Abstract
Tafazzin (TAZ) is a phospholipid transacylase that catalyzes the remodeling of cardiolipin, a mitochondrial phospholipid required for oxidative phosphorylation. Mutations of TAZ cause Barth syndrome, which is characterized by mitochondrial dysfunction and dilated cardiomyopathy, leading to premature death. However, the molecular mechanisms underlying the cause of mitochondrial dysfunction in Barth syndrome remain poorly understood. Here we investigated the role of TAZ in regulating mitochondrial function and mitophagy. Using primary mouse embryonic fibroblasts (MEFs) with doxycycline-inducible knockdown of Taz, we showed that TAZ deficiency in MEFs caused defective mitophagosome biogenesis, but not other autophagic processes. Consistent with a key role of mitophagy in mitochondria quality control, TAZ deficiency in MEFs also led to impaired oxidative phosphorylation and severe oxidative stress. Together, these findings provide key insights on mitochondrial dysfunction in Barth syndrome, suggesting that pharmacological restoration of mitophagy may provide a novel treatment for this lethal condition.
Keywords: autophagy, Barth syndrome, cardiolipin, mitochondrial dysfunction, mitophagosome, mitophagy, tafazzin
Abbreviations
- AdGFP-LC3
recombinant adenovirus expressing GFP tagged MAP1LC3B
- AdTAZ
recombinant adenovirus expressing Myc-tagged TAZ
- BafA1
bafilomycin A1
- BTHS
Barth syndrome
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
- CL
cardiolipin
- Dox
doxycycline
- FCCP
carbonyl cyanide p-triflouromethoxyphenylhydrazone
- LTG
LysoTracker Green
- MAP1LC3B/LC3B
microtubule-associated protein 1 light chain 3 beta
- MEF
mouse embryonic fibroblast
- MLCL
monolysocardiolipin
- MTR
MitoTracker Red
- PARK2
parkin RBR E3 ubiquitin protein ligase
- PINK1
PTEN-induced putative kinase 1
- SOD2
superoxide dismutase 2 mitochondrial
- TAZ
tafazzin
- TLCL
tetralinoleoyl-cardiolipin
Introduction
Cardiolipin (CL) is a mitochondrial phospholipid present both in ATP-producing inner membrane as well as in the outer membrane of mitochondria.1 CL plays a critical role in maintaining respiratory chain enzyme function, membrane stability, and mitochondrial dynamics.2-4 CL is synthesized exclusively in the mitochondria inner membrane as immature CL and is remodeled into mature CL with 4 linoleolic acid chains (C18:2), or tetralinoleoyl-cardiolipin (TLCL).5-7 Depletion of linoleolic acid content from CL is associated with aging and various aging-related diseases.7-9 In addition to modulating bioenergetics, CL is required in many steps of mitochondrial quality control processes, from fusion/fission dynamics to mitophagy. Recent studies have implicated CL in selective autophagy of mitochondria, a critical step in maintaining mitochondrial quality control and cell survival by removing and preventing the accumulation of damaged mitochondria.10 Defective mitophagy causes inefficient mitochondrial respiration and a high level of oxidative stress.11 Additionally, CL can promote membrane fusion critical for normal mitochondrial dynamics as a non-bilayer forming lipid, modulate the activities of autophagy-related proteins, and mediate mitochondria-lysosome interactions.10,12-14 Furthermore, externalized CL serves as a signal for mitophagy, allowing autophagic proteins to recognize and engulf dysfunctional mitochondria for degradation.10
Barth syndrome (BTHS) is a lethal, X-linked, disease whose characteristic defect is CL deficiency and alteration of CL species as a result of mutation of the Tafazzin (TAZ) gene.15-17 The resulting deficiency in mature CL and accumulation of monolysocardiolipin (MLCL) is believed to cause mitochondrial dysfunction and, consequentially, cardioskeletal myopathy, neutropenia, and growth defects.15 Previous studies have reported the accumulation of structurally abnormal mitochondria with inefficient oxidative phosphorylation in BTHS patient tissues and animal models of TAZ depletion, leading to increased levels of reactive oxygen species.18-21
The molecular mechanisms by which TAZ deficiency and accumulation of MLCL in BTHS cause mitochondrial dysfunction remain poorly defined.3,22,23 The persistence of an increased number of abnormal mitochondria suggests that the clearance of damaged mitochondria via mitophagy is impaired in BTHS patients and experimental models, in addition to defects in mitochondrial function.18,24 We hypothesize that TAZ is critical to mitochondrial quality control, and that the deficiency of TAZ as seen in BTHS results in a reduced capacity to initiate degradation of dysfunctional mitochondria. Our results show that Taz knockdown inhibits initiation of mitophagy and leads to impaired oxidative phosphorylation and oxidative stress.
Results
Inducible TAZ depletion results in defective mitophagy in MEFs
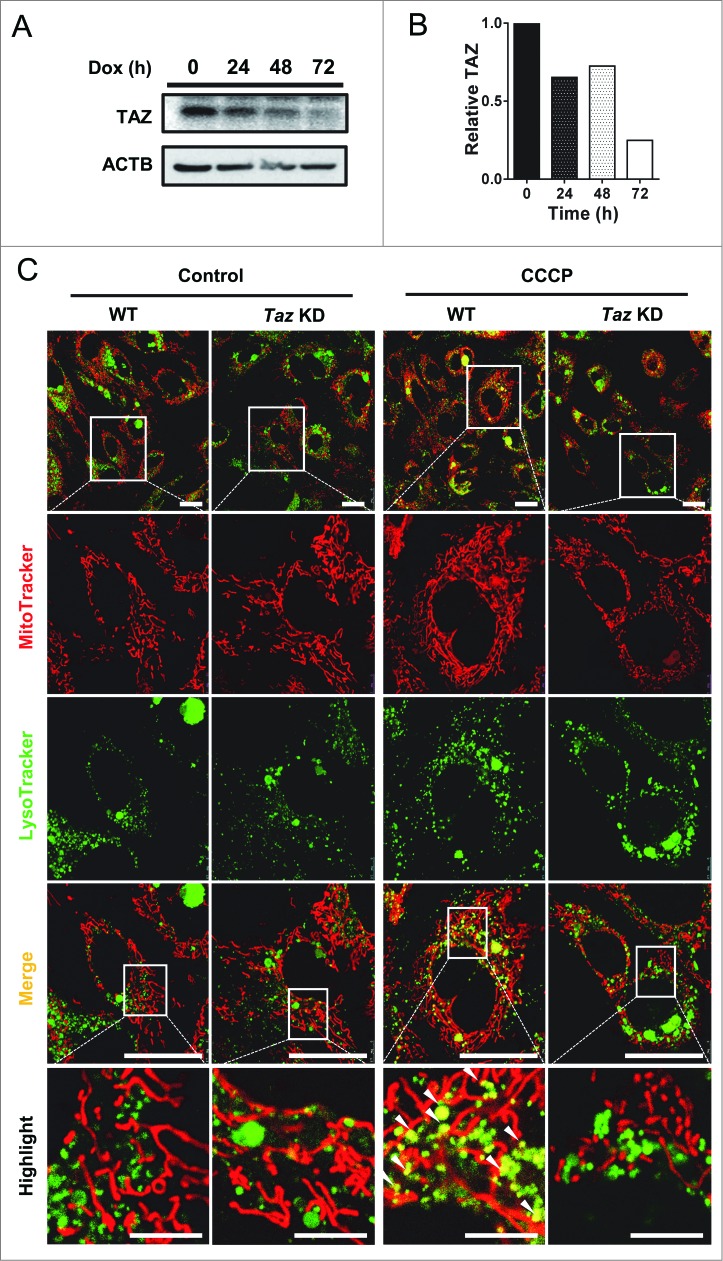
Using primary mouse embryonic fibroblasts (MEFs) isolated from mice with transgenic expression of doxycycline (Dox)-inducible shRNA targeted to Taz mRNA (Taz KD),25 we investigated the role of TAZ depletion in regulating the onset of mitophagy. This approach overcomes the growth defect and compensatory metabolic changes commonly associated with immortalized fibroblasts derived from BTHS patients. We first analyzed the efficiency of Dox-induced depletion of endogenous TAZ in MEFs by western blot analysis, which showed a time-dependent depletion of endogenous TAZ protein in response to Dox induction (Fig. 1A, quantified in Fig. 1B).
