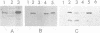Abstract
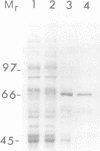
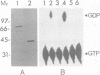
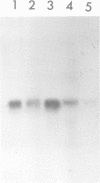
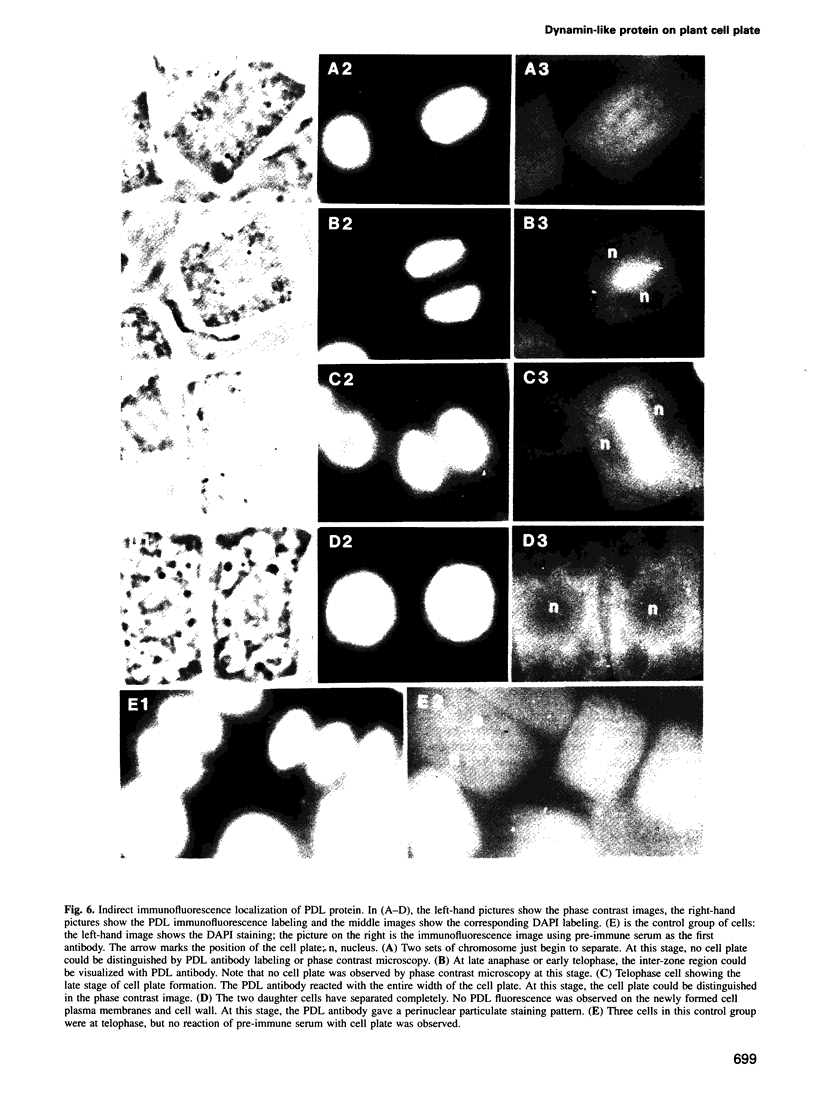
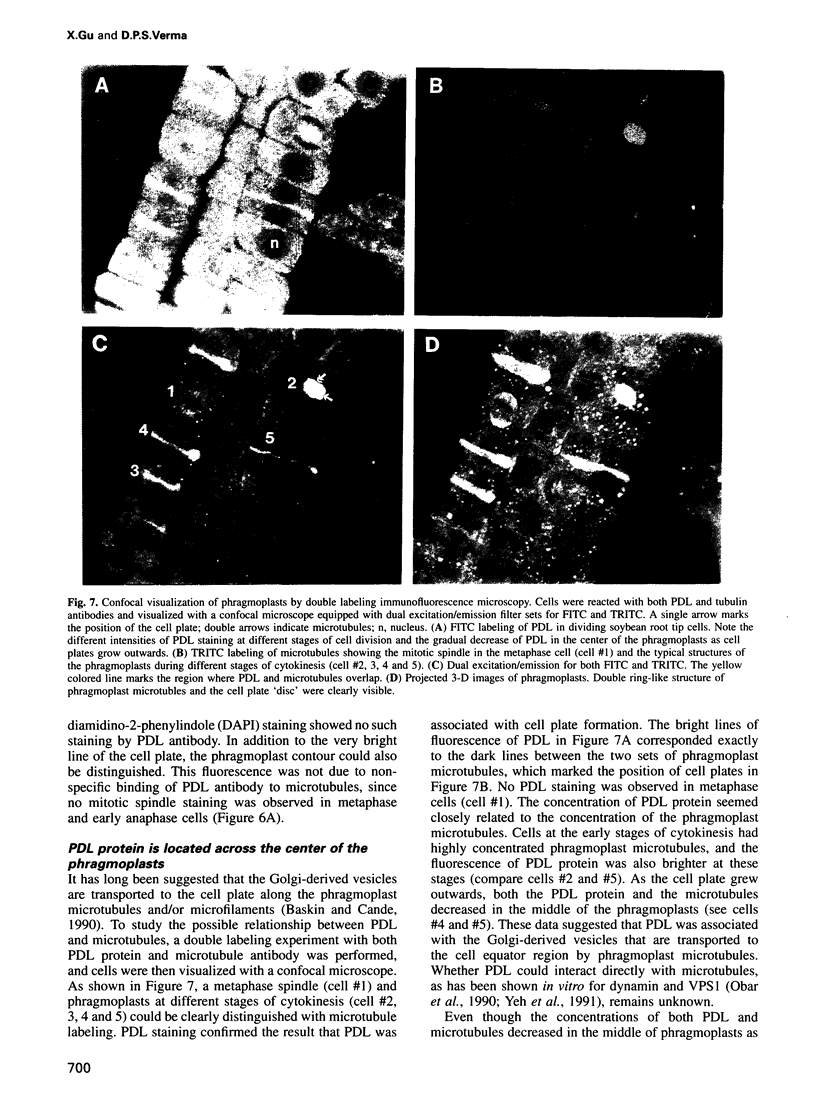
Cytokinesis in a plant cell is accomplished by the formation of a cell plate in the center of the phragmoplast. Little is known of the molecular events associated with this process. In this study, we report the identification of a dynamin-like protein from soybean and demonstrate that this protein is associated with the formation of the cell plate. Plant dynamin-like (PDL) protein contains 610 amino acids showing high homology with other members of the dynamin protein family. Western blot experiments demonstrated that it is associated with the non-ionic detergent-resistant fraction of membranes. Indirect immunofluorescence microscopy localized PDL to the cell plate in dividing soybean root tip cells. Double labeling experiments demonstrated that, unlike phragmoplast microtubules which are concentrated on the periphery of the forming plate, PDL is located across the whole width of the newly formed cell plate. Based on the temporal and spatial organization of PDL in the phragmoplast, we termed this protein 'phragmoplastin'. The data suggest that phragmoplastin may be associated with exocytic vesicles that are depositing cell plate material during cytokinesis in the plant cell.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Chen M. S., Obar R. A., Schroeder C. C., Austin T. W., Poodry C. A., Wadsworth S. C., Vallee R. B. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991 Jun 13;351(6327):583–586. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- Fishkind D. J., Wang Y. L. New horizons for cytokinesis. Curr Opin Cell Biol. 1995 Feb;7(1):23–31. doi: 10.1016/0955-0674(95)80041-7. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout I., Dhand R., Hiles I. D., Fry M. J., Panayotou G., Das P., Truong O., Totty N. F., Hsuan J., Booker G. W. The GTPase dynamin binds to and is activated by a subset of SH3 domains. Cell. 1993 Oct 8;75(1):25–36. [PubMed] [Google Scholar]
- Gunning B. E., Wick S. M. Preprophase bands, phragmoplasts, and spatial control of cytokinesis. J Cell Sci Suppl. 1985;2:157–179. doi: 10.1242/jcs.1985.supplement_2.9. [DOI] [PubMed] [Google Scholar]
