and promotes cell death in vitro. Cancer Res. 2009;69:4851–60. doi: 10.1158/0008-5472.CAN-08-4043. [DOI] [PubMed] [Google Scholar]
- 37.Huang L, Luo J, Cai Q, et al. MicroRNA-125b suppresses the development of bladder cancer by targeting E2F3. Int J Cancer. 2011;128:1758–69. doi: 10.1002/ijc.25509. [DOI] [PubMed] [Google Scholar]
- 38.Pan X, Zhao J, Zhang WN, et al. Induction of SOX4 by DNA damage is critical for p53 stabilization and function. Proc Natl Acad Sci U S A. 2009;106:3788–93. doi: 10.1073/pnas.0810147106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aaboe M, Birkenkamp-Demtroder K, Wiuf C, et al. SOX4 expression in bladder carcinoma: clinical aspects and in vitro functional characterization. Cancer Res. 2006;66:3434–42. doi: 10.1158/0008-5472.CAN-05-3456. [DOI] [PubMed] [Google Scholar]
- 40.Pignot G, Cizeron-Clairac G, Vacher S, et al. microRNA expression profile in a large series of bladder tumors: Identification of a 3-miRNA signature associated with aggressiveness of muscle-invasive bladder cancer. Int J Cancer. 2013;132:2479–91. doi: 10.1002/ijc.27949. [DOI] [PubMed] [Google Scholar]
- 41.Rosenberg E, Baniel J, Spector Y, et al. Predicting progression of bladder urothelial carcinoma using microRNA expression. BJU Int. 2013 doi: 10.1111/j.1464-410X.2012.11748.x. [DOI] [PubMed] [Google Scholar]
- 42.Pass HI, Goparaju C, Ivanov S, et al. hsa-miR-29c* is linked to the prognosis of malignant pleural mesothelioma. Cancer Res. 2010;70:1916–24. doi: 10.1158/0008-5472.CAN-09-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han Y, Liu Y, Gui Y, Cai Z. Inducing cell proliferation inhibition and apoptosis via silencing Dicer, Drosha, and Exportin 5 in urothelial carcinoma of the bladder. J Surg Oncol. 2013;107:201–5. doi: 10.1002/jso.23214. [DOI] [PubMed] [Google Scholar]
- 44.Lamy P, Andersen CL, Dyrskjot L, Torring N, Orntoft T, Wiuf C. Are microRNAs located in genomic regions associated with cancer? Br J Cancer. 2006;95:1415–8. doi: 10.1038/sj.bjc.6603381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tatarano S, Chiyomaru T, Kawakami K, et al. miR-218 on the genomic loss region of chromosome 4p15.31 functions as a tumor suppressor in bladder cancer. Int J Oncol. 2011;39:13–21. doi: 10.3892/ijo.2011.1012. [DOI] [PubMed] [Google Scholar]
- 46.Zhu J, Jiang Z, Gao F, et al. A systematic analysis on DNA methylation and the expression of both mRNA and microRNA in bladder cancer. PLoS One. 2011;6:e28223. doi: 10.1371/journal.pone.0028223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saito Y, Friedman JM, Chihara Y, Egger G, Chuang JC, Liang G. Epigenetic therapy upregulates the tumor suppressor microRNA-126 and its host gene EGFL7 in human cancer cells. Biochem Biophys Res Commun. 2009;379:726–31. doi: 10.1016/j.bbrc.2008.12.098. [DOI] [PubMed] [Google Scholar]
- 48.Yoshitomi T, Kawakami K, Enokida H, et al. Restoration of miR-517a expression induces cell apoptosis in bladder cancer cell lines. Oncol Rep. 2011;25:1661–8. doi: 10.3892/or.2011.1253. [DOI] [PubMed] [Google Scholar]
- 49.Brabletz T. To differentiate or not--routes towards metastasis. Nat Rev Cancer. 2012;12:425–36. doi: 10.1038/nrc3265. [DOI] [PubMed] [Google Scholar]