Figure 1.
TAZ deficiency impairs the formation of mitophagosomes. (A) Primary MEFs from Taz KD mice and WT controls were cultured in medium supplemented with doxycycline (Dox) to induce knockdown of Taz, followed by western blot analysis of endogenous TAZ expression in Taz KD MEFs in response to Dox induction at 0, 24, 48, or 72 h using ACTB/β-actin as internal control. (B) Quantification of relative expression level of TAZ after normalization with ACTB in panel (A). (C) After 72 h of induction with Dox, cells were treated with vehicle (control) or CCCP, stained with MitoTracker Red and LysoTracker Green, and imaged by confocal microscopy. Quantitative analysis of MitoTracker-LysoTracker colocalization is presented in Fig. S1A. White arrowheads highlight colocalization of mitochondria and lysosomes. Scale bars: 7.5 μm for highlighted images, and 25 μm for the rest of the images.
To determine the effect of TAZ deficiency on mitophagosome biogenesis, the primary MEFs from Taz KD mice and wild-type (WT) controls were stained with MitoTracker Red and LysoTracker Green, followed by confocal imaging of mitochondria, lysosomes, and colocalization of both markers as an indicator of mitophagosome biogenesis. The results showed that WT and Taz KD MEFs were indistinguishable in mitochondrial and lysosomal staining under basal conditions (Fig. 1C). In contrast, TAZ depletion significantly impaired the formation of mitophagosomes when compared with MEFs from WT control mice in response to treatment with carbonyl cyanide m-chlorophenylhydrazone (CCCP) which was used to induce mitophagy, suggesting that TAZ is required for mitophagy (highlighted by arrows, Fig. 1C, quantified in Fig. S1A). The defect is not caused by the transgene, since there are no significant differences between WT and Taz KD MEFs in mitophagy in the absence of Dox induction (Fig. S2). The results suggest 2 possible causes for the lack of mitophagosomes in Taz KD. The first scenario is that TAZ deficiency could cause a defect in autophagosome biogenesis, because mitochondrial phospholipids have been shown to be a source for nascent autophagosomal membranes.26 Alternatively TAZ deficiency could cause a defective initiation of mitophagy, since CL is implicated in the recognition of damaged mitochondria.10
TAZ deficiency does not alter autophagosome biogenesis
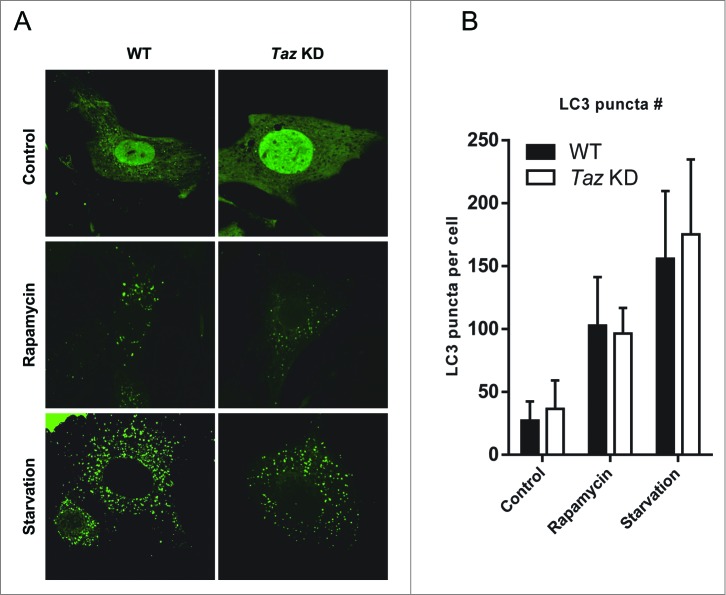
To test the first scenario, we used recombinant adenoviruses expressing GFP-LC3B fusion protein (AdGFP-LC3) to track the number of LC3 puncta as a surrogate for autophagosome biogenesis. We found that under basal conditions, there were very few, if any, LC3 puncta formed in MEF cells (Fig. 2A). In contrast, both WT and Taz KD MEFs exhibited significantly higher number of LC3 puncta in response to treatment of rapamycin or starvation, which were used to induce autophagosome formation. However, the results showed that WT and Taz KD MEFs had similar numbers of LC3 puncta in each condition (quantified in Fig. 2B), implicating that TAZ is not required for autophagosome biogenesis.
Figure 2.
TAZ deficiency does not alter autophagy under basal and starvation conditions. (A) Primary MEFs from Taz KD mice and WT controls were cultured in medium supplemented with doxycycline for 72 h to induce knockdown of Taz. The cells were infected with recombinant adenoviruses expressing GFP-LC3B fusion protein (AdGFP-LC3), treated with culture medium in absence (Control) or presence of 100 nM rapamycin, or 1x HBSS (starvation) for 2 h, and analyzed for LC3 puncta by confocal imaging. (B) Quantitative analysis of LC3 puncta in panel (A) using ImageJ (n > 50, mean ± SD).
