Abstract
The vast majority of approved antidepressants and antipsychotics exhibit a complex pharmacology. The mechanistic understanding of how these psychotropic medications are related to adverse drug reactions (ADRs) is crucial for the development of novel drug candidates and patient adherence. This study aims to associate in vitro assessed binding affinity profiles (39 compounds, 24 molecular drug targets) and ADRs (n=22) reported in clinical trials of antidepressants and antipsychotics (n>59.000 patients) by the use of robust multivariate statistics. Orthogonal projection to latent structures (O-PLS) regression models with reasonable predictability were found for several frequent ADRs such as nausea, diarrhea, hypotension, dizziness, headache, insomnia, sedation, sleepiness, increased sweating, and weight gain. Results of the present study support many well-known pharmacological principles such as the association of hypotension and dizziness with α1-receptor or sedation with H1-receptor antagonism. Moreover, the analyses revealed novel or hardly investigated mechanisms for common ADRs including the potential involvement of 5-HT6-antagonism in weight gain, muscarinic receptor antagonism in dizziness, or 5-HT7-antagonism in sedation. To summarize, the presented study underlines the feasibility and value of a multivariate data mining approach in psychopharmacological development of antidepressants and antipsychotics.
Keywords: Schizophrenia, Depressive disorder, Antidepressive agents, Antipsychotic agents, Clinical pharmacology, Adverse effects
1. Introduction
The development of more effective treatments of major neuropsychiatric disorders such as major depressive disorder (MDD) or schizophrenia is urgently needed due to their leading and unchanged role as contributors to global disease burden over the past two decades (Vos et al., 2012). Despite the doubtless beneficial effects of currently available anti-depressants (ADs) and antipsychotics (APs), reported medication discontinuation rates are much higher than acceptable. Patient adherence is severely affected by adverse drug reactions (ADRs) (Insel, 2012), which are also an important factor leading to the attrition of clinical trials during drug development (Lounkine et al., 2012). Well-studied ADRs among many others include weight gain, sedation, extrapyramidal motor symptoms (EPMS), sexual dysfunction, and QT-interval prolongation (Castro et al., 2013). In contrast to other medical fields, pharmacological development in psychiatry is challenged by its unknown etiology and lack of biological meaningful diagnostic categories (Insel, 2012). Hence, ADs and APs have not been engineered based on prior knowledge of molecular pathways, but were identified by serendipity within the class of anti-histaminergic drugs (Lopez-Munoz and Alamo, 2009). Therefore, it is not surprising that numerous available ADs and APs target a broad spectrum of membrane proteins comprising receptors, transporters, and ion channels that result in complex pharmacological actions (Roth et al., 2004).
Over the past years, there has been an ongoing debate among scientists with regard to the benefits and harms of selective versus non-selective (Roth et al., 2004) as well as unimodal versus multimodal (Nutt, 2009) medications and their impact on observed efficacy and tolerability of psychopharmacological drug treatment. A major argument in favor of selectivity has been the broad clinical use of unimodal psychotropic medications such as selective serotonin re-uptake inhibitors (SSRIs) that demonstrated a more favorable side effect profile than older non-selective tri-cyclic antidepressants (TCAs) or early multimodal medications such as trazodone (Bauer et al., 2013; Grunze et al., 2013; Nutt, 2009). Considering the limitations of available meta-analyses (Huf et al., 2011) it has been suggested that increased selectivity has not lead to an overall improved antidepressive efficacy (Bauer et al., 2013), but instead led to a more acceptable side effect profile (Baghai et al., 2012). On the other hand, several non-selective medications including second-generation APs have been found to provide acceptable treatment efficacy without jeopardizing drug tolerability (Hasan et al., 2013). Given these counterintuitive results with respect to drug selectivity, it becomes apparent that further research is urgently needed that facilitates the understanding on how receptor profiles of psychopharmacological medications translate into clinically observed ADRs. Ideally, inferences should be made from clinical trials investigating the occurrence of ADRs during the application of highly selective drugs that bind to single drug targets. However, most psychopharmacological compounds licensed for medical use are not selective and bind to a variety of drug targets. Therefore, inferences on the relationship between a specific receptor system and a specific ADR are difficult to extrapolate from a single compound. Hence, complimentary evidence stems from numerous drug safety studies in animals that inferred undesired drug effects from occupancy research to humans, a conclusion that has been criticized in the past.
Recently, alternative approaches have been developed including computational strategies that utilize information on the chemical structure of investigational drugs in order to predict ADRs (Bender et al., 2007; Lounkine et al., 2012). While such strategies are definitely novel and promote the understanding of drug structure-ADR relationships, methodological limitations exist including a restriction to single drug- (or off-) targets as well as the qualitative nature of these techniques. Since ADRs are likely to result from drug actions on multiple protein targets interacting with a variety of signaling pathways, a quantitative multivariate approach applied to large data sets may be more suitable and might be capable to detect associations that are otherwise impossible to deconvolute.
To demonstrate the feasibility and clinical plausibility of such a quantitative multivariate data analysis strategy, we performed an orthogonal projections to latent structures (O-PLS) analysis with the goal to associate binding affinity profiles of 39 antidepressants and antipsychotics licensed for medical use with high-quality ARDs data compiled from clinical trials comprising more than 59,000 patients. Both, binding and clinical data were extracted from publicly available databases. Our unprecedented approach verified some associations that had previously been suspected or less appreciated, e.g., a prominent role of 5HT6-receptor blockage in triggering weight gain or a surprisingly large number of targets linked to sedation.
2. Experimental procedures
2.1. Data sources
Biological activities (Ki values) were retrieved from the openly accessible PDSP database (Psychoactive Drug Screening Program, http://pdsp.med.unc.edu). Reported side effect frequencies were extracted from the publically available Cochrane Database of Systematic Reviews (http://www.thecochranelibrary.com).
2.2. Data selection
2.2.1. Binding affinity data
The following inclusion criteria were applied to PDSP data: (1) psychotropic medications had been licensed for clinical use by the Food and Drug Administration (FDA) or European Medicines Agency (EMA), (2) psychotropic medications showed significant binding activities (Ki<10 μM) for at least two different receptor systems. In case of the availability of binding affinity data from multiple sources, human data were preferred over animal data and averaged (86.4% human vs. 13.6% non-human). Average binding affinities for adrenergic receptors were determined regardless of receptor sub-types. A maximum Ki of 10 μM was used as cut-off for low-affinity interactions. Fifty-two compounds fulfilled the above mentioned inclusion criteria and were further used for the selection of clinical trials (see Supplementary material, Table S1).
2.2.2. Clinical data
The following inclusion criteria were applied to reported side effect frequencies in the Cochrane Database of Systematic Reviews: (1) systematic reviews investigating side effect frequencies of the above-defined 52 compounds (n=71); (2) reviews were excluded that investigated combination therapy or dose finding studies. In addition, we included exclusively oral psychotropic medications thereby discarding clinical trials of depot antipsychotics (n=22). This procedure resulted in 49 systematic reviews comprising randomized and placebo- (39%) or active reference-controlled (61%) clinical trials that were considered eligible for this study; included reviews contained ADRs frequency data for 39 different psychotropic medications (19 antidepressants and 20 antipsychotics) in more than 59,000 patients (Table S2). The weighted average (based on the number of study participants) ADR frequency (in %) of all included studies was extracted for each substance resulting in a vector consisting of mean ADRs (column) for different medications (rows). Data of side effect terms were merged in case of high inter-correlations, typically applying to terms that are clinically indistinguishable or at least very similar (e.g. agitation and anxiety, dizziness and vertigo/faintness, nausea and vomiting, sleepiness and drowsiness, abnormal vision and blurred vision). The complete matrix is shown in the Supplementary material, Table S3.
