Abstract
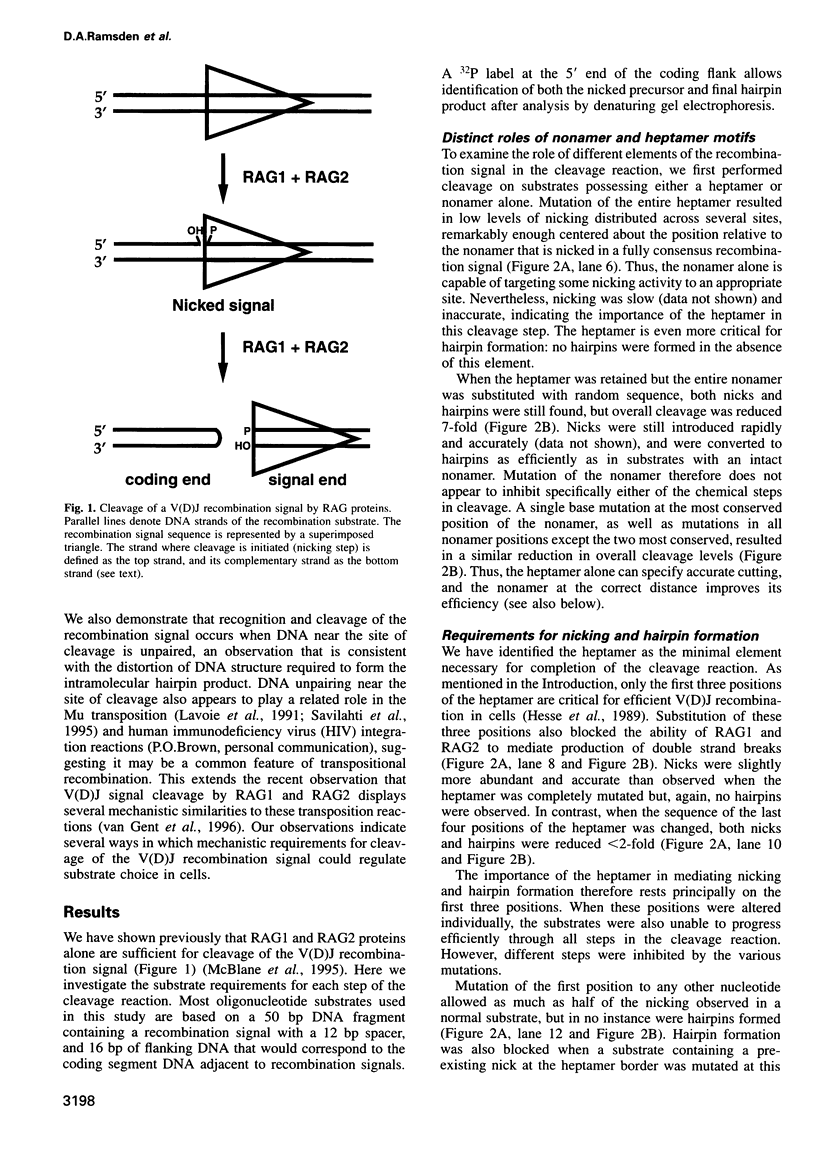
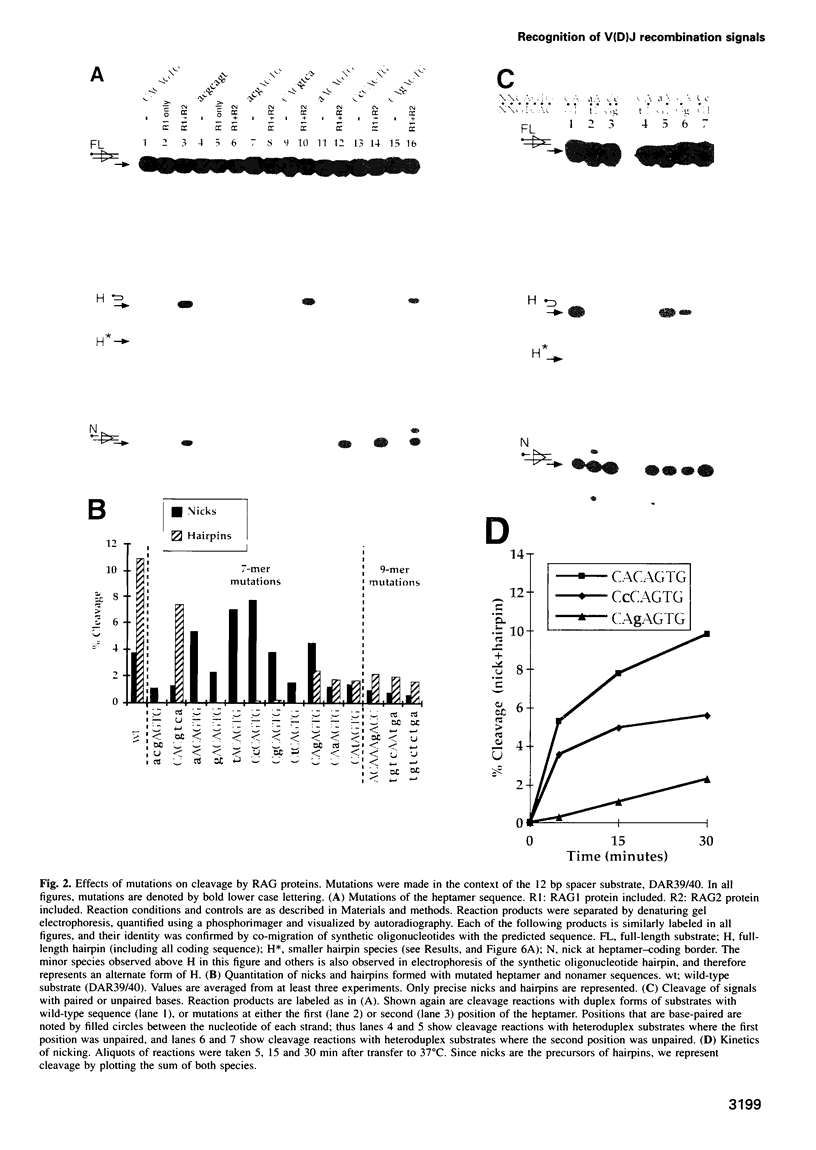
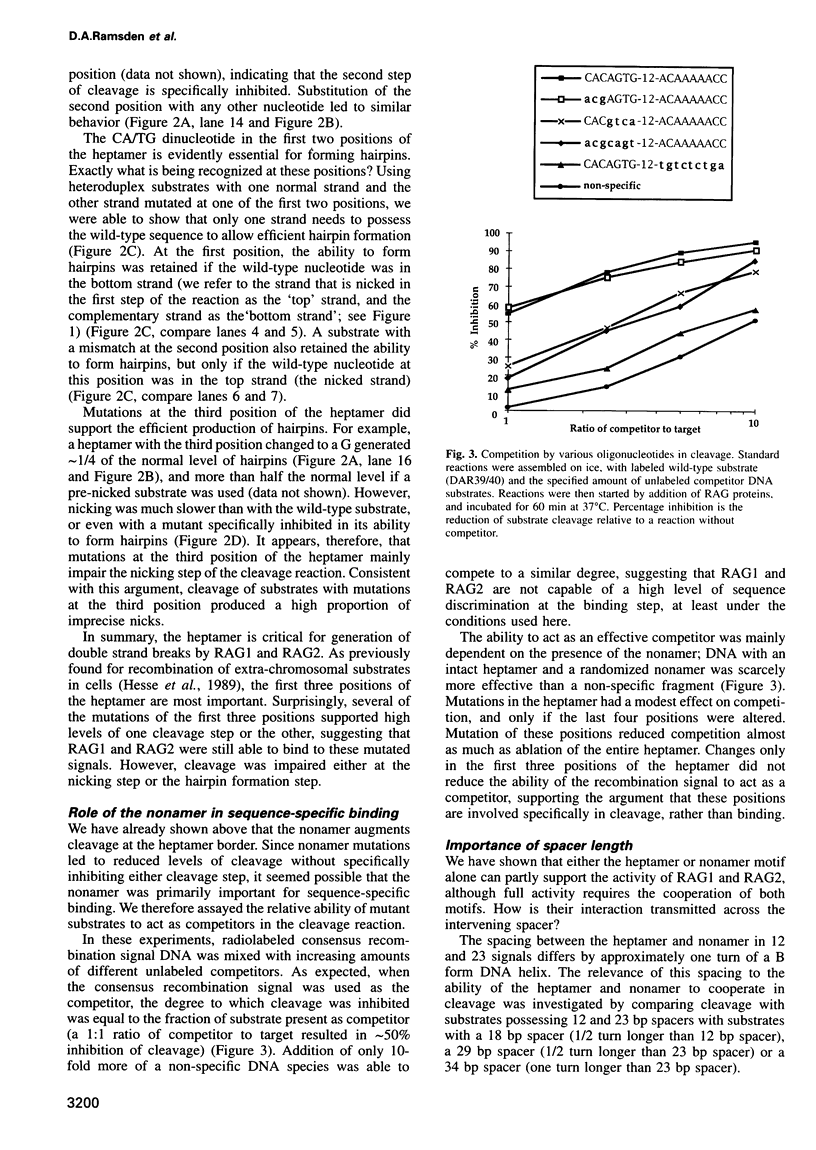
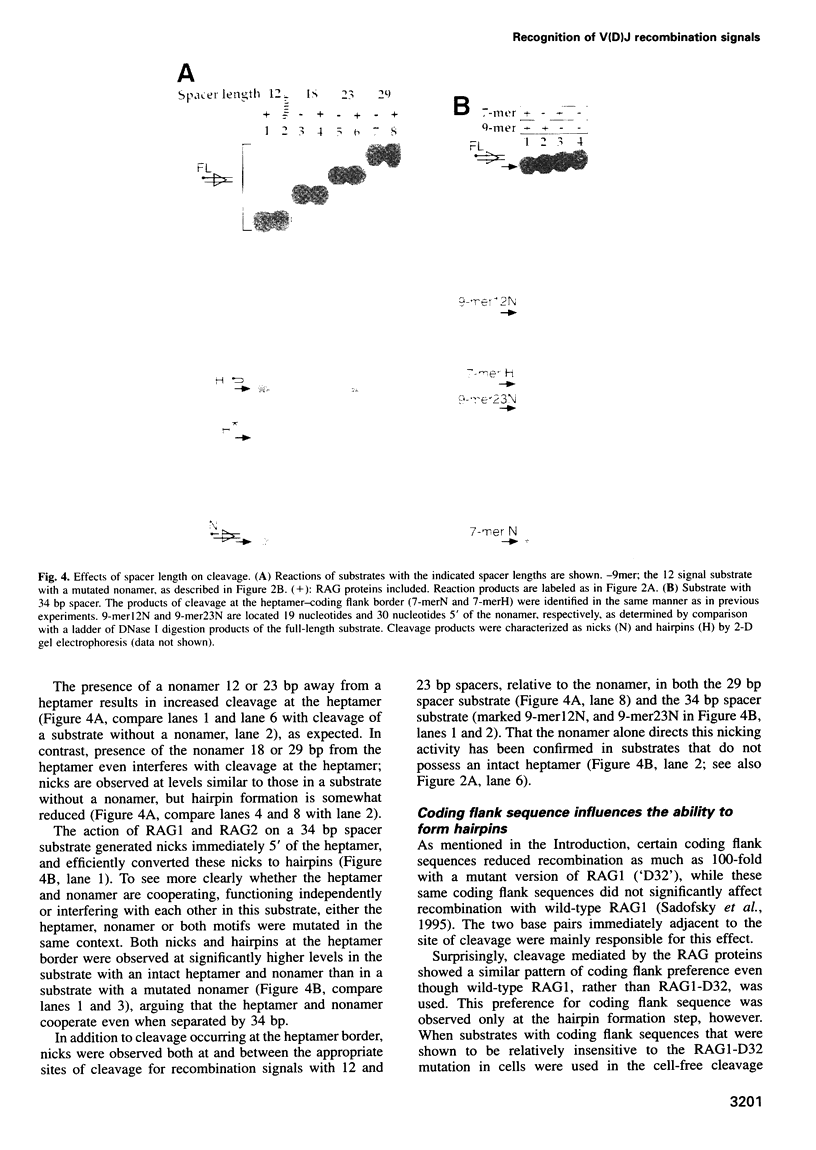
Cleavage of V(D)J recombination signals by purified RAG1 and RAG2 proteins permits the dissection of DNA structure and sequence requirements. The two recognition elements of a signal (nonamer and heptamer) are used differently, and their cooperation depends on correct helical phasing. The nonamer is most important for initial binding, while efficient nicking and hairpin formation require the heptamer sequence. Both nicking and hairpin formation are remarkably tolerant of variations in DNA structure. Certain flanking sequences inhibit hairpin formation, but this can be bypassed by base unpairing, and even a completely single-stranded signal sequence is well utilized. We suggest that DNA unpairing around the signal-coding border is essential for the initiation of V(D)J combination.
Full text
PDF
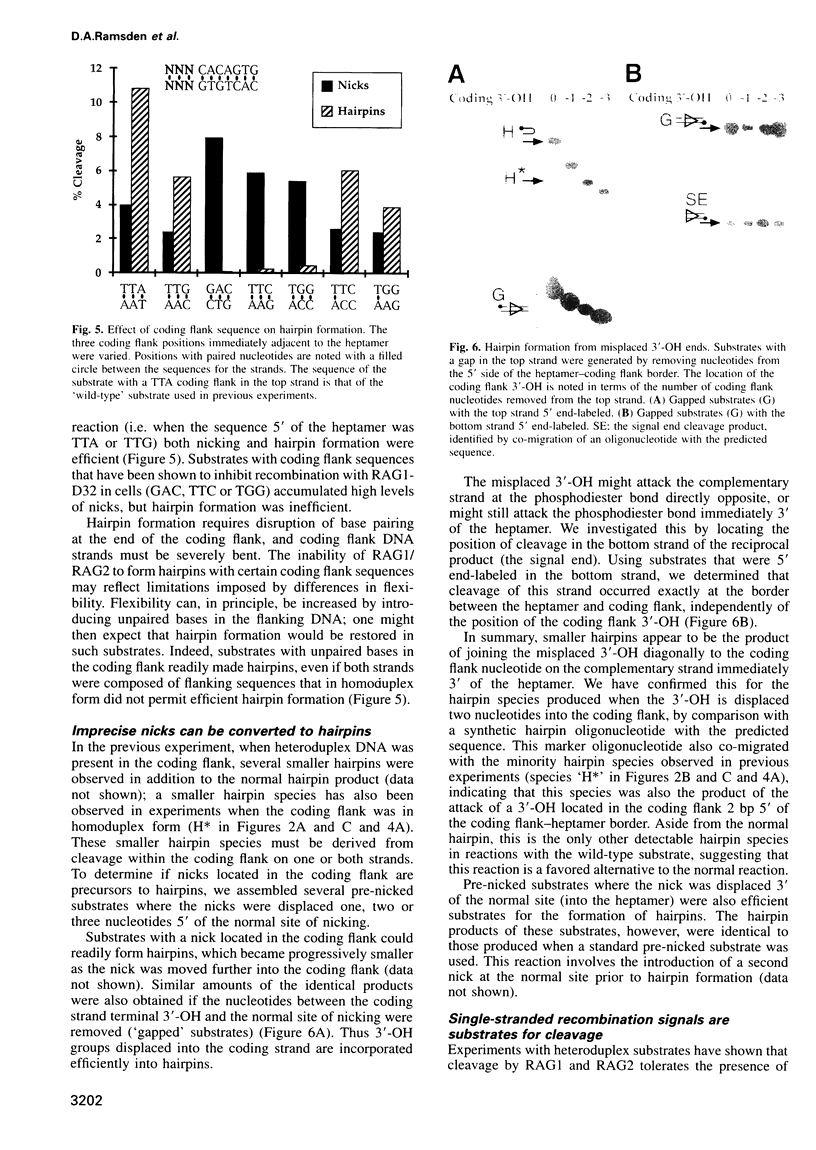
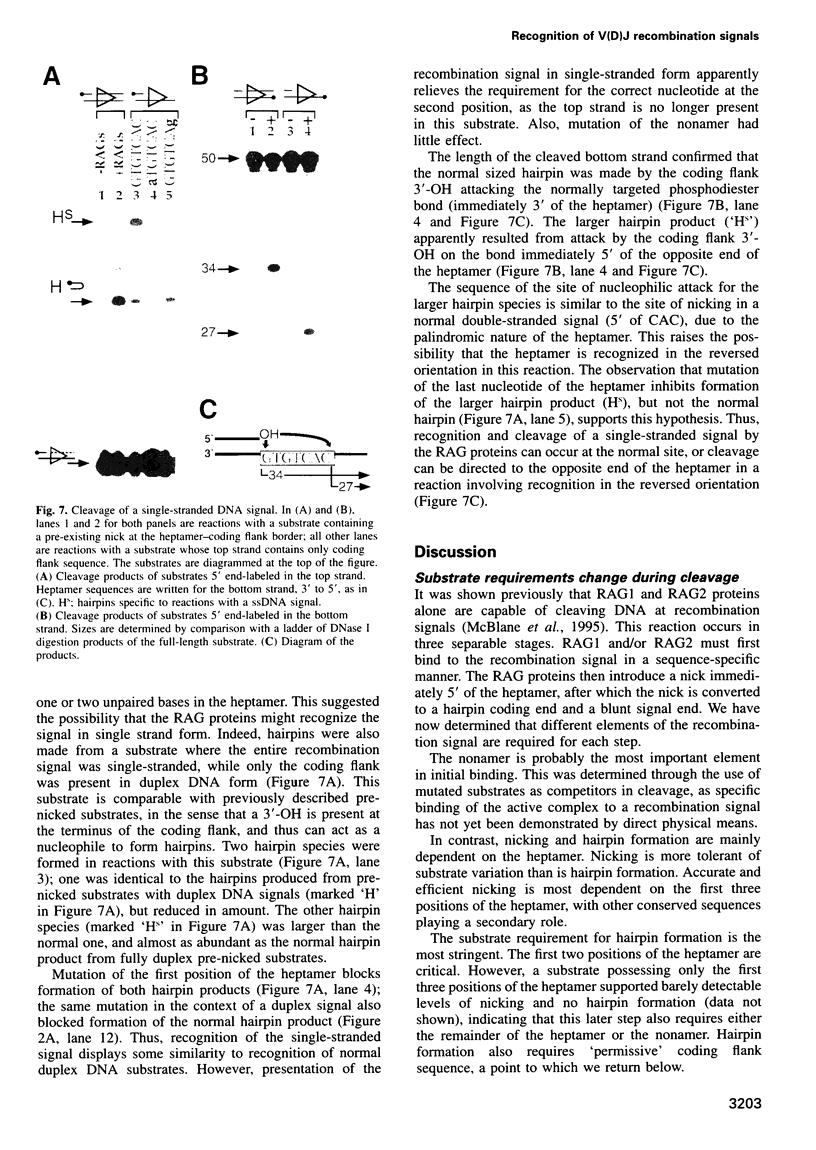