TAZ is required for the initiation of mitophagy
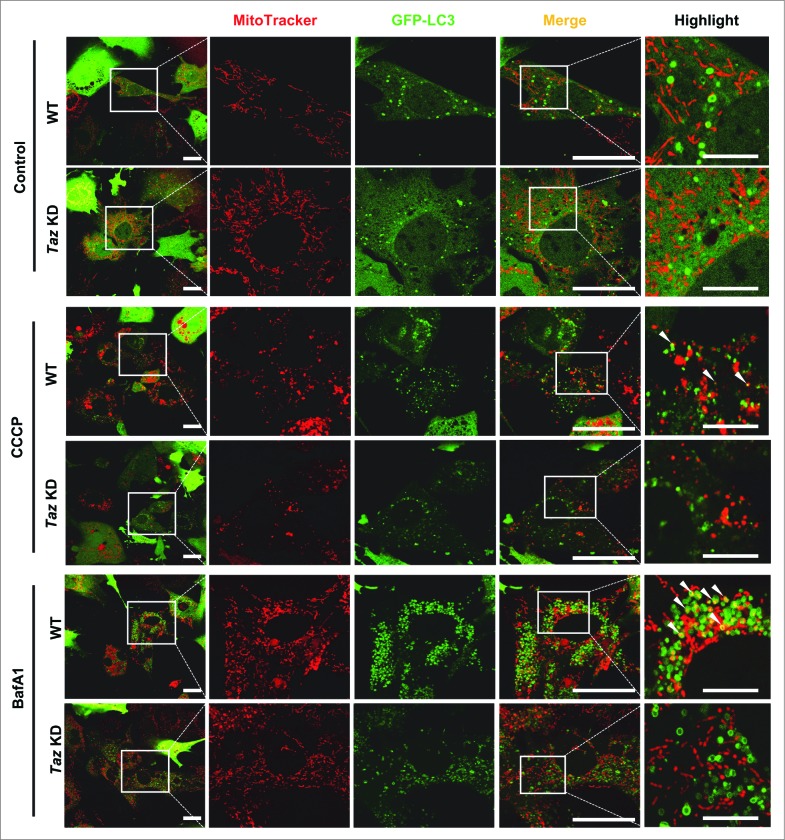
We tested the alternative scenario whether the defective mitophagy in Taz KD MEFs was caused by defective initiation of mitophagy. WT and Taz KD MEFs were infected with AdGFP-LC3 recombinant adenoviruses, stained with MitoTracker Red, starved with Hank's balanced salt solution (HBSS) supplemented with CCCP or bafilomycin A1 (BafA1), and analyzed for colocalization of LC3 puncta with mitochondria (Fig. 3, yellow color, highlighted by arrows) as a surrogate for mitophagosomes. Consistent with our observation in Figure 2, there were no significant differences between WT and Taz KD in autophagosome biogenesis, as evidenced by a similar number of LC3 puncta (quantified in Fig. S1B) under the control condition. Additionally, there were very few mitophagosomes in both groups in response to starvation alone. In contrast, WT MEFs exhibited a significant increase in mitophagosomes in response to treatment with CCCP (highlighted by arrows in Fig. 3, quantified in Fig. S1C and S1D). Furthermore, WT MEFs exhibited a dramatic increase in the number of mitophagosomes in response to treatment with BafA1, which prevents autophagic consumption of mitophagosomes by inhibiting fusion between autophagosomes and lysosomes. Strikingly, TAZ deficiency caused a dramatic decrease in the formation of mitophagosomes in response to treatment with CCCP, which is more pronounced in response to treatment with BafA1.
Figure 3.
TAZ deficiency results in selective decrease in the formation of mitophagosomes. After induction with Dox for 72 h, WT and Taz KD MEFs were infected with AdGFP-LC3, stained with MitoTracker Red (50 nM, 15 min), starved in HBSS medium in the absence (control) and presence of 20 μM CCCP (CCCP) or 100 nM bafilomycin A1 (BafA1), followed by confocal imaging analysis. Quantitative analysis of the number of LC3 puncta, and LC3-MitoTracker Red colocalization is presented in Figure S1B to D. White arrows highlight mitophagosomes. Scale bars: 25 μm, and 7.5 μm for highlighted images.
Phosphorylation of PARK2/Parkin by PTEN-induced putative kinase 1 (PINK1) plays an important role in the initiation of mitophagy.27 We next tested whether the defective mitophagy in TAZ deficient MEFs was due to changes in PINK1 or PARK2 protein levels, since depolarized mitochondria are recognized by a PINK1 and PARK2 dependent mechanism.28 WT and Taz KD MEFs were treated with BafA1, with or without CCCP to measure the accumulation of PINK1 and PARK2 protein levels. Although Taz KD MEFs showed a slight reduction in the levels of PINK1 in Taz KD compared to WT MEFs, there were no significant changes in levels of total PARK2 (Fig. S3A). Consistent with the previous report, CCCP treatment stabilized PINK1 levels in both WT and Taz KD MEFs. Taken together, the results suggest that defective mitophagy in Taz KD MEFs was primarily caused by a failure of recognition of mitophagosomes by LC3 vesicles, which is consistent with previous reported role of CL in autophagic initiation.10
Exogenous TAZ rescues defective mitophagosome formation
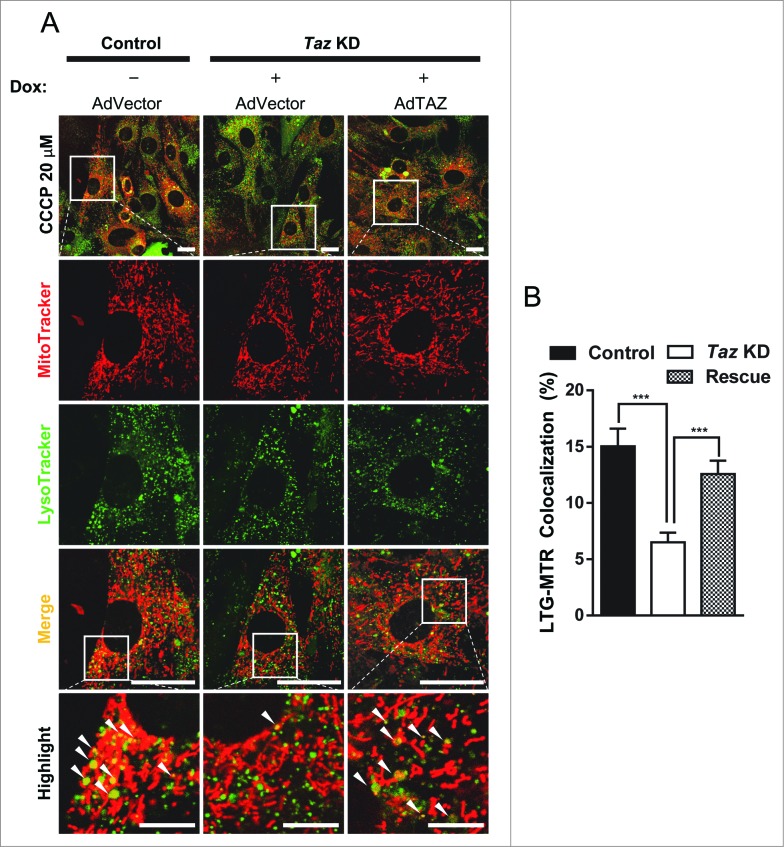
Although the Taz KD transgenic mice have previously been used in other studies,25 it remains a possibility that the shRNA could have off-target activity. To address this issue, we next asked whether exogenous Taz could rescue the defect in mitophagy. We constructed an adenovirus expressing Myc-TAZ fusion protein (AdTAZ) to deliver the expression of exogenous TAZ to the Taz KD MEFs. Western blot analysis of Taz KD MEFs transfected with AdTAZ or an empty vector adenovirus showed that in Taz KD MEFs, AdTAZ was able to restore TAZ protein levels to approximately 46% of WT levels (Fig. S3C). We next infected Taz KD and control MEFs with AdTAZ or an empty vector adenovirus. The infected Taz KD MEFs were cultured in the presence of Dox, followed by analysis of mitophagy. As shown in Figure 4, exogenous TAZ were able to rescue the decrease in mitophagosome formation seen with Taz KD MEFs (quantified in Fig. 4B), despite the observation that TAZ protein levels were not completely restored to WT levels. The results suggest that depletion of TAZ was directly responsible for defective mitophagy in Taz KD MEFs.