- Herskovits J. S., Burgess C. C., Obar R. A., Vallee R. B. Effects of mutant rat dynamin on endocytosis. J Cell Biol. 1993 Aug;122(3):565–578. doi: 10.1083/jcb.122.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits J. S., Shpetner H. S., Burgess C. C., Vallee R. B. Microtubules and Src homology 3 domains stimulate the dynamin GTPase via its C-terminal domain. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11468–11472. doi: 10.1073/pnas.90.24.11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw J. E., Schmid S. L. Dynamin self-assembles into rings suggesting a mechanism for coated vesicle budding. Nature. 1995 Mar 9;374(6518):190–192. doi: 10.1038/374190a0. [DOI] [PubMed] [Google Scholar]
- Jones M. G., Payne H. L. Cytokinesis in Impatiens balsamina and the effect of caffeine. Cytobios. 1978;20(78):79–91. [PubMed] [Google Scholar]
- Kelly R. B. Endocytosis. Ringing necks with dynamin. Nature. 1995 Mar 9;374(6518):116–117. doi: 10.1038/374116a0. [DOI] [PubMed] [Google Scholar]
- Liu J. P., Powell K. A., Südhof T. C., Robinson P. J. Dynamin I is a Ca(2+)-sensitive phospholipid-binding protein with very high affinity for protein kinase C. J Biol Chem. 1994 Aug 19;269(33):21043–21050. [PubMed] [Google Scholar]
- Mahmoudi M., Lin V. K. Comparison of two different hybridization systems in northern transfer analysis. Biotechniques. 1989 Apr;7(4):331-2, 334. [PubMed] [Google Scholar]
- Melén K., Ronni T., Broni B., Krug R. M., von Bonsdorff C. H., Julkunen I. Interferon-induced Mx proteins form oligomers and contain a putative leucine zipper. J Biol Chem. 1992 Dec 25;267(36):25898–25907. [PubMed] [Google Scholar]
- Miki H., Miura K., Matuoka K., Nakata T., Hirokawa N., Orita S., Kaibuchi K., Takai Y., Takenawa T. Association of Ash/Grb-2 with dynamin through the Src homology 3 domain. J Biol Chem. 1994 Feb 25;269(8):5489–5492. [PubMed] [Google Scholar]
- Morin N., Abrieu A., Lorca T., Martin F., Dorée M. The proteolysis-dependent metaphase to anaphase transition: calcium/calmodulin-dependent protein kinase II mediates onset of anaphase in extracts prepared from unfertilized Xenopus eggs. EMBO J. 1994 Sep 15;13(18):4343–4352. doi: 10.1002/j.1460-2075.1994.tb06754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M., Nagata K., Kato A., Ishihama A. Interferon-inducible mouse Mx1 protein that confers resistance to influenza virus is GTPase. J Biol Chem. 1991 Nov 15;266(32):21404–21408. [PubMed] [Google Scholar]
- Nakayama M., Yazaki K., Kusano A., Nagata K., Hanai N., Ishihama A. Structure of mouse Mx1 protein. Molecular assembly and GTP-dependent conformational change. J Biol Chem. 1993 Jul 15;268(20):15033–15038. [PubMed] [Google Scholar]