2.2.3. Data reduction
The above mentioned selection process resulted in 39 psychotropic medications with available binding affinity profiles for 31 drug targets in total and 69 reported side effects. In order to guarantee statistical robustness, drug targets were excluded from further analysis, if the pertinent Ki values were not available for more than half of the 39 included psychotropic medications (n=7). Application of these criteria resulted in an array of drug targets including three monoamine transporters (serotonin transporter (5-HTT), norepinephrine transporter (NET), and dopamine transporter (DAT)), 20G protein-coupled receptors (5-HT1A, 5-HT1B, 5-HT1D, 5-HT2A, 5-HT2C, 5-HT6, 5-HT7; α1, α2, β1; M1, M2, M3, M4, M5; D1, D2, D3, D4; H1), and one ligand-gated ion channel receptor (5-HT3). Average binding affinities (Ki values) were converted to their negative common logarithm (pKi values). The resulting binding affinity data matrix is shown in Table S4. In an analogous manner, ADR frequencies reported in clinical trials were only included if based on a sample of more than 50 study participants. Moreover, specific side effects were excluded, if data were available for less than 15 compounds. Hence, the following side effects were considered in this study: agitation, akathisia, constipation, diarrhea, dizziness, dry mouth, dystonia, fatigue, gastrointestinal symptoms, headache, hypotension, insomnia, sexual dysfunction, nausea, increased salivation, sedation, sleepiness, increased sweating, tremor, urination disorders, abnormal vision, and weight gain. Table S5 displays the ADR profile matrix.
2.3. Statistics
2.3.1. Orthogonal projections to latent structures (O-PLS)
O-PLS regression analysis was used for associating clinical endpoints (ADR frequencies) with receptor binding profiles of ADs and APs. Briefly, similar to its predecessor partial least squares (PLS) regression, O-PLS is a method for relating two data matrices, X and Y within a linear multivariate model. The O-PLS method (Trygg and Wold, 2002) is designed to handle systematic variation in X that is not subject to linear correlation with Y (Y-orthogonal variation). The systematic variation in X is divided into two parts, one that is linearly related (and therefore predictive) to Y, and one that is orthogonal to Y. Removal of Y-orthogonal variation usually facilitates better interpretation of the model, but does not affect its predictive power (Trygg and Wold, 2002).
2.3.2. Data matrices
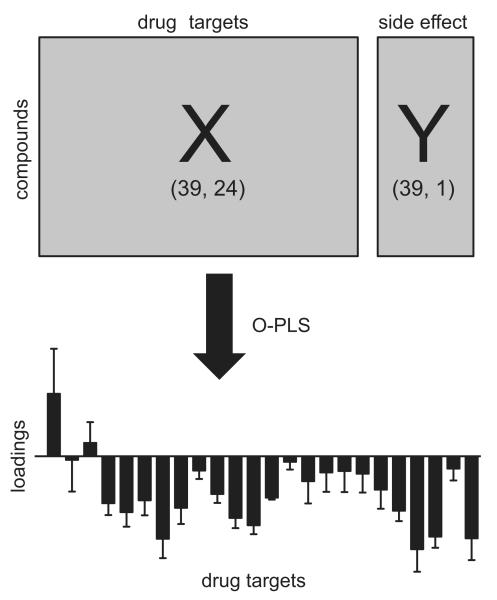
The selection process outlined above resulted in an m-by-n X matrix comprising binding affinities (pKi values) of 39 compounds assessed on 24 drug targets. Elements of the m-by-1 Y matrix represent reported frequencies of a specific side effect for the included compounds. A separate O-PLS model was calculated for each specific side effect (n=22). The dimensions of the X and Y matrices employed for each side effect were variable ranging from 15 (dystonia) to 34 rows (dry mouth) depending on the availability of reported ADRs.
2.3.3. Statistical modeling
SIMCA-P+ (Version 12.0, Umetrics, Umea, Sweden) was used for calculating the O-PLS models. All X variables were centered but not scaled. The goodness-of-fit of the model was determined by the squared correlation coefficient (R2) and the goodness-of-prediction by the 7-fold cross-validated R2 (Q2; Eq. (1)).
| (1) |
Eq. (1): Equation for Q2, the fraction of Y that can be predicted by a component, as estimated by cross-validation [PRESS, prediction error sum of squares; SD, standard deviation].
A value of one represents either a complete explanation of the dependent variable by the regression model (R2) or a perfect match of predicted and observed values in leave-n-out cross validation runs. Q2 is typically lower than R2 and a large difference between these two parameters might indicate chance correlations. A negative Q2 implies that the model is not predictive at all. The most suitable number of components for each O-PLS model was estimated by cross-validation. For each O-PLS model loadings and variable importance plots were constructed to study whether binding affinities correlated positively or negatively, significantly or non-significantly, with Y variables (frequency of ADR).
3. Results
3.1. Descriptive statistics
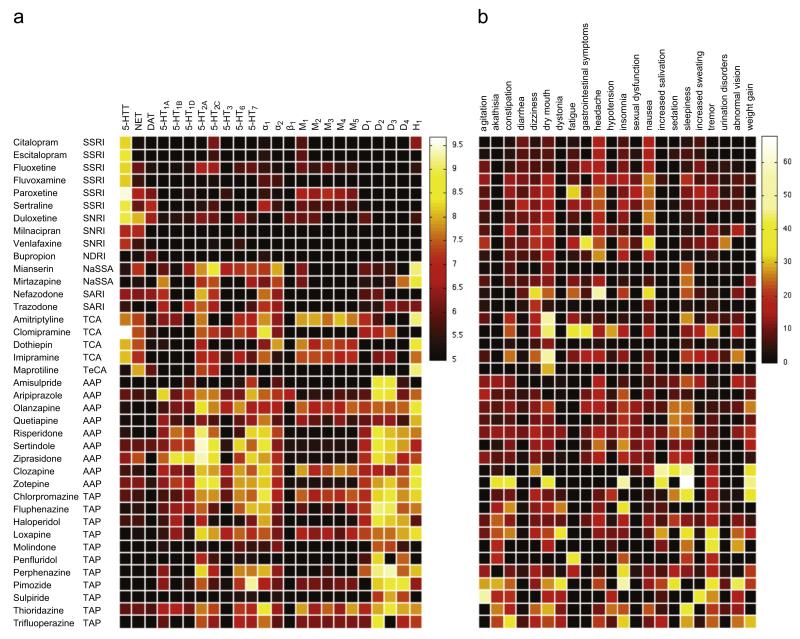
3.1.1. Binding affinities of studied ADs and APs
We compiled information on binding affinity data (pKi values) of 39 psychoactive compounds comprising several drug classes. This comprehensive list highlights specific and well-known profiles, in which psychotropic medications differ in both, qualitative and quantitative traits (Table S4, Figure 1a). The heat map representation (Figure 1a) shows comprehensively the fact that the vast majority of psychotropic medications are likely to interact with many targets when administered at clinically relevant doses. Even SSRIs, which are thought to be the most selective class of psychoactive medications, show quite unique binding pro-files. (1) Citalopram shows mild anti-histaminergic properties, which do not occur in its S-enantiomer escitalopram, the most selective SSRI. (2) Highest 5-HTT affinity is found in paroxetine, in addition to anti-muscarinergic and NET-inhibiting properties. (3) Sertraline with its relatively high DAT. (4) Fluoxetine with significantly high NET as well as 5-HT2C-receptor affinity reflect known differences of SSRI binding profiles that might eventually be transferred to a clinical level. Similarly, binding profiles of SNRIs are as expected exhibiting a strong and balanced NET and 5-HTT inhibition for milnacipran followed by duloxetine, and rather low NET inhibition for venlafaxine. Buproprion displays low DAT-inhibition compared to NET-inhibition. Mirtazapine and its precursor mianserin have been found to exhibit α-antagonistic, anti-serotonergic and anti-histaminergic properties as anticipated. SARIs such as trazodone and nefazodone are displaying 5-HT antagonist action at 5-HT2A and 5-HT2C-receptors, 5-HTT re-uptake inhibition as well as α2-antagonism. In addition, binding profiles highlight subtle differences between these psychotropic medications such as low 5-HTT inhibition for trazodone and a moderate NET-inhibition for nefazodone. In contrast, tricyclic antidepressants (TCAs) included in this study exhibit a potent NET and 5-HTT-inhibition as well as high affinities for M1, α1 and H1-receptors, which are responsible for accompanying ADRs.