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boubnov N. V., Wills Z. P., Weaver D. T. Coding sequence composition flanking either signal element alters V(D)J recombination efficiency. Nucleic Acids Res. 1995 Mar 25;23(6):1060–1067. doi: 10.1093/nar/23.6.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlingame R. P., Obukowicz M. G., Lynn D. L., Howe M. M. Isolation of point mutations in bacteriophage Mu attachment regions cloned in a lambda::mini-Mu phage. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6012–6016. doi: 10.1073/pnas.83.16.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung S., Arndt K., Lu P. Correlation of lac operator DNA imino proton exchange kinetics with its function. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3665–3669. doi: 10.1073/pnas.81.12.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuomo C. A., Oettinger M. A. Analysis of regions of RAG-2 important for V(D)J recombination. Nucleic Acids Res. 1994 May 25;22(10):1810–1814. doi: 10.1093/nar/22.10.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekiel U. R., Engler P., Stern D., Storb U. Asymmetric processing of coding ends and the effect of coding end nucleotide composition on V(D)J recombination. Immunity. 1995 Apr;2(4):381–389. doi: 10.1016/1074-7613(95)90146-9. [DOI] [PubMed] [Google Scholar]
- Gellert M. Molecular analysis of V(D)J recombination. Annu Rev Genet. 1992;26:425–446. doi: 10.1146/annurev.ge.26.120192.002233. [DOI] [PubMed] [Google Scholar]
- Gerstein R. M., Lieber M. R. Coding end sequence can markedly affect the initiation of V(D)J recombination. Genes Dev. 1993 Jul;7(7B):1459–1469. doi: 10.1101/gad.7.7b.1459. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Mizuuchi K., Gellert M. V(D)J recombination: a functional definition of the joining signals. Genes Dev. 1989 Jul;3(7):1053–1061. doi: 10.1101/gad.3.7.1053. [DOI] [PubMed] [Google Scholar]
- Kleinfield R., Hardy R. R., Tarlinton D., Dangl J., Herzenberg L. A., Weigert M. Recombination between an expressed immunoglobulin heavy-chain gene and a germline variable gene segment in a Ly 1+ B-cell lymphoma. 1986 Aug 28-Sep 3Nature. 322(6082):843–846. doi: 10.1038/322843a0. [DOI] [PubMed] [Google Scholar]
- LaFemina R. L., Callahan P. L., Cordingley M. G. Substrate specificity of recombinant human immunodeficiency virus integrase protein. J Virol. 1991 Oct;65(10):5624–5630. doi: 10.1128/jvi.65.10.5624-5630.