Figure 4.
TAZ expression with recombinant adenovirus rescues the defect in autolysosome formation. (A) Primary MEFs from Taz KD mice and WT controls were cultured in medium supplemented with or without doxycycline (Dox) for 24 h, followed by infection with recombinant adenovirus expressing Myc-TAZ (AdTAZ, rescue) or an empty vector control (AdVector) in the presence of Dox, treated with 20 μM CCCP, and analyzed by confocal imaging. (B) Quantification of colocalization of lysosomes and mitochondria of the experiment in panel A, n > 31. White arrowheads highlight colocalization of mitochondria and lysosomes. Scale bars: 7.5 μm for highlighted images, and 25 μm for the rest of the images. All values are mean ± SEM. ***P < 0.0001.
TAZ deficiency leads to oxidative stress and mitochondrial dysfunction
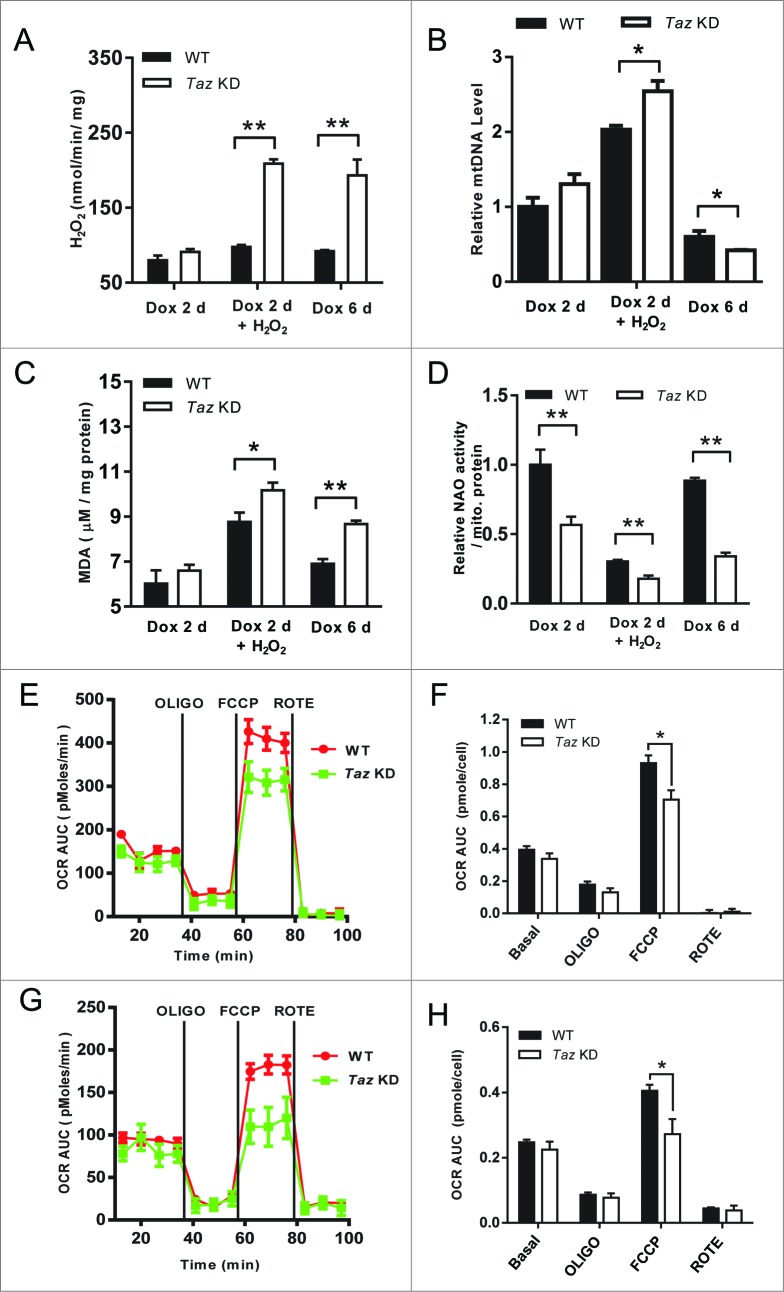
Mitophagy is required for mitochondrial quality control.29 The impaired mitophagy in Taz KD MEFs prompted us to investigate the consequences of TAZ deficiency on mitochondrial dysfunction in response to oxidative stress. The results showed that 48-h induction of TAZ depletion led to severe oxidative stress (Fig. 5A) and lipid peroxidation (Fig. 5C), which were exacerbated in response to treatment with H2O2. TAZ deficiency also significantly increased mitochondrial DNA copy number (Fig. 5B), likely as a compensatory response to depletion of CL, as evidenced by decreased staining of the fluorescent dye 10-N-nonyl acridine orange, a cardiolipin specific dye which is commonly used to monitor CL level in live cells (Fig. 5D). Interestingly, all the defects were further exacerbated with extended Dox-induction without the treatment with H2O2, suggesting TAZ depletion was the primary cause for oxidative stress. Consistent with increased oxidative stress, TAZ deficiency significantly decreased the expression of SOD2, a mitochondria-specific superoxide dismutase that plays a key role in antioxidative stress response (Fig. S3B). Consequently, TAZ deficiency significantly impaired mitochondrial respiration both at early (Fig. 5E, quantified in Fig. 5F) and late passages (Fig. 5G, quantified in Fig. 5H), as evidenced by decreased oxygen consumption rate in response to treatment with carbonyl cyanide p-triflouromethoxyphenylhydrazone (FCCP), a mitochondrial uncoupler commonly used to measure maximum respiration. The results are consistent with the recent study on induced pluripotent stem cell-derived cardiomyocytes demonstrating a large reduction in respiratory capacity from BTHS patients.21
Figure 5.