Figure 1.
Heat maps displaying binding affinities (pKi values) (a) and adverse drug reaction (ADR) (%) profiles (b) for 39 psychotropic medications comprising different drug classes (SSRI, selective serotonin re-uptake inhibitor; SNRI, serotonin-norepinephrine re-uptake inhibitor; NDRI, norepinephrine-dopamine re-uptake inhibitor; NaSSA, noradrenergic and specific serotonergic anti-depressant; SARI, serotonin antagonist and re-uptake inhibitor; TeCA, tetracyclic antidepressant; TCA, tricyclic antidepressant; AAP, atypical antipsychotic; TAP, typical antipsychotic). Binding affinities (in pKi values) range from 5 (inactive, black) to 9.68 (highly active, white), whereas ADR frequencies (in %) range from 0% (no occurrence, black) to 67.9% (very frequent, white). Binding affinity profiles were extracted from the Psychoactive Drugs Screening Program (PDSP) database and side effect frequencies are based on clinical trial data extracted from the Cochrane Database of Systematic Reviews (CDSR).
Most antipsychotics can easily be distinguished within this table due to their complex and numerous affinities at various receptor systems. Differences between typical (TAP) and most atypical (AAP) antipsychotics can also be seen such as lower D2 and higher 5-HT2A binding affinity for AAPs compared to TAPs. It is noteworthy that atypicality is defined by the frequency of EPMS and hyperprolactinaemia, but not by a specific mechanism of action. Currently known mechanisms include muscarinergic antagonism, partial 5-HT1A-receptor agonism as well as antagonistic actions at several 5-HT sub-receptors. Similar to TCAs, TAPs and to a lesser degree AAPs are frequently accompanied by ADRs. This can also be derived from their binding affinity profiles: 5-HT2C, M3 and H1 receptor antagonism leads frequently to metabolic ADRs, and M1, H1, and α1-receptor antagonism leads to sedation and cognitive malfunctioning.
3.1.2. Adverse drug reactions (ADRs) in clinical trials
ADRs were organized in an analogous way for the identical set of psychotropic medications as presented for binding profiles (Table S5, Figure 1b). Many well-known ADRs that are related to specific drug classes can easily be derived from this ‘real-world’ data matrix. Headache and nausea are frequently reported under SSRI treatment. In accordance to differing receptor profiles of several SSRIs, certain ADRs occur more frequently such as fatigue, dry mouth, and constipation for paroxetine, or a relatively broad spectrum of ADRs for fluvoxamine, which might be related to studies of its initial short-release formulation. Sexual dysfunction has been reported as expected with most SSRIs as well as venlafaxine and basically no other class of antidepressants. SNRIs differ only slightly from the ADR patterns seen in SSRIs with marginally higher number of dry mouth, dizziness, and urination problems as well as agitation for venlafaxine, and abnormal vision for milnacipran. Mirtazapine and mianserin showed in accordance with their H1-antagonistic action an increase in excessive daytime sleepiness reports. Well-studied TCAs such as clomipramine showed the expected higher frequency of typical TCA-related side effects compared to newer antidepressants. Similarly, but more striking, are the differences in ADRs between TAPs and AAPs which is clearly in favor of AAPs for basically all ADRs with the exception of older compounds such as clozapine and zotepine. Clearly, akathisia and dystonia are more prevalent in TAPs in line with their D2-antagonistic properties. However, reports of sedation and sleepiness occurring under AAP treatment including zotepine, clozapine, olanzapine; and quetiapine; surpassed several of older and typically high-potent TAPs.
3.2. Quantitative statistics
3.2.1. O-PLS model quality
Heat maps shown in Figure 1 allow for the visual detection of obvious associations between binding profiles and ADRs as detailed above. However, in most instances, inferences are difficult to make due to the complexity of relationships between binding profiles and individual ADRs. Accordingly, O-PLS regression was used to link binding affinities of these 39 psychotropic medications for the array of selected targets to the clinically reported ADRs frequencies (Figure 2). Model quality of 22 calculated O-PLS models for each specific ADR varied substantially (Figure S1): e.g., the O-PLS model for sedation resulted in an R2 value of 0.97 and a Q2 value of 0.77. However, other regression models had negative Q2 values e.g. abnormal vision, akathisia, agitation, dystonia, increased salivation, tremor, urination disorders and sexual dysfunction. The lack of predictive power of these models indicated that the O-PLS analysis did not support the presence of any link between the available set of binding affinity profiles and reported ADRs. The number of observations and components as well as R2X, R2Y, and Q2Y values for each model are provided in the Supplementary material Table S6. Only O-PLS models (n=10) with reasonable quality (R2>0.2, Q2>0.01) were further investigated including the following ADRs: nausea, diarrhea, hypotension, dizziness, headache, insomnia, sedation, daytime sleepiness, increased sweating, and weight gain. Loadings plots for the predictive component of these models are shown in Figure 3 and reflect statistical significant associations between receptor binding profiles and ADRs. Furthermore, variable importance values reflecting the adverse and beneficial effects of antagonistic action at common drug targets are shown in Table 1 and Figure S2.
Figure 2.