- Obar R. A., Collins C. A., Hammarback J. A., Shpetner H. S., Vallee R. B. Molecular cloning of the microtubule-associated mechanochemical enzyme dynamin reveals homology with a new family of GTP-binding proteins. Nature. 1990 Sep 20;347(6290):256–261. doi: 10.1038/347256a0. [DOI] [PubMed] [Google Scholar]
- Poodry C. A., Hall L., Suzuki D. T. Developmental properties of Shibire: a pleiotropic mutation affecting larval and adult locomotion and development. Dev Biol. 1973 Jun;32(2):373–386. doi: 10.1016/0012-1606(73)90248-0. [DOI] [PubMed] [Google Scholar]
- Robinson J. S., Klionsky D. J., Banta L. M., Emr S. D. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol Cell Biol. 1988 Nov;8(11):4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P. J., Sontag J. M., Liu J. P., Fykse E. M., Slaughter C., McMahon H., Südhof T. C. Dynamin GTPase regulated by protein kinase C phosphorylation in nerve terminals. Nature. 1993 Sep 9;365(6442):163–166. doi: 10.1038/365163a0. [DOI] [PubMed] [Google Scholar]
- Rothman J. H., Raymond C. K., Gilbert T., O'Hara P. J., Stevens T. H. A putative GTP binding protein homologous to interferon-inducible Mx proteins performs an essential function in yeast protein sorting. Cell. 1990 Jun 15;61(6):1063–1074. doi: 10.1016/0092-8674(90)90070-u. [DOI] [PubMed] [Google Scholar]
- Seedorf K., Kostka G., Lammers R., Bashkin P., Daly R., Burgess W. H., van der Bliek A. M., Schlessinger J., Ullrich A. Dynamin binds to SH3 domains of phospholipase C gamma and GRB-2. J Biol Chem. 1994 Jun 10;269(23):16009–16014. [PubMed] [Google Scholar]
- Shpetner H. S., Vallee R. B. Dynamin is a GTPase stimulated to high levels of activity by microtubules. Nature. 1992 Feb 20;355(6362):733–735. doi: 10.1038/355733a0. [DOI] [PubMed] [Google Scholar]
- Shpetner H. S., Vallee R. B. Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules. Cell. 1989 Nov 3;59(3):421–432. doi: 10.1016/0092-8674(89)90027-5. [DOI] [PubMed] [Google Scholar]
- Staeheli P., Pitossi F., Pavlovic J. Mx proteins: GTPases with antiviral activity. Trends Cell Biol. 1993 Aug;3(8):268–272. doi: 10.1016/0962-8924(93)90055-6. [DOI] [PubMed] [Google Scholar]
- Takei K., McPherson P. S., Schmid S. L., De Camilli P. Tubular membrane invaginations coated by dynamin rings are induced by GTP-gamma S in nerve terminals. Nature. 1995 Mar 9;374(6518):186–190. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- Vallee R. B., Okamoto P. M. The regulation of endocytosis: identifying dynamin's binding partners. Trends Cell Biol. 1995 Feb;5(2):43–47. doi: 10.1016/s0962-8924(00)88937-0. [DOI] [PubMed] [Google Scholar]