TAZ deficiency exacerbates oxidative stress, leading to mitochondrial dysfunction. (A to D), After 48 h (2 d) or 144 h (6 d) induction with Dox, WT, and Taz KD MEFs were treated with either medium alone (control) or supplemented with 0.5 mM H2O2 for 4 h, followed by analysis of mitochondrial H2O2 production by Amplex Red assay (A), mitochondrial DNA (mtDNA) copy number by qPCR analysis (B), lipid peroxidation level in the form of malondialdehyde (MDA) by TBARS assay (C), and relative CL level by staining with 10-N-nonyl-acridine orange (NAO) (D). (E, G) Analysis of mitochondrial oxygen consumption rate (OCR) in WT and Taz KD MEFs in passage 2 and 5, respectively, in response to treatment with oligomycin, FCCP, and rotenone with a Seahorse XF24 Flux Analyzer. (F, H) Quantitative analysis of area under the curve (AUC) of the data presented in panels (E and G). All values are mean ± SEM. *P < 0.05. **P < 0.001.
Discussion
BTHS is a clinically devastating disease caused by mutations in the TAZ gene, and resulting in CL depletion, accumulation of dysfunctional mitochondria, and early lethality.15,17 However, the observed accumulation of swollen and abnormal mitochondria that implicates defective mitochondrial quality control as a key disease mechanism has not yet been explored. Here we report in primary cell cultures that mitophagy is severely diminished in TAZ-deficient cells compared to WT cells. We found that basal and depolarization-stimulated levels of mitophagy were reduced, as assessed by colocalization of LC3 puncta with mitochondria. We provide evidence that suggests, for the first time, that defective mitophagy may be one of the underlying mechanisms that is permissive for the severe mitochondrial dysfunction seen in BTHS patients and rodent models of BTHS. However, it should be noted that defective mitophagy may represent one of several molecular mechanisms by which TAZ deficiency causes mitochondrial dysfunction, since CL is required for dynamic biological processes of mitochondria, from the assembly of respiratory chain supercomplexes, to the function of various inner and outer membrane proteins, and for mitochondrial biogenesis.1,8,30-32 Additionally, alteration in acyl chain compositions away from TLCL reduces efficiency of respiratory complex components,33,34 whereas the loss of TLCL as a result of TAZ mutations destabilizes respiratory chain supercomplexes in BTHS patients.35
Mitophagy is a critical aspect of mitochondrial quality control along with fusion/fission dynamics that permits clearance of damaged and dysfunctional organelles.36 We showed that mitochondria from TAZ-deficient MEFs have significantly greater mitochondrial H2O2 production and lipid peroxidation in response to oxidative stress. This suggests that TAZ-deficient cells are more sensitive to the “vicious cycle” of reactive oxygen species-induced injury and, when paired with defective mitophagy, results in cells retaining damaged mitochondria that would have otherwise been degraded. While depolarized mitochondria are typically targeted for degradation via a PINK1 and PARK2 dependent pathway,37 mitochondria with extremely low membrane potentials are present and retained in cells derived from BTHS patients.38 In line with a defect in mitochondrial quality control, TAZ-deficient MEFs exhibited significantly decreased mitochondrial respiratory capacity that worsened with increasing cell passage. This is consistent with a recent study of BTHS patients that show diminished O2 consumption in muscle and impaired cardiac reserve resulting in exercise intolerance.39 Our findings suggest that a defect in mitophagy, apart from respiratory defects arising from TLCL deficiency, may underlie the pathophysiology seen in BTHS patients.
One of the major unresolved issues in autophagy research is the origin of the phagophore membrane for autophagosome initiation. Phospholipids extracted from mitochondria were believed to provide the major source of lipids for autophagic membranes, and the outer mitochondrial membrane has been shown to contain up to 25% of the total mitochondrial CL.26 Our inducible TAZ depletion model affords us the opportunity to test this hypothesis, since mutation of TAZ depletes CL.17 Surprisingly, our findings indicate that TAZ is not required for autophagosome biogenesis. Furthermore, in contrast to previous findings which suggested that CL is required for various stages of autophagy, 10,12-14 our data demonstrate that TAZ is specifically required for the initiation of mitophagy, and TAZ depletion did not impair other autophagic processes. However, key questions remain on how TAZ regulates the initiation of mitophagy. One possible explanation is provided by a recent study by Chu, et al.10 They show that externalization of CL from mitochondrial inner membrane to outer membrane surface serves as a recognition signal that directs damaged mitochondria to mitophagy.10 They further show that TLCL, but not MLCL, directly bind to LC3, which is required for cargo recognition by the autophagic system. Consistent with those findings, our recent report also shows that increased TLCL content significantly stimulates mitophagy.9 Together, our findings identified a pivotal role of TAZ in regulating the initiation of mitophagy in primary MEFs, which is required for mitochondrial quality control process, as depicted by a model in Fig. 6. More importantly, our data suggest that pharmacological restoration of mitophagy, but not autophagy per se, may provide a novel treatment for Barth syndrome, a debilitating disease that leads to premature death.