Statistical approach for relating binding affinity profiles to clinically reported adverse drug reactions (ADRs). Elements of the X matrix represent binding affinities (pKi values) for 39 psychotropic medications comprising 24 drug targets. Elements of the Y vector represent frequencies of a single ADR reported in clinical trials of these 39 psychotropic medications. For each specific ADR (n=22) a separate orthogonal projection to latent structures (O-PLS) model was calculated. Principal component 1 (p[1]) loadings plots were used to interpret the relationship between drug target interactions and ADR frequencies. Positive p[1] loadings indicate that binding affinities correlate positively with the Y variable (frequency of ADR), whereas negative p[1] loadings indicate a negative correlation. p[1] loadings close to zero demonstrate the absence of any correlation between binding affinities and ADR frequencies.
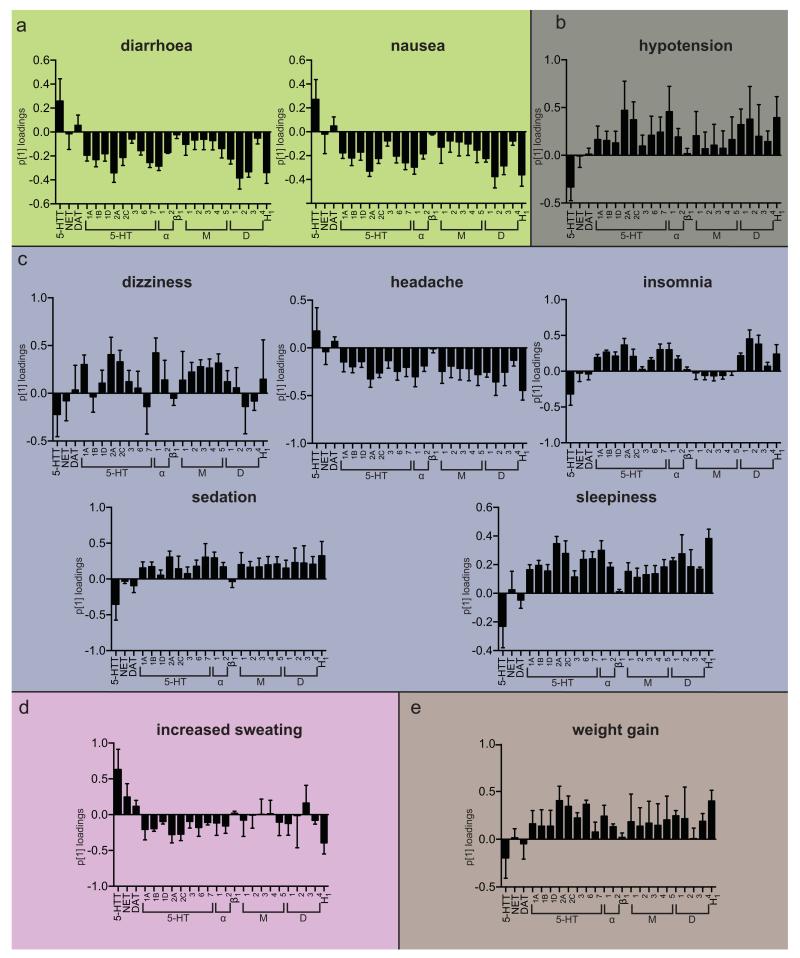
Figure 3.
Principal component 1 loadings (p[1]) for selected orthogonal projection to latent structures (O-PLS) models with good predictabilities (diarrhea, nausea, hypotension, dizziness, headache, insomnia, sedation, sleepiness, increased sweating and weight gain). The O-PLS models were calculated by relating pKi values of 39 psychotropic medications on included drug targets (X variables) to the frequency of adverse drug reactions (ADRs) in percent (Y variable). Positive p[1] loadings indicate that binding affinities correlate positively with Y variable (frequency of ADR), whereas negative p1 loadings indicate a negative correlation. p[1] loadings close to zero demonstrate the absence of any correlation between binding affinities and ADR frequencies. Error bars represent 95% confidence intervals calculated by jack-knifing. (a) gastrointestinal, (b) cardiovascular, (c) neuropsychiatric, (d) dermatological and (e) Other.
Table 1.
Adverse and beneficial effects of antagonistic action at common drug targets of antidepressants and antipsychotics. Variable importance values are displayed (Figure S2) with values greater than one in the O-PLS analysis. (+) indicates that inhibition of the target is positively correlated to any specific adverse drug reaction (ADR) and possibly causal, whereas (−) stands for a negative correlation with the ADR putatively reflecting protective properties. (+ +) and (− −) denote variable importance values higher than 1.5.
| Drug. target | Diarrhea | Nausea | Hypotension | Dizziness | Headache | Insomnia | Sedation | Sleepiness | Increased sweating | Weight gain |
|---|---|---|---|---|---|---|---|---|---|---|
| 5-HTT | + | + | − | − − | − | + + | − | |||
| NET | + | |||||||||
| DAT | + | |||||||||
| 5-HT1A | − | + | ||||||||
| 5-HT1B | + | |||||||||
| 5-HT1D | ||||||||||
| 5-HT2A | − − | − | + + | + | + + | + | − | + + | ||
| 5-HT2C | − | + | + | − | + + | |||||
| 5-HT3 | + | |||||||||
| 5-HT6 | + | − | + + | |||||||
| 5-HT7 | − | − | + + | + | ||||||
| α 1 | − | + + | + + | + | + | + | + | |||
| α 2 | ||||||||||
| β 1 | ||||||||||
| M1 | + | − − | ||||||||
| M2 | + | − | ||||||||
| M3 | + | + + | ||||||||
| M4 | + | − | ||||||||
| M5 | + | + + | − | + | ||||||
| D1 | − | + | − | |||||||
| D2 | − − | − − | + + | − | + + | + | + | + | ||
| D3 | − − | − | + + | + + | + | |||||
| D4 | + | |||||||||
| H1 | − − | − − | − − | + + | + + | − − | + + |
Results of associations of receptor binding profiles and individual ADRs with sufficient model quality will be summarized in the following paragraphs:
Gastrointestinal ADRs (Figure 3a)
Results of nausea and diarrhea associations have been grossly identical and will therefore be reported together. O-PLS analysis showed that 5-HTT re-uptake inhibition appears to be the main reason for nausea and diarrhea within the array of studied receptors and transporters. Interestingly, inhibition of the overwhelming majority of all other receptors and transporters resulted in opposing effects. Highest negative correlations were seen for H1-, 5-HT2A-, or D2-receptors indicating a reduced chance to suffer from nausea and diarrhea, when these systems are blocked.
Cardiovascular ADRs (Figure 3b)
Antagonism of α1-receptors has been associated with reports of hypotension as expected. It is noteworthy, that 5-HT2A-, 5-HT2C-, D1-, D2-, and H1-receptors have also been found to correlate with hypotension. Interestingly, our analyses showed a reduced chance of hypotension, when 5-HTT inhibition has been present.