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie B. D., Chan B. S., Allison R. G., Chaconas G. Structural aspects of a higher order nucleoprotein complex: induction of an altered DNA structure at the Mu-host junction of the Mu type 1 transpososome. EMBO J. 1991 Oct;10(10):3051–3059. doi: 10.1002/j.1460-2075.1991.tb07856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. M. The mechanism of V(D)J joining: lessons from molecular, immunological, and comparative analyses. Adv Immunol. 1994;56:27–150. doi: 10.1016/s0065-2776(08)60450-2. [DOI] [PubMed] [Google Scholar]
- McBlane J. F., van Gent D. C., Ramsden D. A., Romeo C., Cuomo C. A., Gellert M., Oettinger M. A. Cleavage at a V(D)J recombination signal requires only RAG1 and RAG2 proteins and occurs in two steps. Cell. 1995 Nov 3;83(3):387–395. doi: 10.1016/0092-8674(95)90116-7. [DOI] [PubMed] [Google Scholar]
- Ramsden D. A., Baetz K., Wu G. E. Conservation of sequence in recombination signal sequence spacers. Nucleic Acids Res. 1994 May 25;22(10):1785–1796. doi: 10.1093/nar/22.10.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden D. A., Gellert M. Formation and resolution of double-strand break intermediates in V(D)J rearrangement. Genes Dev. 1995 Oct 1;9(19):2409–2420. doi: 10.1101/gad.9.19.2409. [DOI] [PubMed] [Google Scholar]