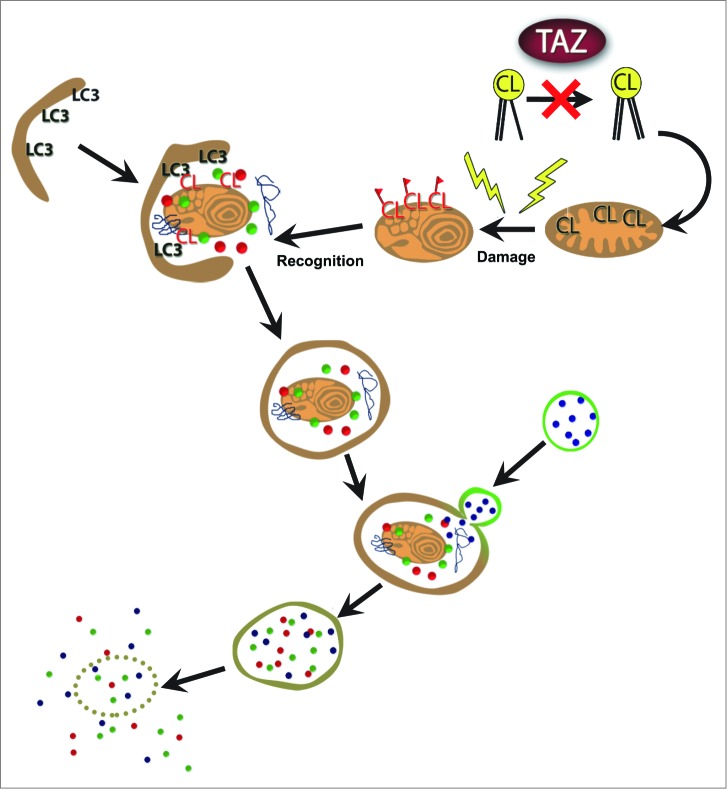
Figure 6.
Proposed mechanism for mitochondrial dysfunction in BTHS. Mutations in TAZ result in defective transacylation and depletion of CL. Loss of CL reduces externalized CL as a signal for mitophagy, leading to accumulation of damaged and dysfunctional mitochondria.
Materials and Methods
Mouse embryonic fibroblast (MEF) isolation, culture, and genotyping
All procedures involving animals were done in accordance with approval of institutional animal care and use protocols according to NIH guidelines (NIH publication No. 86-23, 1985). MEFs were isolated at E12.5-13.5 from the time of copulation of transgenic ROSA26H1/tetO-shRNA:taz(Taz KD) mice. MEFs were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 1x nonessential amino acids, 1x essential amino acids, 100 U/mL penicillin-streptomycin, and 1.25 μg/mL amphotericin B. MEFs were genotyped by PCR analysis of embryonic tissue using primers and conditions previously described.25
Induction of Taz knockdown and autophagy
All experiments used Taz KD MEFs and wild-type (WT) littermate MEFs as controls, unless otherwise specified. Induction of Taz knockdown was achieved by supplementing culture medium with doxycycline (1 μg/mL) for 72 h prior to experimental manipulations. For autophagic induction and analysis, MEFs were treated with Hank's balanced salt solution (HBSS, 2 h), rapamycin (100 nM, 2 h), bafilomycin A1 (BafA1, 100 nM, 2 h), 20 μM carbonyl cyanide m-chlorophenyl hydrazine (CCCP, 20 μM, 4 h), or hydrogen peroxide, (H2O2, 500 μM, 2 h) unless otherwise stated.
Generation of TAZ adenovirus
TAZ adenovirus (AdTAZ) was generated using the AdEasy system with pAdTrack-CMV plasmid system as previously described.40 Briefly, MYC-tagged TAZ was inserted into pAdTrack-CMV and transformed into BJ5183 competent cells by heat-shock. The adenovirus was propagated using HEK 293A cell lines, and collected by repeated freeze-thaw cycles followed by centrifugation to remove cell debris. Cells were infected 48 h prior to experimental manipulations.
Confocal imaging
MEFs were stained with MitoTracker Red CMXRos (50 nM, 15 min, prior to CCCP treatment) to visualize mitochondria, LysoTracker Green DND-26 (50 nM) to visualize lysosomes, or transfected with adenovirus expressing GFP-LC3 for 24 h to visualize LC3 puncta. Images were captured using a Leica TSC SP8 × white light laser confocal microscope (Leica Microsystems, Wetzlar, Germany), a 63×/1.4 NA oil immersion objective at 1024 × 1024 resolution with excitation lines 488 nM and 578 nM, and Leica LAS AF software (Leica Microsystems, Wetzlar, Germany) unless otherwise stated. Multiple fields of view were selected at random and imaged for further analysis in ImageJ.
Oxidative stress and lipid peroxidation assays
The amount of reactive oxygen species generated from the mitochondria of MEFs was detected indirectly by quantitatively measuring H2O2 with Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit as previously described.9 Lipid peroxidation products in the MEFs were quantified by measuring the level of malondialdehyde using a TBARS kit according to the manufacturer's instructions.
Mitochondrial DNA (mtDNA) copy number assay
mtDNA copy number assays were performed as previously described 9. Analysis of mitochondrial copy number in MEFs was carried out using mitochondrion-encoded NADH dehydrogenase 1 (mt-Nd1) as the mtDNA marker and Ppia (peptidylprolyl isomerase A, also known as cyclophilin A) as a genomic marker. The primer pairs used included: mt-Nd1 (forward, 5′-TGACCCATAGCCATAATATGATTT-3′; reverse, 5′-CTCTACGTTAAACCCTGATACTAA-3′), and Ppia (forward, 5′-ACACGCCATAATGGCACTCC-3'; reverse, 5′-CAGTCTTGGCAGTGCAGAT-3′).
Mitochondrial respiration assay
Mitochondrial respiration assays were performed using the Seahorse XF24 analyzer (Seahorse Bioscience, North Billerica, MA), as described previously.7 Briefly, MEFs cells were seeded in XF24 cell culture microplates (10,000 cells/well) and cultured overnight. MEFs were treated sequentially with oligomycin (OLIGO, 1 μM), p-trifluoromethoxy carbonyl cyanide phenyl hydrazone (FCCP, 0.3 μM), and rotenone (ROTE, 1 μM). Oxygen consumption rates were monitored in real time, and the area under the oxygen consumption rates curve after each compound treatment was quantified.
10-N-nonyl-acridine orange (NAO) staining
NAO was used to bind non-oxidized cardiolipin in vitro. Mitochondria isolated from MEFs were fixed in fixative buffer (0.25 M sucrose, 10 mM Tris-HCl, and 1% formaldehyde) for 15 min and stained with NAO (100 nM) for 20 min, followed by washing with PBS (10 mM phosphate, pH 7.4) and measuring fluorescence at 485/530 nm.
Statistical analyses
Data are presented as the mean ± standard error mean (SEM) unless otherwise stated. Statistical analysis was performed using Prism 6.04 (GraphPad Software, Inc.). Statistical significance among groups was tested using the unpaired Student t test, and significance was defined as P < 0.05.