Neuropsychiatric ADRs (Figure 3c)
Reports of dizziness are predominantly correlated with antagonism at α1-, 5-HT2A-, 5-HT2C-, and muscarinergic receptors reflect a variety of reasons for dizziness such as orthostatic hypotension and vestibular suppression among others. Headache was associated with 5-HTT and DAT inhibition. Interestingly, blockage of many receptors appeared to confer a protective effect, most notably H1-receptor inhibition. Reported ADRs frequencies for insomnia showed maximal correlations with D2- and D3- receptor antagonism. Sedation and excessive daytime sleepiness have been unsurprisingly correlated with similar targets due to their inherent relationship with the functionality of the arousal system. Antagonistic action at serotonergic, adrenergic, muscarinergic, dopaminergic, and histaminergic receptors showed to increase sedation and sleepiness. Interestingly, only β1-antagonism has been unrelated. In contrast, inhibition of 5-HT and to a lower degree dopamine re-uptake demonstrated to reduce the likelihood of reporting sedation and sleepiness in investigated clinical trials. Furthermore, our analyses reflected the similarity between sleepiness and insomnia, which are known to be co-occurring symptoms of many sleep disorders (hypovigilance during the day and hyper-vigilance at night). Therefore, we found similar associations for insomnia reports with histaminergic, dopaminergic, adrenergic, and serotonergic receptor antagonism. In is noteworthy that muscarinergic, β1, 5-HT3, DAT, and NET antagonism was unrelated to insomnia in our study. Moreover, we also found that 5-HTT inhibition reduced the risk of insomnia reports in investigated clinical trials.
Dermatological ADRs (Figure 3d)
Reports of increased sweating are mainly correlated with 5-HTT and NET inhibition. On the other hand, blockage of various 5-HT receptors as well as H1-receptor antagonism conferred a reduced chance to report increased sweating.
Metabolic ADRs (Figure 3e)
In addition to well known candidates such as 5-HT2c, M3, H1 receptors, we found a variety of other antagonistic receptor interactions to be linked to weight gain. 5-HT2A and 5-HT6 showed the strongest effects in the loading plots. A few targets showed a lack of association with weight gain such as NET, DAT, β1, and D3. Conversely, 5-HT re-uptake inhibition has been linked to decreased reporting of weight gain.
4. Discussion
The present study supports the idea that in vitro binding affinity profiles can be utilized to gain insights into putative mechanisms of ADRs occurring in clinical psychopharmacological trials. While many findings are well-known, several reported associations are novel and of potential interest for future pharmacological experiments. Our strategy of associating binding affinity profiles with ADRs has proven to be robust and feasible and constitutes a useful strategy for psychopharmacological research (Kroeze et al., 2003; Selent et al., 2010).
The present study was able to link the majority of common and important ADRs observed in clinical trials of ADs and APs to in vitro binding affinity profiles. 5-HTT inhibition has been identified to be the main contributor of nausea and diarrhea. Nausea is frequently observed at the beginning of SSRI treatment (Tuerke et al., 2012). However, the exact mechanism of SSRIs-induced nausea is still unclear. Interestingly, 5-HT3-antagonism, which is effective in chemotherapy-induced nausea has shown little effect on AD-induced nausea (Leatherman et al., 1999), which is in line with our results. Moreover, presented data indicate possible anti-emetic effects of 5-HT7-receptor antagonism in AD-induced nausea, which has not previously been published to our best knowledge. In addition, this study provides human evidence of an anti-emetic effect of 5-HT2A-antagonism rendering animal work (Wolff and Leander, 2000). Furthermore, our analysis was able to detect D2- and H1-mediated anti-emetic effects, which are therapeutically exploited by drugs such as metoclopramide and betahistidine. Diarrhea was highly associated with 5-HTT inhibition in our study. This is not surprising, given the high concentration of 5-HTTs in the GI tract and the prominent role of 5-HT signaling in GI mobility (Gershon, 2004). Interestingly, 5-HT2A- and H1-receptor antagonisms have been found to be more protective against psychotropic medication-induced diarrhea than muscarinergic receptor antagonism, which cannot be anticipated from current understanding of GI physiology.
The present study further reflected the importance of α1-receptor antagonism in orthostatic hypotension and consecutive dizziness. However, orthostatic hypotension is known to result from a much broader range of molecular mechanisms. This is underlined by the fact that several investiged psychotropic medications do not exhibit a significant α1-receptor affinity but still induce hypotension such as sulpiride (Figure 1). In addition to α1-recpetor antagonism, dizziness was predominately associated with muscarinergic and serotonergic antagonism in our study. Acetylcholine (ACH) is the primary neurotransmitter in vestibulocerebellar pathways, which may account for effects of muscarinic antagonists in motion sickness and reported dizziness during ACE-inhibitor treatment (Jones, 2010). Interestingly, M3-antagonism has been linked to dizziness (Zinner et al., 2005) similar to our results. Moreover, this study revealed a link between 5-HT2A-antagonism and dizziness in line with reports of dizziness during ketanserin treatment (Nagatomo et al., 2004). In addition to 5-HTT inhibition, we also observed an association between DAT inhibition and headache. This finding supports recent migraine models postulating ictal dopamine release as trigger for headache, which is based on accompanying dopaminergic symptoms such as yawning, nausea, postdromal somnolence, euphoria, and polyuria (Barbanti et al., 2013). Interestingly, the analysis revealed a negative correlation between H1 and M1 antagonism and headache, indicating that these receptor interactions have protective effects. While the implication of histamine in pain and its potential to trigger headaches has been reported before (Haas et al., 2008), the relationship of M1 antagonism and headache deserves further attention.
At least five neurotransmitters (histamine, dopamine, norepinephrine, 5-HT, and ACH) relevant for our study are known to modulate neuronal arousal (Sehgal and Mignot, 2011). Mirroring this complex regulation, we have identified several mechanisms that are associated with sleepiness, sedation, and insomnia. It is noteworthy that hypovigilance during the day and hypervigilance at night are typically features of sleep disorders and are caused by the same molecular mechanisms. H1- and α1-antagonism were associated with sedation and sleepiness in this study reflecting its use in hypnotic drugs or frequent reports of these ADRs in clinical trials (Mitchell and Weinshenker, 2010). Insomnia in restless leg syndrome is treated by dopamine agonists such as pramipexole (Montplaisir et al., 2006). Caffeine-induced arousal is thought to be mediated by D2-receptors, which are coupled with purine receptors (Ferre, 2010). Hence, our findings of increased insomnia due to dopaminergic antagonism are supported by previous studies. Sleep symptoms such as insomnia occurring during a depressive episode are also affected by 5-HT and are reduced in MDD patients under chronic SSRIs treatment. Recent research suggests that 5-HT2A-receptor antagonism (e.g. ritanserin) might be effective in staying asleep but not falling asleep (Vanover and Davis, 2010). We have observed a linkage between 5-HT2A antagonism and sedation, which is in line with pharmacological trials of nelotanserin (Al-Shamma et al., 2010). 5-HT7-mediated effects on sleepiness or sedation are further highlighted by our study. Although 5-HT7-dependent regulation of sleep as well as its pharmacological exploitation (Lopez-Rodriguez et al., 2003) have been suggested before, available human evidence is very limited. Furthermore, we found a linkage between 5-HT2C antagonism and sleepiness. The role of the 5-HT2C receptor in promoting sleep has previously been proposed for drugs such as melatonin and agomelatin (Mitchell and Weinshenker, 2010).
