Abstract
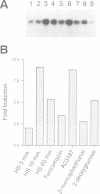
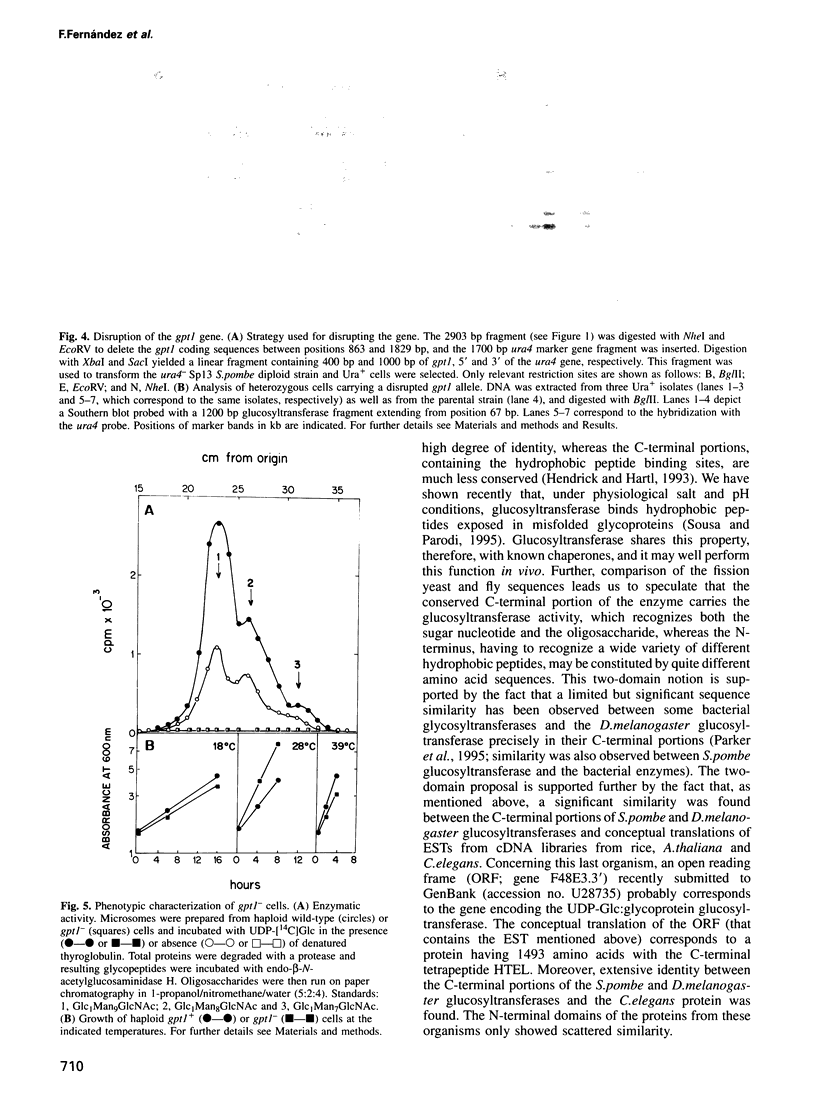
We have identified and begun the characterization of the gene encoding UDP-Glc:glycoprotein glucosyltransferase in Schizosaccharomyces pombe. This gene, here designated gpt1, codes for a polypeptide having a signal peptide of 18 amino acids followed by 1429 amino acids with no transmembrane domain, as expected for a soluble protein of the endoplasmic reticulum (ER). The C-terminal tetrapeptide PDEL most probably corresponds to a novel ER retention signal in this fission yeast. Synthesis of the corresponding mRNA was induced 2- to 9-fold by conditions known to affect glycoprotein folding in the ER (e.g. heat shock, culture in the presence of a Ca2+ionophore, 2-mercaptoethanol or inhibitors of protein N-glycosylation such as tunicamycin or 2-deoxyglucose). This is the first evidence obtained in vivo that supports the proposed involvement of the enzyme in the quality control of glycoprotein folding in the ER. Thus far, the said involvement was inferred solely from the ability of the enzyme to glucosylate misfolded but not native glycoproteins in cell-free assays. The gpt1 gene was disrupted and gpt1- cells were found to be viable. Moreover, no significant differences in the growth rate patterns at 18, 28 or 39 degrees C or in cell morphology between gpt1+ and gpt1- cells were observed, although they differed slightly in size.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cox J. S., Shamu C. E., Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993 Jun 18;73(6):1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- Fernández F. S., Trombetta S. E., Hellman U., Parodi A. J. Purification to homogeneity of UDP-glucose:glycoprotein glucosyltransferase from Schizosaccharomyces pombe and apparent absence of the enzyme fro Saccharomyces cerevisiae. J Biol Chem. 1994 Dec 2;269(48):30701–30706. [PubMed] [Google Scholar]
- Hammond C., Braakman I., Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert D. N., Foellmer B., Helenius A. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell. 1995 May 5;81(3):425–433. doi: 10.1016/0092-8674(95)90395-x. [DOI] [PubMed] [Google Scholar]
- Helenius A. How N-linked oligosaccharides affect glycoprotein folding in the endoplasmic reticulum. Mol Biol Cell. 1994 Mar;5(3):253–265. doi: 10.1091/mbc.5.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman U., Wernstedt C., Góez J., Heldin C. H. Improvement of an "In-Gel" digestion procedure for the micropreparation of internal protein fragments for amino acid sequencing. Anal Biochem. 1995 Jan 1;224(1):451–455. doi: 10.1006/abio.1995.1070. [DOI] [PubMed] [Google Scholar]
- Hendrick J. P., Hartl F. U. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- Jannatipour M., Rokeach L. A. The Schizosaccharomyces pombe homologue of the chaperone calnexin is essential for viability. J Biol Chem. 1995 Mar 3;270(9):4845–4853. doi: 10.1074/jbc.270.9.4845. [DOI] [PubMed] [Google Scholar]