- Reth M., Gehrmann P., Petrac E., Wiese P. A novel VH to VHDJH joining mechanism in heavy-chain-negative (null) pre-B cells results in heavy-chain production. 1986 Aug 28-Sep 3Nature. 322(6082):840–842. doi: 10.1038/322840a0. [DOI] [PubMed] [Google Scholar]
- Roth D. B., Menetski J. P., Nakajima P. B., Bosma M. J., Gellert M. V(D)J recombination: broken DNA molecules with covalently sealed (hairpin) coding ends in scid mouse thymocytes. Cell. 1992 Sep 18;70(6):983–991. doi: 10.1016/0092-8674(92)90248-b. [DOI] [PubMed] [Google Scholar]
- Roth D. B., Nakajima P. B., Menetski J. P., Bosma M. J., Gellert M. V(D)J recombination in mouse thymocytes: double-strand breaks near T cell receptor delta rearrangement signals. Cell. 1992 Apr 3;69(1):41–53. doi: 10.1016/0092-8674(92)90117-u. [DOI] [PubMed] [Google Scholar]
- Roth D. B., Zhu C., Gellert M. Characterization of broken DNA molecules associated with V(D)J recombination. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10788–10792. doi: 10.1073/pnas.90.22.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadofsky M. J., Hesse J. E., Gellert M. Definition of a core region of RAG-2 that is functional in V(D)J recombination. Nucleic Acids Res. 1994 May 25;22(10):1805–1809. doi: 10.1093/nar/22.10.