Antidepressant-induced sweating is commonly related to noradrenergic stimulation (Stahl et al., 2005). Surprisingly, in this study increased sweating has mainly been attributed to 5-HTT inhibition and only to a lesser degree to NET inhibition. It is noteworthy that medication-induced changes of thermoregulation are poorly understood and likely result from altered hypothalamic 5-HT signaling, which is in line with our findings (Ghaleiha et al., 2012). Apart from well-studied candidates such as 5-HT2C-, M3-, H1-receptors (Kroeze et al., 2003; Silvestre and Prous, 2005), we report further molecular targets to be potentially linked to weight gain such as 5-HT2A and specifically 5-HT6. It is generally accepted that 5-HT-ergic neurotransmission impinges on food intake. This is also substantiated by the clinical efficacy of fluoxetine in the treatment of bulimia. However, the specific post-synaptic effector sites are still under debate: 5-HT6-receptor antagonists have been observed to reduce food intake in rodents (Halford et al., 2007). In rats, this has been linked to an action at 5-HT6-receptors expressed in the paraventricular nucleus of the hypothalamus and/or the nucleus tractus solitarii but not at the arcuate nucleus (Garfield et al., 2014). Surprisingly, our observations suggest that blockage of 5-HT6-receptors is associated with weight gain in patients. At present, it is obviously impossible to resolve this discrepancy. It is worth noting, though, that there are large interspecies differences in the distribution of 5-HT6-receptors (Hirst et al., 2003). This suggests that murine data cannot be readily extrapolated to the situation in people. Furthermore, several paradigms that examined rodent feeding behavior focused on short term hypohagic responses to 5-HT6-receptor antagonists (see e.g., (Garfield et al., 2014)). Long term exposure to 5-HT6-receptor agonist, however, causes a hypophagic response in rats (Fisas et al., 2006). This observation predicts that long-term treatment with 5-HT6-receptor antagonists results in hyperphagia and weight gain, which – in fact – emerged from our analysis. Furthermore, the human 5-HT6-receptor has a high constitutive activity, while the rat receptor is silent under basal conditions (Fisas et al., 2006). The high basal activity of the unliganded human receptor ought to augment effects resulting from antagonist occupancy. Last but not least it is important to note that our analyses included depressive and schizophrenic patients and not humans suffering from obesity. Moreover, acute depression and psychosis are frequently associated with underweight due to lack of appetite and malnutrition (Suzuki et al., 2014). Hence, our finding of 5-HT6-related weight gain might indicate important differences between psychiatric and obesity patient populations. Furthermore, 5-HT2A antagonism was identified as a major contributor to weight gain. The role of 5-HT2A in obesity has previously been proposed based on an association between genetic variability within the 5-HT2A receptor gene and food as well as alcohol intake in obese patients (Aubert et al., 2000). Finally, we found that NET and 5-HTT inhibition are either not linked to or even protective against weight gain. This is in line with SNRI studies that resulted in the licensing as anti-obesity drugs (e.g. sibutramine). Notably, other mechanisms such as peripheral increased lipogenesis and decreased insulin sensitivity (Sacher et al., 2008) are also thought to be involved in antipsychotic-induced metabolic side effects.
Our approach is not without limitations. While we have been able to reproduce many known mechanisms for ADRs on a human as well as on an animal level, several ADRs (see results section) could not be significantly linked to in vitro receptor- and transporter binding profiles. This lack of association is not surprising and does not argue against the sensitivity of our approach. One reason for this failure is the fact that many reported symptoms such as fatigue and sexual dysfunction are frequent manifestations of the disease itself rather than ADRs. Therefore, these symptoms are also reported to occur in placebo-treated control groups. Ideally ADR frequencies should be normalized to a ‘baseline-frequency’. However, this was not possible since the majority of clinical trials included in this study were reference-controlled trials. Within this context it is important to note that reported Q2 values indicate the likelihood that such random correlations between drug target interaction profiles and drug-unrelated symptoms can occur. Hence, results showing moderate or high Q2 values are very likely to represent associations between drug targets and “true” ADRs. Another bias is the lack of standardized reporting tools for ADRs in clinical trials. In most clinical trials, patients have to spontaneously report ADRs. This leads to considerably lower reports than when ADRs are continuously assessed using a questionnaire or scale during each study visit. For example, the occurrence of sexual dysfunction in males during SSRI treatment is known to be very common (Perlis et al., 2009). However, patients are often reluctant to report this symptom spontaneously. On the other hand, a rigorous assessment of certain ADRs may lead to over-estimation. For example, in trials comparing SSRIs to TCAs, reports of TCA-typical ADRs in the SSRI group tend to be higher, since clinicians specifically focus on the assessment of their potential occurrence. Hence, it needs to be mentioned that the measurement of in vitro binding profiles is possible with relatively high precision, whereas ADR profiles are less precise.
Moreover, our model did not include all possible biological or pharmacological determinants that might affect the occurrence of ADRs. The presence of active metabolites has not been considered within this study for e.g. effects of quetiapine are thought to be mediated by the actions of both, quetiapine and its active metabolite norquetiapine. In contrast to quetiapine, norquetiapine has high affinity to NET, which may contribute to its broad spectrum of efficacy (Nyberg et al., 2013). Similarly, (2S, 3S)-hydroxybupropion, a major metabolite of bupropion, is believed to contribute to its antidepressant activity. Compared with bupropion, (2S, 3S)-hydroxybupropion produces similar or stronger inhibition of DA and NE uptake (Damaj et al., 2004). Furthermore, some ADRs may only occur under high-dose treatment, whereas others occur even at low doses. In addition, certain ADRs are dependent on the duration of the drug treatment. For example, gastrointestinal symptoms are more common during the initial phase of SSRI treatment, whereas sexual dysfunction or anticholinergic ADRs also occur during chronic treatment. However, dose or treatment duration were not separately analyzed in this study due to the lack of specific information available in the Cochrane reviews. Similarly, genetic susceptibility to ADRs has not been analyzed (Rabl et al., 2010).
Furthermore, this study did not consider interactions between possible drug target combinations due to the astronomic number of possibilities. This is likely the reason why we did for example not detect the protective effect of 5-HT2A-receptor antagonism against extrapyramidal motor symptoms, which only occurs in the presence of D2-receptor antagonism (Tauscher et al., 2002).
Due to above-mentioned reasons most models showed only moderate predictive power. However, even models with low Q2 values can be plausible such as for constipation. Since diarrhea is frequently caused by SSRIs (Gartlehner et al., 2008), 5-HTT inhibition is likely protective against constipation. This relationship was well-preserved within this study despite a very low Q2 value.
ADRs provide a glimpse into the dynamics of human molecular biology that is otherwise inaccessible. This is in particular true for the human brain and specifically mental disorders, which are difficult to study in animal experiments. Mining psychotropic medication-induced ADRs in patient samples provides an ethically sound and informative approach to probe the molecular mechanisms of ADRs occurring in patient populations, which lead frequently to treatment discontinuation.
In conclusion, this study provides compelling evidence for a multitude of associations between molecular targets and ADRs that are observed in clinical trials of ADs and APs. While many mechanisms have been reported before, this study was able to identify potential novel causes for ADRs, which should be considered in the development of future psychotropic medications, such as 5-HT6-receptor antagonism in weight gain, and 5-HT7-receptor antagonism in sleepiness. Furthermore, the present study demonstrates that many frequent ADRs such as hypotension, dizziness, sleepiness, insomnia and sedation are resulting from a variety of molecular mechanisms.