- Kohno K., Normington K., Sambrook J., Gething M. J., Mori K. The promoter region of the yeast KAR2 (BiP) gene contains a regulatory domain that responds to the presence of unfolded proteins in the endoplasmic reticulum. Mol Cell Biol. 1993 Feb;13(2):877–890. doi: 10.1128/mcb.13.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Transport of secretory and membrane glycoproteins from the rough endoplasmic reticulum to the Golgi. A rate-limiting step in protein maturation and secretion. J Biol Chem. 1988 Feb 15;263(5):2107–2110. [PubMed] [Google Scholar]
- Loo T. W., Clarke D. M. Prolonged association of temperature-sensitive mutants of human P-glycoprotein with calnexin during biogenesis. J Biol Chem. 1994 Nov 18;269(46):28683–28689. [PubMed] [Google Scholar]
- Margolese L., Waneck G. L., Suzuki C. K., Degen E., Flavell R. A., Williams D. B. Identification of the region on the class I histocompatibility molecule that interacts with the molecular chaperone, p88 (calnexin, IP90). J Biol Chem. 1993 Aug 25;268(24):17959–17966. [PubMed] [Google Scholar]
- Meaden P., Hill K., Wagner J., Slipetz D., Sommer S. S., Bussey H. The yeast KRE5 gene encodes a probable endoplasmic reticulum protein required for (1----6)-beta-D-glucan synthesis and normal cell growth. Mol Cell Biol. 1990 Jun;10(6):3013–3019. doi: 10.1128/mcb.10.6.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Mori K., Ma W., Gething M. J., Sambrook J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993 Aug 27;74(4):743–756. doi: 10.1016/0092-8674(93)90521-q. [DOI] [PubMed] [Google Scholar]
- Normington K., Kohno K., Kozutsumi Y., Gething M. J., Sambrook J. S. cerevisiae encodes an essential protein homologous in sequence and function to mammalian BiP. Cell. 1989 Jun 30;57(7):1223–1236. doi: 10.1016/0092-8674(89)90059-7. [DOI] [PubMed] [Google Scholar]
- Ou W. J., Cameron P. H., Thomas D. Y., Bergeron J. J. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature. 1993 Aug 26;364(6440):771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- Parker C. G., Fessler L. I., Nelson R. E., Fessler J. H. Drosophila UDP-glucose:glycoprotein glucosyltransferase: sequence and characterization of an enzyme that distinguishes between denatured and native proteins. EMBO J. 1995 Apr 3;14(7):1294–1303. doi: 10.1002/j.1460-2075.1995.tb07115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi A. J., Behrens N. H., Leloir L. F., Carminatti H. The role of polyprenol-bound saccharides as intermediates in glycoprotein synthesis in liver. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3268–3272. doi: 10.1073/pnas.69.11.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi A. J., Mendelzon D. H., Lederkremer G. Z., Martin-Barrientos J. Evidence that transient glucosylation of protein-linked Man9GlcNAc2, Man8GlcNAc2, and Man7GlcNAc2 occurs in rat liver and Phaseolus vulgaris cells. J Biol Chem. 1984 May 25;259(10):6351–6357. [PubMed] [Google Scholar]
- Parodi A. J., Mendelzon D. H., Lederkremer G. Z. Transient glucosylation of protein-bound Man9GlcNAc2, Man8GlcNAc2, and Man7GlcNAc2 in calf thyroid cells. A possible recognition signal in the processing of glycoproteins. J Biol Chem. 1983 Jul 10;258(13):8260–8265. [PubMed] [Google Scholar]
