Abstract
Chloroquine (CQ) is exploited in clinical trials as an autophagy blocker to potentiate anticancer therapy, but it is unknown if it solely acts by inhibiting cancer cell-autonomous autophagy. Our recent study shows that besides blocking cancer cell growth, CQ also affects endothelial cells (ECs) and promotes tumor vessel normalization. This vessel normalizing effect of CQ reduces tumor hypoxia, cancer cell intravasation, and metastasis, while improving the delivery and response to chemotherapy. By compromising autophagy in melanoma cells or using mice with a conditional knockout of ATG5 in ECs, we found that the favorable effects of CQ on the tumor vasculature do not rely on autophagy. CQ-induced vessel normalization relies mainly on altered endolysosomal trafficking and sustained NOTCH1 signaling in ECs. Remarkably these CQ-mediated effects are abrogated when tumors are grown in mice harboring EC-specific deletion of NOTCH1. The autophagy-independent vessel normalization by CQ leading to improved delivery and tumor response to chemotherapy further advocates its clinical use in combination with anticancer treatments.
Keywords: anti-cancer therapy, ATG5, autophagy, chloroquine, clinical trials, melanoma, NOTCH1, vessel normalization
One of the latest issues of Autophagy, dedicated to translational or clinical research, highlighted the promising anticancer therapy potentiating effects of CQ or hydroxychloroquine (HCQ) emerging from ongoing clinical trials. CQ, an old antimalarial agent with acceptable safety profile and recognized evidence of efficacy, is currently being tested in multiple clinical trials for numerous late-stage cancers including breast, lung, prostate, and melanoma, either as monotherapy or combined with other anticancer modalities. CQ is a weak base that accumulates in acidic organelles, for example, late endosomes and lysosomes, where it affects fusion events and disrupts degradation by alkalinizing these compartments, thus compromising autophagosome turnover.
The propagation of CQ/HCQ in clinical trials is based on the assumption that when malignant cells become reliant on autophagy for survival and growth, CQ/HCQ will sensitize cancer cells to killing by anticancer treatments by curtailing autophagy. In support of this, a plethora of in vitro studies have validated the autophagy-inhibitory efficacy of CQ, and its ability to phenocopy the effects of more specific approaches, obtained by knocking down or knocking out essential autophagy genes, in vivo. However, a concern has been growing during recent years in the autophagy community that besides inhibiting autophagy in cancer cells, CQ may also (or perhaps mainly) exert its anticancer effects through autophagy-independent functions. Through blockage of lysosomal functions and endosomal trafficking, CQ could indeed have other anticancer effects, but their nature remained largely unidentified. Moreover, current efforts to explore the effects of CQ mainly focused on malignant cells, whereas it is becoming increasingly evident that the crosstalk between cancer and stromal cells influences tumor behavior and the responses to anticancer drugs.
Driven by these uncertainties, we asked whether CQ affects tumor growth by inhibiting only autophagy, and whether the cancer cells in the tumor stroma represent the major targets of the therapeutic effects of CQ. Using melanoma, a known autophagy-sustained cancer model, we found that CQ treatment of tumor-bearing mice dose-dependently decreases tumor growth, in line with previous reports. Curiously, however, we noticed that CQ fails to affect primary tumor growth, at doses that still blunt metastasis. When analyzing the tumor stroma, we observed that CQ treatment not only ameliorates the tumor micro-environment and reduces tumor hypoxia, but also decreases cancer cell invasion, intravasation, and dissemination. Furthermore, CQ improves the abnormal structure and function of tumor blood vessels, through a process known as ‘vessel normalization.’ At the structural level, CQ reduces vessel density and tortuosity and improves endothelial cell alignment and tight junction formation. This CQ-induced vessel normalization effect ameliorates tumor vessel function, characterized by improved perfusion, reduced leakiness and decreased intratumoral hypoxia, properties that are accompanied by reduced cancer cell intravasation, increased drug delivery, and enhanced chemotherapy response.