Supplementary Material
Acknowledgments
This study was funded by the project program grant SFB35 of the Austrian Science Fund (FWF).
Role of funding source
Funding for this study was provided by the project program grant SFB35 of the Austrian Science Fund (FWF). The FWF had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Abbreviations
- 5-HT
5-hydroxy tryptamine (serotonin)
- 5-HTT
serotonin transporter
- AAP
atypical antipsychotics
- ACH
acetylcholine
- ADR
adverse drug reaction
- AP
antipsychotic
- CDSR
cochrane database of systematic reviews
- CTZ
chemotrigger zone
- DAT
dopamine transporter
- EMA
european medicines agency
- EPMS
extrapyramidal motor symptoms
- FDA
food and drug administration
- GI
gastrointestinal
- Ki
inhibition constant
- MAO
monoamine oxidase
- MDD
major depressive disorder
- NDRI
norepinephrine-dopamine re-uptake inhibitor
- NET
norepinephrine transporter
- O-PLS
orthogonal projection to latent structures
- PDSP
psychoactive drugs screening program
- p1
principal component 1
- pKi
negative common logarithm of Ki
- PRESS
prediction error sum of squares
- SARI
serotonin antagonist and re-uptake inhibitor
- SD
standard deviation
- SNRI
serotonin norepinephrine re-uptake inhibitor
- SSRI
selective serotonin re-uptake inhibitor
- TAP
typical antipsychotic
- TCA
tricyclic antidepressant
- TeCA
tetracyclic antidepressant
Footnotes
Conflict of interest
All authors declare that they have no conflicts of interest.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.euroneuro.2014.06.013.
References
- Al-Shamma HA, Anderson C, Chuang E, Luthringer R, Grottick AJ, Hauser E, Morgan M, Shanahan W, Teegarden BR, Thomsen WJ, Behan D. Nelotanserin, a novel selective human 5-hydroxytryptamine2A inverse agonist for the treatment of insomnia. J. Pharmacol. Exp. Ther. 2010;332:281–290. doi: 10.1124/jpet.109.160994. [DOI] [PubMed] [Google Scholar]
- Aubert R, Betoulle D, Herbeth B, Siest G, Fumeron F. 5-HT2A receptor gene polymorphism is associated with food and alcohol intake in obese people. Int. J. Obes. Relat. Metab. Disord. 2000;24:920–924. doi: 10.1038/sj.ijo.0801253. [DOI] [PubMed] [Google Scholar]
- Baghai TC, Blier P, Baldwin DS, Bauer M, Goodwin GM, Fountoulakis KN, Kasper S, Leonard BE, Malt UF, Stein DJ, Versiani M, Moller HJ. Executive summary of the report by the WPA section on pharmacopsychiatry on general and comparative efficacy and effectiveness of antidepressants in the acute treatment of depressive disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2012;262:13–22. doi: 10.1007/s00406-011-0274-7. [DOI] [PubMed] [Google Scholar]
- Barbanti P, Fofi L, Aurilia C, Egeo G. Dopaminergic symptoms in migraine. Neurol. Sci. 2013;34(Suppl 1):S67–S70. doi: 10.1007/s10072-013-1415-8. [DOI] [PubMed] [Google Scholar]
- Bauer M, Pfennig A, Severus E, Whybrow PC, Angst J, Moller HJ. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J. Biol. Psychiatry. 2013;14:334–385. doi: 10.3109/15622975.2013.804195. [DOI] [PubMed] [Google Scholar]
- Bender A, Scheiber J, Glick M, Davies JW, Azzaoui K, Hamon J, Urban L, Whitebread S, Jenkins JL. Analysis of pharmacology data and the prediction of adverse drug reactions and off-target effects from chemical structure. ChemMedChem. 2007;2:861–873. doi: 10.1002/cmdc.200700026. [DOI] [PubMed] [Google Scholar]
- Castro VM, Clements CC, Murphy SN, Gainer VS, Fava M, Weilburg JB, Erb JL, Churchill SE, Kohane IS, Iosifescu DV, Smoller JW, Perlis RH. QT interval and anti-depressant use: a cross sectional study of electronic health records. Br. Med. J. 2013:346. doi: 10.1136/bmj.f288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, Lukas RJ, Martin BR. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol. Pharmacol. 2004;66:675–682. doi: 10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- Ferre S. Role of the central ascending neurotransmitter systems in the psychostimulant effects of caffeine. J. Alzheimers Dis. 2010;20(Suppl 1):S35–S49. doi: 10.3233/JAD-2010-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisas A, Codony X, Romero G, Dordal A, Giraldo J, Merce R, Holenz J, Heal D, Buschmann H, Pauwels PJ. Chronic 5-HT6 receptor modulation by E-6837 induces hypophagia and sustained weight loss in diet-induced obese rats. Br. J. Pharmacol. 2006;148:973–983. doi: 10.1038/sj.bjp.0706807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield AS, Burke LK, Shaw J, Evans ML, Heisler LK. Distribution of cells responsive to 5-HT6 receptor antagonist-induced hypophagia. Behav. Brain Res. 2014;266:201–206. doi: 10.1016/j.bbr.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartlehner G, Gaynes BN, Hansen RA, Thieda P, DeVeaugh-Geiss A, Krebs EE, Moore CG, Morgan L, Lohr KN. Comparative benefits and harms of second-generation antidepressants: background paper for the American College of Physicians. Ann. Intern. Med. 2008;149:734–750. doi: 10.7326/0003-4819-149-10-200811180-00008. [DOI] [PubMed] [Google Scholar]
- Gershon MD. Review article: serotonin receptors and transporters – roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol. Ther. 2004;20(Suppl 7):3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- Ghaleiha A, Jahangard L, Sherafat Z, Ahmadpanah M, Brand S, Holsboer-Trachsler E, Bajoghli H, Haghighi M. Oxybutynin reduces sweating in depressed patients treated with sertraline: a double-blind, placebo-controlled, clinical study. Neuropsychiatr. Dis. Treat. 2012;8:407–412. doi: 10.2147/NDT.S36329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunze H, Vieta E, Goodwin GM, Bowden C, Licht RW, Moller HJ, Kasper S, Treatment, W.T.F. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2012 on the long-term treatment of bipolar disorder. World J. Biol. Psychiatry. 2013;14:154–219. [Google Scholar]
- Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol. Rev. 2008;88:1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- Halford JC, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. Serotonergic drugs: effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67:27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Thibaut F, Moller HJ, Guideli, W.T.F.T. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, Part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J. Biol. Psychiatry. 2013;14:2–44. doi: 10.3109/15622975.2012.739708. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Abrahamsen B, Blaney FE, Calver AR, Aloj L, Price GW, Medhurst AD. Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling. Mol. Pharmacol. 2003;64:1295–1308. doi: 10.1124/mol.64.6.1295. [DOI] [PubMed] [Google Scholar]
- Huf W, Kalcher K, Pail G, Friedrich ME, Filzmoser P, Kasper S. Meta-analysis: fact or fiction? How to interpret meta-analyses. World J. Biol. Psychiatry. 2011;12:188–200. doi: 10.3109/15622975.2010.551544. [DOI] [PubMed] [Google Scholar]