- Partaledis J. A., Berlin V. The FKB2 gene of Saccharomyces cerevisiae, encoding the immunosuppressant-binding protein FKBP-13, is regulated in response to accumulation of unfolded proteins in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5450–5454. doi: 10.1073/pnas.90.12.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S., Xu Y., Brenner M. B. Retention of unassembled components of integral membrane proteins by calnexin. Science. 1994 Jan 21;263(5145):387–390. doi: 10.1126/science.8278814. [DOI] [PubMed] [Google Scholar]
- Ray M. K., Yang J., Sundaram S., Stanley P. A novel glycosylation phenotype expressed by Lec23, a Chinese hamster ovary mutant deficient in alpha-glucosidase I. J Biol Chem. 1991 Dec 5;266(34):22818–22825. [PubMed] [Google Scholar]
- Rokeach L. A., Jannatipour M., Haselby J. A., Hoch S. O. Primary structure of a human small nuclear ribonucleoprotein polypeptide as deduced by cDNA analysis. J Biol Chem. 1989 Mar 25;264(9):5024–5030. [PubMed] [Google Scholar]
- Rose M. D., Misra L. M., Vogel J. P. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989 Jun 30;57(7):1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- Sousa M. C., Ferrero-Garcia M. A., Parodi A. J. Recognition of the oligosaccharide and protein moieties of glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. Biochemistry. 1992 Jan 14;31(1):97–105. doi: 10.1021/bi00116a015. [DOI] [PubMed] [Google Scholar]
- Sousa M., Parodi A. J. The molecular basis for the recognition of misfolded glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. EMBO J. 1995 Sep 1;14(17):4196–4203. doi: 10.1002/j.1460-2075.1995.tb00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta S. E., Bosch M., Parodi A. J. Glucosylation of glycoproteins by mammalian, plant, fungal, and trypanosomatid protozoa microsomal membranes. Biochemistry. 1989 Oct 3;28(20):8108–8116. doi: 10.1021/bi00446a022. [DOI] [PubMed] [Google Scholar]
- Trombetta S. E., Gañan S. A., Parodi A. J. The UDP-Glc:glycoprotein glucosyltransferase is a soluble protein of the endoplasmic reticulum. Glycobiology. 1991 Mar;1(2):155–161. doi: 10.1093/glycob/1.2.155. [DOI] [PubMed] [Google Scholar]
- Tuite M. F., Bossier P., Fitch I. T. A highly conserved sequence in yeast heat shock gene promoters. Nucleic Acids Res. 1988 Dec 23;16(24):11845–11845. doi: 10.1093/nar/16.24.11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware F. E., Vassilakos A., Peterson P. A., Jackson M. R., Lehrman M. A., Williams D. B. The molecular chaperone calnexin binds Glc1Man9GlcNAc2 oligosaccharide as an initial step in recognizing unfolded glycoproteins. J Biol Chem. 1995 Mar 3;270(9):4697–4704. doi: 10.1074/jbc.270.9.4697. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Tector M., Salter R. D. Calnexin recognizes carbohydrate and protein determinants of class I major histocompatibility complex molecules. J Biol Chem. 1995 Feb 24;270(8):3944–3948. doi: 10.1074/jbc.270.8.3944. [DOI] [PubMed] [Google Scholar]