Reagents and antibodies
Cell culture reagents: amphotericin B (1.25 μg/mL; Invitrogen, 15290-018); bafilomycin A1 (BafA1; Tocris, 1334); carbonyl cyanide m-chlorophenylhydrazone (CCCP; Sigma, C2759); doxycycline (Dox; Sigma, D3447); Dulbecco's modified Eagle's medium (Sigma, D5796, lot RNBD4518); 50× essential animo acids (Invitrogen, 11130-051); fetal bovine serum (Atlanta Biologicals, S11550, lot E12070); Hank's balanced salt solution (HBSS; Corning, 21-023-CM, lot 21023091); 100x nonessential amino acids (Invitrogen, 11140-050); penicillin-streptomycin (100 U/mL; Invitrogen, 15140-163); phosphate-buffered saline PBS 10x (Corning, 46-013-CM, lot 46013042); rapamycin (Tocris, 1292).
Fluorescent markers
10-N-nonyl-acridine orange (NAO; Molecular Probes, A1372); MitoTracker Red CMXRos (Molecular Probes, M-7512); LysoTracker Green DND-26 (Molecular Probes, L-7526).
Mitochondria function and oxidative stress
carbonyl cyanide p-triflouromethoxyphenylhydrazone (FCCP; Sigma, C2920); oligomycin (Sigma, 75351); rotenone (Sigma, R8875); Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (Invitrogen, A22188); Thiobarbituric acid reactive substances (TBARS) kit (Cayman Chemical Company, 10009055).
Primary antibodies
ACTB (Sigma, A5441); GAPDH (Santa Cruz Biotechnology, sc-32233); PARK2 (Abcam, ab15954); PINK1 (Abnova, H00065018-A01); SOD2 (Santa Cruz Biotechnology, sc-30080); TAZ (gift from Dr. Steve Claypool, Johns Hopkins University).
Funding
This study was supported in part by grants from NIH (DK076685, YS) and Barth Syndrome Foundation (YS).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Gebert N, Joshi AS, Kutik S, Becker T, McKenzie M, Guan XL, Mooga VP, Stroud DA, Kulkarni G, Wenk MR, et al.. Mitochondrial cardiolipin involved in outer-membrane protein biogenesis: implications for Barth syndrome. Curr Biol 2009;19:2133-9; PMID:19962311; http://dx.doi.org/ 10.1016/j.cub.2009.10.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol 2007;292:C33-44; PMID:16899548; http://dx.doi.org/ 10.1152/ajpcell.00243.2006 [DOI] [PubMed] [Google Scholar]
- 3.Claypool SM, Koehler CM. The complexity of cardiolipin in health and disease. Trends Biochem Sci 2012; 37:32-41; PMID:22014644; http://dx.doi.org/ 10.1016/j.tibs.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlame M. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J Lipid Res 2008; 49: 1607–20; PMID:18077827; http://dx.doi.org/ 10.1194/jlr.R700018-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Y, Kelley RI, Blanck TJ, Schlame M. Remodeling of cardiolipin by phospholipid transacylation. J Biol Chem 2003; 278: 51380–5; PMID:14551214; http://dx.doi.org/ 10.1074/jbc.M307382200 [DOI] [PubMed] [Google Scholar]
- 6.Taylor WA, Hatch GM. Identification of the human mitochondrial linoleoyl-coenzyme A monolysocardiolipin acyltransferase (MLCL AT-1). J Biol Chem 2009; 284: 30360–71; PMID:19737925; http://dx.doi.org/ 10.1074/jbc.M109.048322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Romestaing C, Han X, Li Y, Hao X, Wu Y, Sun C, Liu X, Jefferson LS, Xiong J, et al.. Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell Metab 2010; 12: 154–65; PMID:20674860; http://dx.doi.org/ 10.1016/j.cmet.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Liu X, Wang H, Zhang W, Chan DC, Shi Y. Lysocardiolipin acyltransferase 1 (ALCAT1) controls mitochondrial DNA fidelity and biogenesis through modulation of MFN2 expression. Proc Natl Acad Sci U S A 2012; 109: 6975–80; PMID:22509026; http://dx.doi.org/ 10.1073/pnas.1120043109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Ye B, Miller S, Yuan H, Zhang H, Tian L, Nie J, Imae R, Arai H, Li Y, et al.. Ablation of ALCAT1 mitigates hypertrophic cardiomyopathy through effects on oxidative stress and mitophagy. Mol Cell Biol 2012; 32: 4493–504; PMID:22949503; http://dx.doi.org/ 10.1128/MCB.01092-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, et al.. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol 2013; 15: 1197–205; PMID:24036476; http://dx.doi.org/ 10.1038/ncb2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, et al.. PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell 2009; 33: 627–38; PMID:19285945; http://dx.doi.org/ 10.1016/j.molcel.2009.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S, Tarsio M, Kane PM, Greenberg ML. Cardiolipin mediates cross-talk between mitochondria and the vacuole. Mol Biol Cell 2008; 19:5047-58; PMID:18799619; http://dx.doi.org/ 10.1091/mbc.E08-05-0486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang W, Choi W, Hu W, Mi N, Guo Q, Ma M, Liu M, Tian Y, Lu P, Wang FL, et al.. Crystal structure and biochemical analyses reveal Beclin 1 as a novel membrane binding protein. Cell Res 2012; 22: 473–89; PMID:22310240; http://dx.doi.org/ 10.1038/cr.2012.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshi AS, Thompson MN, Fei N, Huttemann M, Greenberg ML. Cardiolipin and mitochondrial phosphatidylethanolamine have overlapping functions in mitochondrial fusion in Saccharomyces cerevisiae. J Biol Chem 2012; 287: 17589–97; PMID:22433850; http://dx.doi.org/ 10.1074/jbc.M111.330167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barth PG, Scholte HR, Berden JA, Van der Klei-Van Moorsel JM, Luyt-Houwen IE, Van't Veer-Korthof ET, Van der Harten JJ, Sobotka-Plojhar MA. An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J Neurol Sci 1983; 62: 327–55; PMID:6142097; http://dx.doi.org/ 10.1016/0022-510X(83)90209-5 [DOI] [PubMed] [Google Scholar]
- 16.Vreken P, Valianpour F, Nijtmans LG, Grivell LA, Plecko B, Wanders RJ, Barth PG. Defective remodeling of cardiolipin and phosphatidylglycerol in Barth syndrome. Biochem Biophys Res Commun 2000; 279: 378–82; PMID:11118295; http://dx.doi.org/ 10.1006/bbrc.2000.3952 [DOI] [PubMed] [Google Scholar]