- Insel TR. Next-generation treatments for mental disorders. Sci. Transl. Med. 2012;4:155ps119. doi: 10.1126/scitranslmed.3004873. [DOI] [PubMed] [Google Scholar]
- Jones RW. A review comparing the safety and tolerability of memantine with the acetylcholinesterase inhibitors. Int. J. Geriatr. Psychiatry. 2010;25:547–553. doi: 10.1002/gps.2384. [DOI] [PubMed] [Google Scholar]
- Kroeze WK, Hufeisen SJ, Popadak BA, Renock S, Steinberg SA, Ernsberger P, Jayathilake K, Meltzer HY, Roth BL. HI-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28:519–526. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- Leatherman ME, Bebchuk JM, Ekstrom RD, Heine AD, Carson SW, Golden RN. The effects of serotonin (3) receptor blockade on the psychobiological response to intravenous clomipramine in healthy human subjects. Biol. Psychiatry. 1999;45:238–240. doi: 10.1016/s0006-3223(98)00045-6. [DOI] [PubMed] [Google Scholar]
- Lopez-Munoz F, Alamo C. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr. Pharm. Des. 2009;15:1563–1586. doi: 10.2174/138161209788168001. [DOI] [PubMed] [Google Scholar]
- Lopez-Rodriguez ML, Porras E, Morcillo MJ, Benhamu B, Soto LJ, Lavandera JL, Ramos JA, Olivella M, Campillo M, Pardo L. Optimization of the pharmacophore model for 5-HT7R antagonism. Design and synthesis of new naphtholactam and naphthosultam derivatives. J. Med. Chem. 2003;46:5638–5650. doi: 10.1021/jm030841r. [DOI] [PubMed] [Google Scholar]
- Lounkine E, Keiser MJ, Whitebread S, Mikhailov D, Hamon J, Jenkins JL, Lavan P, Weber E, Doak AK, Cote S, Shoichet BK, Urban L. Large-scale prediction and testing of drug activity on side-effect targets. Nature. 2012;486:361–367. doi: 10.1038/nature11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell HA, Weinshenker D. Good night and good luck: norepinephrine in sleep pharmacology. Biochem. Pharmacol. 2010;79:801–809. doi: 10.1016/j.bcp.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montplaisir J, Fantini ML, Desautels A, Michaud M, Petit D, Filipini D. Long-term treatment with pramipexole in restless legs syndrome. Eur. J. Neurol. 2006;13:1306–1311. doi: 10.1111/j.1468-1331.2006.01459.x. [DOI] [PubMed] [Google Scholar]
- Nagatomo T, Rashid M, Abul Muntasir H, Komiyama T. Functions of 5-HT2A receptor and its antagonists in the cardiovascular system. Pharmacol. Ther. 2004;104:59–81. doi: 10.1016/j.pharmthera.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Nutt DJ. Beyond psychoanaleptics - can we improve antidepressant drug nomenclature? (vol 23, pg 343, 2009) J. Psychopharmacol. 2009;23:861. doi: 10.1177/0269881109105498. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Jucaite A, Takano A, Kagedal M, Cselenyi Z, Halldin C, Farde L. Norepinephrine transporter occupancy in the human brain after oral administration of quetiapine XR. Int. J. Neuropsychopharmacol. 2013;16:2235–2244. doi: 10.1017/S1461145713000680. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Laje G, Smoller JW, Fava M, Rush AJ, McMahon FJ. Genetic and clinical predictors of sexual dysfunction in citalopram-treated depressed patients. Neuropsychopharmacology. 2009;34:1819–1828. doi: 10.1038/npp.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabl U, Scharinger C, Muller M, Pezawas L. Imaging genetics: implications for research on variable antidepressant drug response. Expert Rev. Clin. Pharmacol. 2010;3:471–489. doi: 10.1586/ecp.10.35. [DOI] [PubMed] [Google Scholar]
- Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat. Rev. Drug Discov. 2004;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- Sacher J, Mossaheb N, Spindelegger C, Klein N, Geiss-Granadia T, Sauermann R, Lackner E, Joukhadar C, Muller M, Kasper S. Effects of olanzapine and ziprasidone on glucose tolerance in healthy volunteers. Neuropsychopharmacology. 2008;33:1633–1641. doi: 10.1038/sj.npp.1301541. [DOI] [PubMed] [Google Scholar]
- Sehgal A, Mignot E. Genetics of sleep and sleep disorders. Cell. 2011;146:194–207. doi: 10.1016/j.cell.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selent J, Bauer-Mehren A, Lopez L, Loza MI, Sanz F, Pastor M. A novel multilevel statistical method for the study of the relationships between multireceptorial binding affinity profiles and in vivo endpoints. Mol. Pharmacol. 2010;77:149–158. doi: 10.1124/mol.109.060103. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Prous J. Research on adverse drug events. I. Muscarinic M3 receptor binding affinity could predict the risk of antipsychotics to induce type 2 diabetes. Methods Find. Exp. Clin. Pharmacol. 2005;27:289–304. doi: 10.1358/mf.2005.27.5.908643. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Grady MM, Moret C, Briley M. SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectr. 2005;10:732–747. doi: 10.1017/s1092852900019726. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Sugai T, Fukui N, Watanabe J, Ono S, Tsuneyama N, Saito M, Someya T. High prevalence of underweight and undernutrition in Japanese inpatients with schizophrenia. Psychiatry Clin. Neurosci. 2014;68:78–82. doi: 10.1111/pcn.12082. [DOI] [PubMed] [Google Scholar]
- Tauscher J, Kufferle B, Asenbaum S, Tauscher-Wisniewski S, Kasper S. Striatal dopamine-2 receptor occupancy as measured with [123I]iodobenzamide and SPECT predicted the occurrence of EPS in patients treated with atypical antipsychotics and haloperidol. Psychopharmacology. 2002;162:42–49. doi: 10.1007/s00213-002-1082-6. [DOI] [PubMed] [Google Scholar]
- Trygg J, Wold S. Orthogonal projections to latent structures (O-PLS) J. Chemom. 2002;16:119–128. [Google Scholar]
- Tuerke KJ, Limebeer CL, Fletcher PJ, Parker LA. Double dissociation between regulation of conditioned disgust and taste avoidance by serotonin availability at the 5-HT3 receptor in the posterior and anterior insular cortex. J. Neurosci. 2012;32:13709–13717. doi: 10.1523/JNEUROSCI.2042-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanover KE, Davis RE. Role of 5-HT2A receptor antagonists in the treatment of insomnia. Nat. Sci. Sleep. 2010;2:139–150. doi: 10.2147/nss.s6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure L, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fevre EM, Finucane MM, Flax-man S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta MN, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope 3rd CA, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leon FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MC, Leander JD. A comparison of the behavioural effects of 5-HT2A and 5-HT2C receptor agonists in the pigeon. Behav. Pharmacol. 2000;11:355–364. doi: 10.1097/00008877-200008000-00001. [DOI] [PubMed] [Google Scholar]
- Zinner N, Tuttle J, Marks L. Efficacy and tolerability of darifenacin, a muscarinic M3 selective receptor antagonist (M3 SRA), compared with oxybutynin in the treatment of patients with overactive bladder. World J. Urol. 2005;23:248–252. doi: 10.1007/s00345-005-0507-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.