Abstract
Autophagy is a spatially regulated process in axons; autophagosomes form preferentially in the distal axon tip then move actively and processively toward the cell body. Despite the primarily unidirectional transport observed in live-cell imaging experiments, both anterograde-directed KIF5/kinesin-1 motors and retrograde-directed dynein motors are tightly associated with axonal autophagosomes. Here, we discuss our recent work identifying the scaffolding protein MAPK8IP1/JIP1 (mitogen-activated protein kinase 8 interacting protein 1) as a key regulator of autophagosome transport in neurons. MAPK8IP1 tightly coordinates motor activity to ensure the fidelity of retrograde autophagosome transport in the axon.
Keywords: autophagosomes, axonal transport, dynactin, dynein, JIP1, kinesin, LC3, LIR, MKP1, motor regulation
Neurons are particularly sensitive to defects in autophagy. Seminal genetic studies in mice showed that knockout of Atg genes essential for autophagosome formation leads to neuronal cell death. Neurons depend heavily on the fidelity of autophagy for 2 reasons. As terminally differentiated cells, neurons no longer undergo mitosis, which can mitigate the accumulation of damaged organelles and misfolded proteins in the cytoplasm. Clearance of both organelles and protein aggregates is further complicated by the extreme nature of neuronal cytoarchitecture, with cellular extensions that can reach lengths of up to 1 m in humans. Indeed, defects in autophagy are associated with multiple neurodegenerative diseases, including Alzheimer, Parkinson, and Huntington diseases, and amyotrophic lateral sclerosis.
Autophagy in the axon is a constitutive process that is spatially regulated. Sandra Maday has shown that axonal autophagosomes in primary neurons cultured from dorsal root ganglia or hippocampus are preferentially generated in the distal axon tip, and then move processively in the retrograde direction with fast speeds and few pauses. Autophagosome transport in the axon strikingly contrasts with the motility of other organelles, including mitochondria and lysosomes, which move in both anterograde and retrograde directions in the axon. As they move distally to proximally along the axon, autophagosomes fuse with lysosomes and become increasingly acidified, suggesting that retrograde transport and autophagic function are tightly coupled. Thus, an important question in axonal autophagosome biology is how the direction of transport is conferred and maintained.
We now identify the scaffolding protein MAPK8IP1/JIP1 as a critical regulator of autophagosome motility. MAPK8IP1 is recruited to autophagosomes by direct binding to LC3 via a phenylalanine-type LIR (LC3-interacting region) motif. Interestingly, 2 other well-characterized adaptors for autophagosomes, OPTN/optineurin, and FYCO1, which facilitate association of the actin-based motor myosin and the anterograde microtubule-based motor kinesin, respectively, also bind to LC3 via phenylalanine-type LIRs, in contrast to tryptophan-type LIRs that function in initial cargo sequestration.
In the axon, autophagosomes form at the distal tips, then exhibit bidirectional or frequent back-and-forth movement initially, followed by processive retrograde transport. MAPK8IP1 does not play a role in initial formation of autophagosomes, as MAPK8IP1-deficient neurons do not contain altered numbers of autophagosomes in the distal tip. Rather, co-migration studies suggest that MAPK8IP1 recruitment to nascent autophagosomes coincides with initiation of unidirectional, retrograde transport. Consistent with this observation, knockdown of MAPK8IP1 leads to accumulation in the distal axon of bidirectional autophagosomes that are unable to effectively transit toward the cell soma.
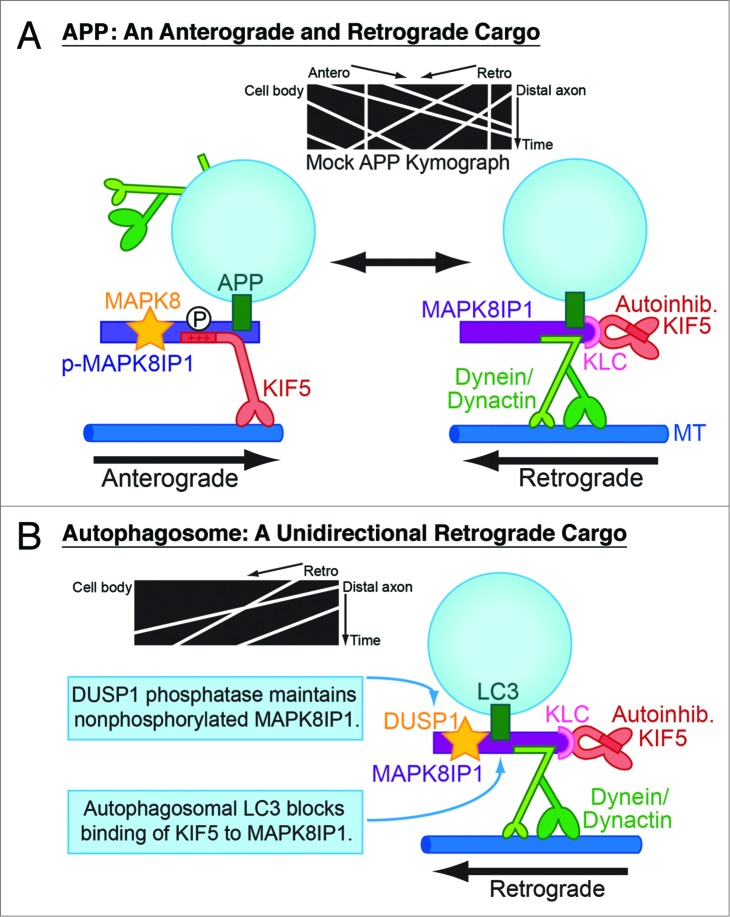
Once MAPK8IP1 is recruited to autophagosomes, retrograde transport is sustained along the length of the axon via 2 distinct molecular mechanisms. As a scaffolding protein, MAPK8IP1 binds directly to both kinesin-1 heavy chain (KIF5/KHC) and the DCTN1/p150Glued subunit of dynactin, the activator for dynein. However, MAPK8IP1 cannot bind simultaneously to both motor complexes. Thus, 2 alternative MAPK8IP1 complexes can be formed—one that facilitates anterograde transport, and another that facilitates retrograde transport. Switching between these 2 complexes is regulated by phosphorylation of MAPK8IP1 at the MAPK8/JNK phosphorylation site, S421, located within the KIF5-binding domain. In previous work we tested this model by demonstrating that phosphomimetic MAPK8IP1-S421D preferentially associates with kinesin and favors anterograde transport of MAPK8IP1's canonical cargo, APP (amyloid beta A4 precursor protein), while phosphodeficient MAPK8IP1-S421A favors retrograde APP transport (Fig. 1A).