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadofsky M. J., Hesse J. E., McBlane J. F., Gellert M. Expression and V(D)J recombination activity of mutated RAG-1 proteins. Nucleic Acids Res. 1993 Dec 11;21(24):5644–5650. doi: 10.1093/nar/21.24.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadofsky M. J., Hesse J. E., van Gent D. C., Gellert M. RAG-1 mutations that affect the target specificity of V(D)j recombination: a possible direct role of RAG-1 in site recognition. Genes Dev. 1995 Sep 1;9(17):2193–2199. doi: 10.1101/gad.9.17.2193. [DOI] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Savilahti H., Rice P. A., Mizuuchi K. The phage Mu transpososome core: DNA requirements for assembly and function. EMBO J. 1995 Oct 2;14(19):4893–4903. doi: 10.1002/j.1460-2075.1995.tb00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlissel M., Constantinescu A., Morrow T., Baxter M., Peng A. Double-strand signal sequence breaks in V(D)J recombination are blunt, 5'-phosphorylated, RAG-dependent, and cell cycle regulated. Genes Dev. 1993 Dec;7(12B):2520–2532. doi: 10.1101/gad.7.12b.2520. [DOI] [PubMed] [Google Scholar]
- Surette M. G., Harkness T., Chaconas G. Stimulation of the Mu A protein-mediated strand cleavage reaction by the Mu B protein, and the requirement of DNA nicking for stable type 1 transpososome formation. In vitro transposition characteristics of mini-Mu plasmids carrying terminal base pair mutations. J Biol Chem. 1991 Feb 15;266(5):3118–3124. [PubMed] [Google Scholar]
- Timsit Y., Vilbois E., Moras D. Base-pairing shift in the major groove of (CA)n tracts by B-DNA crystal structures. Nature. 1991 Nov 14;354(6349):167–170. doi: 10.1038/354167a0. [DOI] [PubMed] [Google Scholar]
- van Gent D. C., McBlane J. F., Ramsden D. A., Sadofsky M. J., Hesse J. E., Gellert M. Initiation of V(D)J recombination in a cell-free system. Cell. 1995 Jun 16;81(6):925–934. doi: 10.1016/0092-8674(95)90012-8. [DOI] [PubMed] [Google Scholar]
- van Gent D. C., Mizuuchi K., Gellert M. Similarities between initiation of V(D)J recombination and retroviral integration. Science. 1996 Mar 15;271(5255):1592–1594. doi: 10.1126/science.271.5255.1592. [DOI] [PubMed] [Google Scholar]
- van den Ent F. M., Vink C., Plasterk R. H. DNA substrate requirements for different activities of the human immunodeficiency virus type 1 integrase protein. J Virol. 1994 Dec;68(12):7825–7832. doi: 10.1128/jvi.68.12.7825-7832.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]