But are these effects of CQ reliant on autophagy inhibition in the cancer cells and/or endothelial cells? To address these relevant questions we genetically interfered with either melanoma cell- or EC-intrinsic autophagy by repressing ATG5 expression in these cells. We observed that compromising melanoma cell-autophagy blunts tumor growth and dissemination at steps of the metastatic cascade downstream to tumor cell intravasation but, most importantly, fails to induce tumor vessel normalization. Strikingly, we found that growing melanoma in mice harboring an EC-specific Atg5 knockout evoked a cancer phenotype hallmarked by a tumor vasculature displaying increased—not decreased—structural and functional abnormalities. These observations altogether demonstrate that the improvement of the tumor vasculature and tumor microenvironment observed after systemic CQ treatment occur largely through autophagy-independent effects.
Which are then the molecular mechanisms underpinning the tumor vasculature normalizing effects of CQ? We focused on lysosomal function and angiogenic receptor trafficking in ECs after CQ treatment in vitro. We found that the enlarged and disturbed late endosomal/lysosomal compartments caused by CQ-treatment result in altered endosomal trafficking and sustained signaling/transcriptional activity of NOTCH1 in ECs. Intriguingly, none of these CQ-mediated effects on NOTCH1 are phenocopied by inhibiting ATG5-mediated autophagy in ECs, again indicating a model whereby CQ affects the EC phenotype through a NOTCH1-reliant, autophagy-dispensable mechanism.
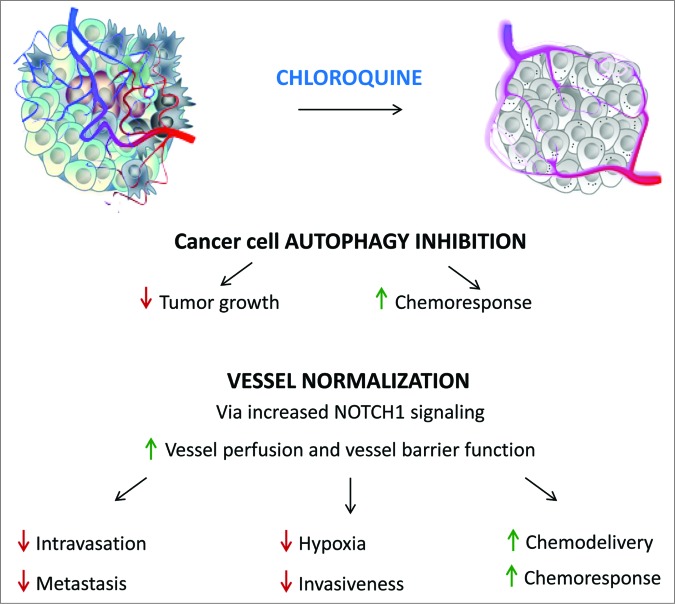
Is NOTCH1 a bona fide target of CQ in vivo? To answer this question we implanted melanoma tumors in mice lacking Notch1 in ECs. Remarkably, both CQ-induced vessel normalization and antimetastatic effects are ablated in these animals, thus validating the vital role of NOTCH1 for the antitumor effects of CQ in vivo. Considering that vessel normalization rather than pruning is emerging as an increasingly important therapeutic goal, especially for its ability to lower intratumoral hypoxia, and the lack of clinically available ‘vessel normalizing’ agents, the effects of CQ are appealing and urge further clinical validation. Moreover, as tumor hypoxia is known to boycott antitumor immunity, CQ, in spite of its mild immunosuppressant activity, may ultimately facilitate immune responses, a conjecture that requires further analysis. Considering that metastasis is the main cause of a cancer patient's death, the improved barrier function of a normalized tumor vasculature, impeding cancer cell dissemination, observed after CQ treatment, is auspicious. In the future, the dual effects of CQ as cancer cell autophagy-inhibitor and vessel-normalizing agent, as depicted in Figure 1, should be considered when developing protocols and CQ-based therapeutic regimens.
Figure 1.
Chloroquine normalizes tumor vasculature and blocks cancer cell-autophagy. The figure schematically illustrates the dual targeting of the tumor by chloroquine. On the one hand, CQ blocks autophagy in cancer cells, thereby reducing cancer cell proliferation and increasing therapeutic response. On the other hand, CQ normalizes the tumor vasculature, thereby reducing cancer cell intravasation, metastasis, tumor invasiveness and hypoxia, and improving chemodelivery and chemoresponse. Please refer to the text for further explanation.
Chloroquine, one of the oldest drugs still used in practice today, reveals its secrets.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.