- Vater C. A., Raymond C. K., Ekena K., Howald-Stevenson I., Stevens T. H. The VPS1 protein, a homolog of dynamin required for vacuolar protein sorting in Saccharomyces cerevisiae, is a GTPase with two functionally separable domains. J Cell Biol. 1992 Nov;119(4):773–786. doi: 10.1083/jcb.119.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd T. C., Dekker B. M., Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989 Mar 25;17(6):2362–2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick S. M., Seagull R. W., Osborn M., Weber K., Gunning B. E. Immunofluorescence microscopy of organized microtubule arrays in structurally stabilized meristematic plant cells. J Cell Biol. 1981 Jun;89(3):685–690. doi: 10.1083/jcb.89.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsbach K., Payne G. S. Vps1p, a member of the dynamin GTPase family, is necessary for Golgi membrane protein retention in Saccharomyces cerevisiae. EMBO J. 1993 Aug;12(8):3049–3059. doi: 10.1002/j.1460-2075.1993.tb05974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolniak S. M., Hepler P. K., Jackson W. T. Detection of the membrane-calcium distribution during mitosis in Haemanthus endosperm with chlorotetracycline. J Cell Biol. 1980 Oct;87(1):23–32. doi: 10.1083/jcb.87.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolniak S. M., Larsen P. M. The timing of protein kinase activation events in the cascade that regulates mitotic progression in Tradescantia stamen hair cells. Plant Cell. 1995 Apr;7(4):431–445. doi: 10.1105/tpc.7.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E., Driscoll R., Coltrera M., Olins A., Bloom K. A dynamin-like protein encoded by the yeast sporulation gene SPO15. Nature. 1991 Feb 21;349(6311):713–715. doi: 10.1038/349713a0. [DOI] [PubMed] [Google Scholar]
- Zhang D. H., Callaham D. A., Hepler P. K. Regulation of anaphase chromosome motion in Tradescantia stamen hair cells by calcium and related signaling agents. J Cell Biol. 1990 Jul;111(1):171–182. doi: 10.1083/jcb.111.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bliek A. M., Meyerowitz E. M. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991 May 30;351(6325):411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- van der Bliek A. M., Redelmeier T. E., Damke H., Tisdale E. J., Meyerowitz E. M., Schmid S. L. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993 Aug;122(3):553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]