- 50.McConkey DJ, Choi W, Marquis L, et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009;28:335–44. doi: 10.1007/s10555-009-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim T, Veronese A, Pichiorri F, et al. p53 regulates epithelial-mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. J Exp Med. 2011;208:875–83. doi: 10.1084/jem.20110235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adam L, Zhong M, Choi W, et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res. 2009;15:5060–72. doi: 10.1158/1078-0432.CCR-08-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiklund ED, Bramsen JB, Hulf T, et al. Coordinated epigenetic repression of the miR-200 family and miR-205 in invasive bladder cancer. Int J Cancer. 2011;128:1327–34. doi: 10.1002/ijc.25461. [DOI] [PubMed] [Google Scholar]
- 54.Kenney PA, Wszolek MF, Rieger-Christ KM, et al. Novel ZEB1 expression in bladder tumorigenesis. BJU Int. 2011;107:656–63. doi: 10.1111/j.1464-410X.2010.09489.x. [DOI] [PubMed] [Google Scholar]
- 55.Friedman JM, Liang G, Liu CC, et al. The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res. 2009;69:2623–9. doi: 10.1158/0008-5472.CAN-08-3114. [DOI] [PubMed] [Google Scholar]
- 56.Li X, Chen J, Hu X, et al. Comparative mRNA and microRNA expression profiling of three genitourinary cancers reveals common hallmarks and cancer-specific molecular events. PLoS One. 2011;6:e22570. doi: 10.1371/journal.pone.0022570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Catto JWF, Alcaraz A, Bjartell AS, et al. MicroRNA in Prostate, Bladder, and Kidney Cancer: A Systematic Review. Eur Urol. 2011;59:671–81. doi: 10.1016/j.eururo.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 58.Bo J, Yang G, Huo K, et al. microRNA-203 suppresses bladder cancer development by repressing bcl-w expression. FEBS J. 2011;278:786–92. doi: 10.1111/j.1742-4658.2010.07997.x. [DOI] [PubMed] [Google Scholar]
- 59.Lu Q, Lu C, Zhou GP, Zhang W, Xiao H, Wang XR. MicroRNA-221 silencing predisposed human bladder cancer cells to undergo apoptosis induced by TRAIL. Urol Oncol. 2010;28:635–41. doi: 10.1016/j.urolonc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 60.Cao Y, Yu SL, Wang Y, Guo GY, Ding Q, An RH. MicroRNA-dependent regulation of PTEN after arsenic trioxide treatment in bladder cancer cell line T24. Tumour Biol. 2011;32:179–88. doi: 10.1007/s13277-010-0111-z. [DOI] [PubMed] [Google Scholar]
- 61.Wang G, Zhang H, He H, et al. Up-regulation of microRNA in bladder tumor tissue is not common. Int Urol Nephrol. 2010;42:95–102. doi: 10.1007/s11255-009-9584-3. [DOI] [PubMed] [Google Scholar]
- 62.Lodygin D, Tarasov V, Epanchintsev A, et al. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7:2591–600. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- 63.Huang L, Luo J, Cai Q, et al. MicroRNA-125b suppresses the development of bladder cancer by targeting E2F3. Int J Cancer. 2011;128:1758–69. doi: 10.1002/ijc.25509. [DOI] [PubMed] [Google Scholar]
- 64.Lin T, Dong W, Huang J, et al. MicroRNA-143 as a tumor suppressor for bladder cancer. J Urol. 2009;181:1372–80. doi: 10.1016/j.juro.2008.10.149. [DOI] [PubMed] [Google Scholar]
- 65.Chen H, Lin YW, Mao YQ, et al. MicroRNA-449a acts as a tumor suppressor in human bladder cancer through the regulation of pocket proteins. Cancer Lett. 2012 doi: 10.1016/j.canlet.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 66.Ueno K, Hirata H, Majid S, et al. Tumor suppressor microRNA-493 decreases cell motility and migration ability in human bladder cancer cells by downregulating RhoC and FZD4. Mol Cancer Ther. 2012;11:244–53. doi: 10.1158/1535-7163.MCT-11-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hirata H, Hinoda Y, Ueno K, Shahryari V, Tabatabai ZL, Dahiya R. MicroRNA-1826 targets VEGFC, beta-catenin (CTNNB1) and MEK1 (MAP2K1) in human bladder cancer. Carcinogenesis. 2012;33:41–8. doi: 10.1093/carcin/bgr239. [DOI] [PMC free article] [PubMed] [Google Scholar]