Figure 1.
MAPK8IP 1 regulates the axonal transport of 2 different organelles, APP-positive vesicles and autophagosomes. (A) MAPK8IP 1 exists as 2 distinct motor complexes—an anterograde complex binds directly to KIF5 and activates kinesin motor activity, and a retrograde complex binds directly to the dynein activator dynactin. Phosphorylation at the S421 site in the KIF5-binding domain of MAPK8IP 1 regulates switching between these 2 complexes and, thus, determines the direction of APP transport. (B) Two mechanisms ensure that autophagosomes move exclusively in the retrograde direction in the axon. The phosphatase DUSP1, which robustly associates with MAPK8IP 1-positive autophagosomes, may maintain MAPK8IP 1 in the nonphosphorylated retrograde motor complex. Binding of LC3 to MAPK8IP 1 further deters binding and activation of KIF5. MT, microtubule.
These observations suggested to us that maintaining MAPK8IP1 in the nonphosphorylated state will sustain retrograde autophagosome transport. We found that in neurons depleted of endogenous MAPK8IP1, exogenous expression of MAPK8IP1-S421A is sufficient to rescue retrograde autophagosome transport. In contrast, expression of the phosphomimetic mutant MAPK8IP1-S421D increases the percentage of anterograde autophagosomes by 10-fold. Immunostaining shows that DUSP1/MKP1 (dual specificity phosphatase 1) robustly associates with MAPK8IP1-positive autophagosomes along the axon, likely acting to sustain MAPK8IP1 in the dephosphorylated state as autophagosomes move back to the cell body. These observations suggest that the phosphorylation of MAPK8IP1 at S421 acts as a molecular switch to regulate the direction of autophagosome transport along the axon (Fig. 1B).
In addition, we found that binding to LC3 decreases the ability of MAPK8IP1 to activate KIF5 motor activity in vitro. Since the MAPK8IP1 LIR is located in close proximity to the KIF5-binding domain, it is likely that steric hindrance upon LC3 binding competitively inhibits KIF5 binding.
Importantly, MAPK8IP1-mediated retrograde autophagosome transport in the axon is tightly coupled to autophagic function. In knockdown and rescue experiments, expression of a mutant form of MAPK8IP1 unable to bind to LC3 disrupts retrograde autophagosome transport, and also decreases autophagosome acidification, indicative of a likely defect in cargo degradation. Thus, the role of MAPK8IP1 in safeguarding the fidelity of retrograde autophagosome transport in the axon prevents impaired transport, which can lead to failures in cargo degradation.
Many questions governing the regulation of autophagosome transport remain. It is not yet clear why the anterograde motor kinesin remains tightly bound to autophagosomes that move processively in the retrograde direction for long distances. One possibility is that autoinhibited KIF5 may be retained on the retrograde MAPK8IP1 complex via its associated adaptor, KLC (kinesin light chain); local activation of KIF5 may be important for circumnavigating road blocks along the axon. Further work is required to fully identify the upstream regulatory mechanisms leading to MAPK8IP1 recruitment and phospho-regulation. Finally, it is also not clear if multiple adaptors may cooperate with each other to facilitate the directed transport of autophagosomes. Our lab has recently shown that the scaffolding protein HTT/huntingtin also regulates autophagosome transport along axons, and that expression of mutant HTT leads to defects in mitophagy. The dynein adaptor SNAPIN, involved in retrograde transport of RAB7-positive late endosomes, may also contribute to autophagosome transport. Thus, several adaptors may need to work in concert to move each retrograde autophagosome.
Funding
This work was supported by NIH funding to MMF (T32GM7517 and F31NS73262) and ELFH (R01GM48661).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.