Abstract
How do viruses spread from cell to cell? Enveloped viruses acquire their surrounding membranes by budding: either through the plasma membrane or an internal membrane of infected cells. Thus, a newly budded enveloped virus finds itself either in the extracellular milieu or in a lumenal compartment from which it can exit the cell by conventional secretion. On the other hand, naked viruses such as poliovirus, nodavirus, adenovirus, and SV40 lack an external membrane. They are simply protein-nucleic acid complexes within the cytoplasm or nucleus of the infected cell, and thus would seem to have no other exit route than cell lysis. We have presented the first documentation of nonlytic spread of a naked virus, and showed the interconnections between this event and the process or components of the autophagy pathway.
Keywords: live imaging, nonlytic, RNA virus, unconventional secretion, virus spread
Infection with poliovirus, rhinovirus, SARS coronavirus, and several other positive-strand RNA viruses induces the formation of double-membraned vesicles decorated with LC3-II. Although viral replication occurs in the cytoplasm, the interior lumen of these vesicles can contain viral particles as well as other cytoplasmic constituents such as ribosomes and mitochondria. During infection with poliovirus, a non-enveloped virus, siRNA-mediated depletion of LC3 shows a greater decrease in extracellular than intracellular virus. Given that this naked virus would conventionally be thought to stay within infected cells until they lysed, this finding argued either that LC3 contributes to cell lysis or to some other form of exit from the cell. This led us to the hypothesis that the virus-induced double-membraned vesicles could serve as vehicles for egress of the virus.
To visualize the cell-to-cell spread of poliovirus, we used time-lapse microscopy to monitor the progress of a viral variant that expresses the DsRed fluorescent protein (Fig. 1). The infected human hepatocytes also expressed GFP-LC3, allowing us to see the abundant GFP-LC3-containing puncta characteristic of poliovirus-infected cells (not shown). In panel A, a living, well-isolated cell infected with poliovirus (cell 1) could be seen at 9 and 10 h after the initial infection. By 10 h, the infection of its neighbor (cell 2) could be visualized; this infection probably occurred 4 h earlier, because that is the period of time required for the DsRed signal to be detected. In any case, cell 1 did not die until 12 h post-infection as evidenced by the Sytox Blue staining, a marker of dead cells. Therefore, the observation that cell 2 was infected at 10 h argues that the infectious material escaped from cell 1 long before it died.
Figure 1.
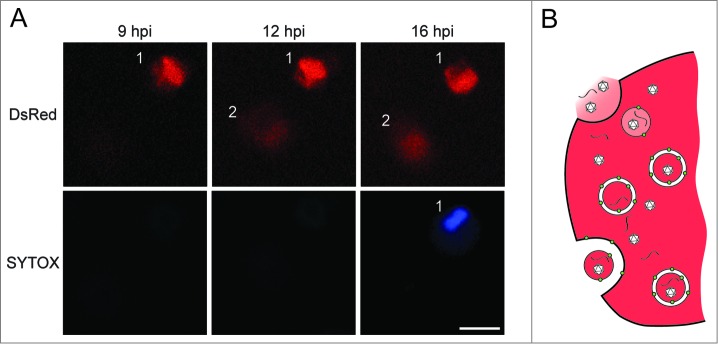
Evidence and proposed mechanism for nonlytic spread of a non-enveloped virus. (A) Top panel shows a single infected cell (1) infecting another cell (2) at 10 h after infection with a poliovirus variant that expresses DsRed. Transfer of infectious material from cell 1 to cell 2 occurs before the death of cell 1, shown by the penetration of Sytox Blue. The numbers of these nonlytic transfer events increase in the presence of autophagy stimulators loperamide or nicardipine. (B) Hypothesis for unconventional secretion of infectious viral material. Double-membraned vesicles induced by poliovirus infection contain virions and, most likely, unencapsidated viral RNA. Upon fusion of the outer membrane with the plasma membrane, the internal contents will be released. Depending upon the state of the inner membrane, the virions and viral RNA will be released free or inside a vesicle. Scale bar = 25 μm. This figure was modified from Fig. 4 published in Bird et al., PNAS 2014 111:13081 copyright National Academy of Sciences (USA).
What is the effect of the autophagy pathway on the nonlytic spread of poliovirus? In untreated cells, conclusively nonlytic spread (Fig. 1A) is an infrequent event. However, when cells are treated with compounds that stimulate autophagy in an MTOR-independent fashion, including loperamide and nicardipine, the frequency of the non-lytic spread events increase dramatically. Loperamide and nicardipine treatment also increase the numbers of infected cells in viral plaques in tissue culture. As expected if this effect is mediated by the autophagy pathway or its components, the increase in plaque size upon loperamide treatment does not occur when LC3 is depleted.
Our hypothesis for the mechanism by which the LC3-containing double-membraned vesicles induced by poliovirus promote nonlytic viral spread is shown in Figure 1B. We suggest that the nonlytic spread of naked cytoplasmic virus is a form of unconventional secretion, in which the outer vesicular membrane fuses with the cellular plasma membrane, releasing the contents into the extracellular milieu. We do not yet know whether the transmitted infectious material is encapsidated or non-encapsidated RNA, within a single-membraned vesicle or not: all these materials are most certainly present, and which are responsible for viral transmission remains to be determined.
In this study, single-cell analysis of individually infected cells allowed the unambiguous observation of cell-to-cell viral transmission without cell death. As in most cases of unconventional secretion, it was difficult to determine whether the secreted products resulted from the nonlytic ‘leakage’ of many cells or the undetected lysis of a few. Single-cell analysis may resolve such issues for other hypothesized examples of unconventional secretion such as the transmission of aggregated proteins and cytoplasmic signaling molecules.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.