- 17.Schlame M, Kelley RI, Feigenbaum A, Towbin JA, Heerdt PM, Schieble T, Wanders RJ, DiMauro S, Blanck TJ. Phospholipid abnormalities in children with Barth syndrome. J Am Coll Cardiol 2003; 42: 1994–9; PMID:14662265; http://dx.doi.org/ 10.1016/j.jacc.2003.06.015 [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Sutachan JJ, Plesken H, Kelley RI, Schlame M. Characterization of lymphoblast mitochondria from patients with Barth syndrome. Lab Invest; J Tech Methods Pathol 2005; 85: 823–30; PMID:15806137; http://dx.doi.org/ 10.1038/labinvest.3700274 [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Condell M, Plesken H, Edelman-Novemsky I, Ma J, Ren M, Schlame M. A Drosophila model of Barth syndrome. Proc Natl Acad Sci U S A 2006; 103: 11584–8; PMID:16855048; http://dx.doi.org/ 10.1073/pnas.0603242103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen S, He Q, Greenberg ML. Loss of tafazzin in yeast leads to increased oxidative stress during respiratory growth. Mol Microbiol 2008; 68: 1061–72; PMID:18430085; http://dx.doi.org/ 10.1111/j.1365-2958.2008.06216.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, et al.. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med 2014; 20: 616–23; PMID:24813252; http://dx.doi.org/ 10.1038/nm.3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saini-Chohan HK, Mitchell RW, Vaz FM, Zelinski T, Hatch GM. Delineating the role of alterations in lipid metabolism to the pathogenesis of inherited skeletal and cardiac muscle disorders: thematic review series: genetics of human lipid diseases. J Lipid Res 2012; 53: 4–27; PMID:22065858; http://dx.doi.org/ 10.1194/jlr.R012120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Y. Emerging roles of cardiolipin remodeling in mitochondrial dysfunction associated with diabetes, obesity, and cardiovascular diseases. J Biomed Res 2010; 24: 6–15; PMID:23554606; http://dx.doi.org/ 10.1016/S1674-8301(10)60003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soustek MS, Falk DJ, Mah CS, Toth MJ, Schlame M, Lewin AS, Byrne BJ. Characterization of a transgenic short hairpin RNA-induced murine model of Tafazzin deficiency. Hum Gene Ther 2011; 22: 865–71; PMID:21091282; http://dx.doi.org/ 10.1089/hum.2010.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acehan D, Vaz F, Houtkooper RH, James J, Moore V, Tokunaga C, Kulik W, Wansapura J, Toth MJ, Strauss A, et al.. Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J Biol Chem 2011; 286: 899–908; PMID:21068380; http://dx.doi.org/ 10.1074/jbc.M110.171439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, Lippincott-Schwartz J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell 2010; 141: 656–67; PMID:20478256; http://dx.doi.org/ 10.1016/j.cell.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu CT. A pivotal role for PINK1 and autophagy in mitochondrial quality control: implications for Parkinson disease. Hum Mol Genet 2010; 19: R28–37; PMID:20385539; http://dx.doi.org/ 10.1093/hmg/ddq143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, et al.. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 2010; 189: 211–21; PMID:20404107; http://dx.doi.org/ 10.1083/jcb.200910140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, et al.. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 2008; 27: 433–46; PMID:18200046; http://dx.doi.org/ 10.1038/sj.emboj.7601963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang M, Mileykovskaya E, Dowhan W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J Biol Chem 2002; 277: 43553–6; PMID:12364341; http://dx.doi.org/ 10.1074/jbc.C200551200 [DOI] [PubMed] [Google Scholar]
- 31.Brandner K, Mick DU, Frazier AE, Taylor RD, Meisinger C, Rehling P. Taz1, an outer mitochondrial membrane protein, affects stability and assembly of inner membrane protein complexes: implications for barth syndrome. Mol Biol Cell 2005; 16: 5202–14; PMID:16135531; http://dx.doi.org/ 10.1091/mbc.E05-03-0256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeVay RM, Dominguez-Ramirez L, Lackner LL, Hoppins S, Stahlberg H, Nunnari J. Coassembly of Mgm1 isoforms requires cardiolipin and mediates mitochondrial inner membrane fusion. J Cell Biol 2009; 186: 793–803; PMID:19752025; http://dx.doi.org/ 10.1083/jcb.200906098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwall CT, Greenwood VL, Alder NN. The stability and activity of respiratory Complex II is cardiolipin-dependent. Biochimica et Biophysica Acta 2012; 1817: 1588–96; PMID:22575443; http://dx.doi.org/ 10.1016/j.bbabio.2012.04.015 [DOI] [PubMed] [Google Scholar]
- 34.van Gestel RA, Rijken PJ, Surinova S, O'Flaherty M, Heck AJ, Killian JA, de Kroon AI, Slijper M. The influence of the acyl chain composition of cardiolipin on the stability of mitochondrial complexes; an unexpected effect of cardiolipin in alpha-ketoglutarate dehydrogenase and prohibitin complexes. J Proteomics 2010; 73: 806–14; PMID:19944197; http://dx.doi.org/ 10.1016/j.jprot.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 35.McKenzie M, Lazarou M, Thorburn DR, Ryan MT. Mitochondrial respiratory chain supercomplexes are destabilized in Barth Syndrome patients. J Mol Biol 2006; 361: 462–9; PMID:16857210; http://dx.doi.org/ 10.1016/j.jmb.2006.06.057 [DOI] [PubMed] [Google Scholar]
- 36.Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet 2012; 46: 265–87; PMID:22934639; http://dx.doi.org/ 10.1146/annurev-genet-110410-132529 [DOI] [PubMed] [Google Scholar]
- 37.Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 2010; 8: e1000298; PMID:20126261; http://dx.doi.org/ 10.1371/journal.pbio.1000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karkucinska-Wieckowska A, Trubicka J, Werner B, Kokoszynska K, Pajdowska M, Pronicki M, Czarnowska E, Lebiedzinska M, Sykut-Cegielska J, Ziolkowska L, et al.. Left ventricular noncompaction (LVNC) and low mitochondrial membrane potential are specific for Barth syndrome. J Inherited Metab Dis 2013; 36: 929–37; PMID:23361305; http://dx.doi.org/ 10.1007/s10545-013-9584-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spencer CT, Byrne BJ, Bryant RM, Margossian R, Maisenbacher M, Breitenger P, Benni PB, Redfearn S, Marcus E, Cade WT. Impaired cardiac reserve and severely diminished skeletal muscle O(2) utilization mediate exercise intolerance in Barth syndrome. Am J Physiol Heart Circ Physiol 2011; 301: H2122–9; PMID:21873497; http://dx.doi.org/ 10.1152/ajpheart.00479.2010 [DOI] [PubMed] [Google Scholar]
- 40.Nie J, Sun C, Faruque O, Ye G, Li J, Liang Q, Chang Z, Yang W, Han X, Shi Y. Synapses of amphids defective (SAD-A) kinase promotes glucose-stimulated insulin secretion through activation of p21-activated kinase (PAK1) in pancreatic beta-cells. J Biol Chem 2012; 287: 26435–44; PMID:22669945; http://dx.doi.org/ 10.1074/jbc.M112.378372 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.